Abstract
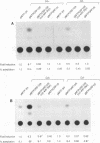
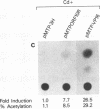
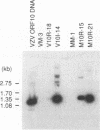
The varicella-zoster virus (VZV) open reading frame 10 (ORF10) protein is the homolog of the herpes simplex virus type 1 (HSV-1) protein VP16. These are two virion tegument proteins that have extensive amino acid sequence identity in their amino-terminal and middle domains. ORF10, however, lacks the acidic carboxy terminus which is critical for transactivation by VP16. Earlier studies showed that VZV ORF10 does not form a tertiary complex with the TAATGARAT regulatory element (where R is a purine) with which HSV-1 VP16 interacts, suggesting that ORF10 may not have transactivating ability. Using transient-expression assays, we show that VZV ORF10 is able to transactivate VZV immediate-early (IE) gene (ORF62) and HSV-1 IE gene (ICP4 and ICP0) promoters. Furthermore, cell lines stably expressing ORF10 complement the HSV-1 mutant in1814, which lacks the transactivating function of VP16, and enhance the de novo synthesis of infectious virus following transfection of HSV-1 virion DNA. These results indicate that ORF10, like its HSV-1 homolog VP16, is a transactivating protein despite the absence of sequences similar to the VP16 carboxy-terminal domain. The transactivating function of the VZV ORF10 tegument protein may be critical for efficient initiation of viral infection.
Full text
PDF


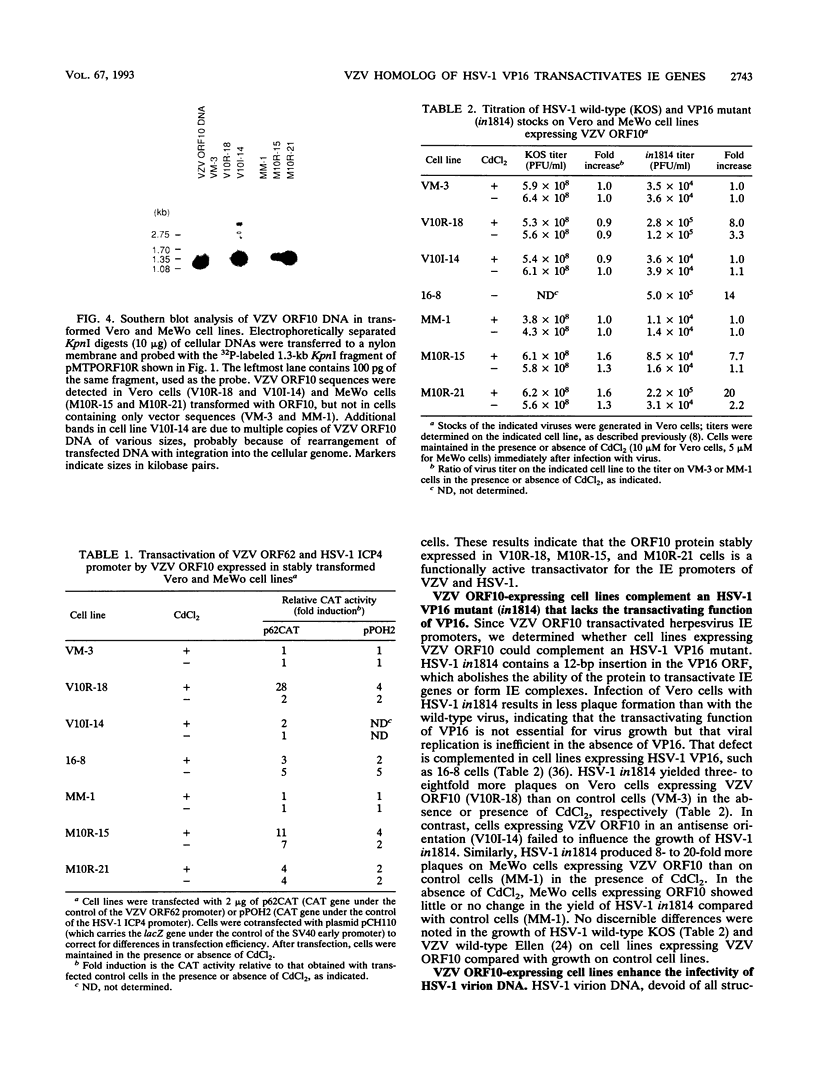
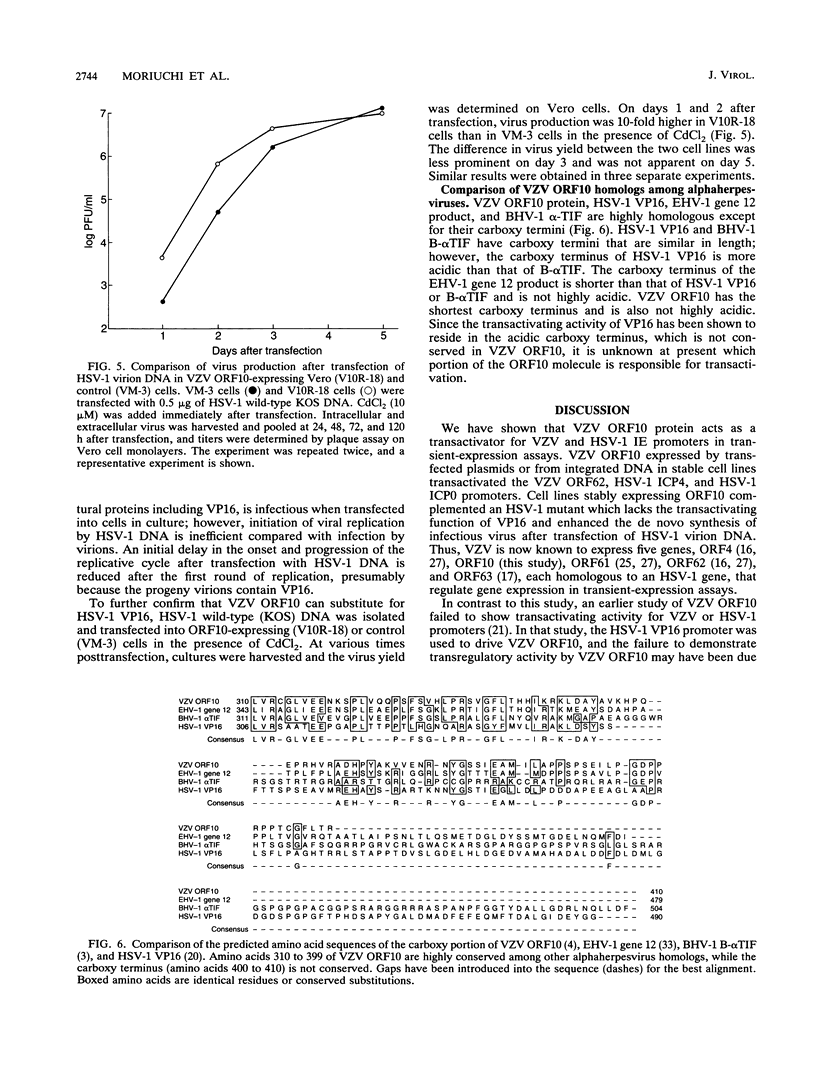


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ace C. I., Dalrymple M. A., Ramsay F. H., Preston V. G., Preston C. M. Mutational analysis of the herpes simplex virus type 1 trans-inducing factor Vmw65. J Gen Virol. 1988 Oct;69(Pt 10):2595–2605. doi: 10.1099/0022-1317-69-10-2595. [DOI] [PubMed] [Google Scholar]
- Batterson W., Roizman B. Characterization of the herpes simplex virion-associated factor responsible for the induction of alpha genes. J Virol. 1983 May;46(2):371–377. doi: 10.1128/jvi.46.2.371-377.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter D. E., Misra V. Sequences of the bovine herpesvirus 1 homologue of herpes simplex virus type-1 alpha-trans-inducing factor (UL48). Gene. 1992 Oct 1;119(2):259–263. doi: 10.1016/0378-1119(92)90280-3. [DOI] [PubMed] [Google Scholar]
- Davison A. J., Scott J. E. The complete DNA sequence of varicella-zoster virus. J Gen Virol. 1986 Sep;67(Pt 9):1759–1816. doi: 10.1099/0022-1317-67-9-1759. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disney G. H., Everett R. D. A herpes simplex virus type 1 recombinant with both copies of the Vmw175 coding sequences replaced by the homologous varicella-zoster virus open reading frame. J Gen Virol. 1990 Nov;71(Pt 11):2681–2689. doi: 10.1099/0022-1317-71-11-2681. [DOI] [PubMed] [Google Scholar]
- Felser J. M., Kinchington P. R., Inchauspe G., Straus S. E., Ostrove J. M. Cell lines containing varicella-zoster virus open reading frame 62 and expressing the "IE" 175 protein complement ICP4 mutants of herpes simplex virus type 1. J Virol. 1988 Jun;62(6):2076–2082. doi: 10.1128/jvi.62.6.2076-2082.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felser J. M., Straus S. E., Ostrove J. M. Varicella-zoster virus complements herpes simplex virus type 1 temperature-sensitive mutants. J Virol. 1987 Jan;61(1):225–228. doi: 10.1128/jvi.61.1.225-228.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelman I. H., Silverstein S. Co-ordinate regulation of herpes simplex virus gene expression is mediated by the functional interaction of two immediate early gene products. J Mol Biol. 1986 Oct 5;191(3):395–409. doi: 10.1016/0022-2836(86)90135-x. [DOI] [PubMed] [Google Scholar]
- Gerster T., Roeder R. G. A herpesvirus trans-activating protein interacts with transcription factor OTF-1 and other cellular proteins. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6347–6351. doi: 10.1073/pnas.85.17.6347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Greaves R., O'Hare P. Separation of requirements for protein-DNA complex assembly from those for functional activity in the herpes simplex virus regulatory protein Vmw65. J Virol. 1989 Apr;63(4):1641–1650. doi: 10.1128/jvi.63.4.1641-1650.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigh A., Greaves R., O'Hare P. Interference with the assembly of a virus-host transcription complex by peptide competition. Nature. 1990 Mar 15;344(6263):257–259. doi: 10.1038/344257a0. [DOI] [PubMed] [Google Scholar]
- Heine J. W., Honess R. W., Cassai E., Roizman B. Proteins specified by herpes simplex virus. XII. The virion polypeptides of type 1 strains. J Virol. 1974 Sep;14(3):640–651. doi: 10.1128/jvi.14.3.640-651.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honess R. W., Roizman B. Regulation of herpesvirus macromolecular synthesis. I. Cascade regulation of the synthesis of three groups of viral proteins. J Virol. 1974 Jul;14(1):8–19. doi: 10.1128/jvi.14.1.8-19.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
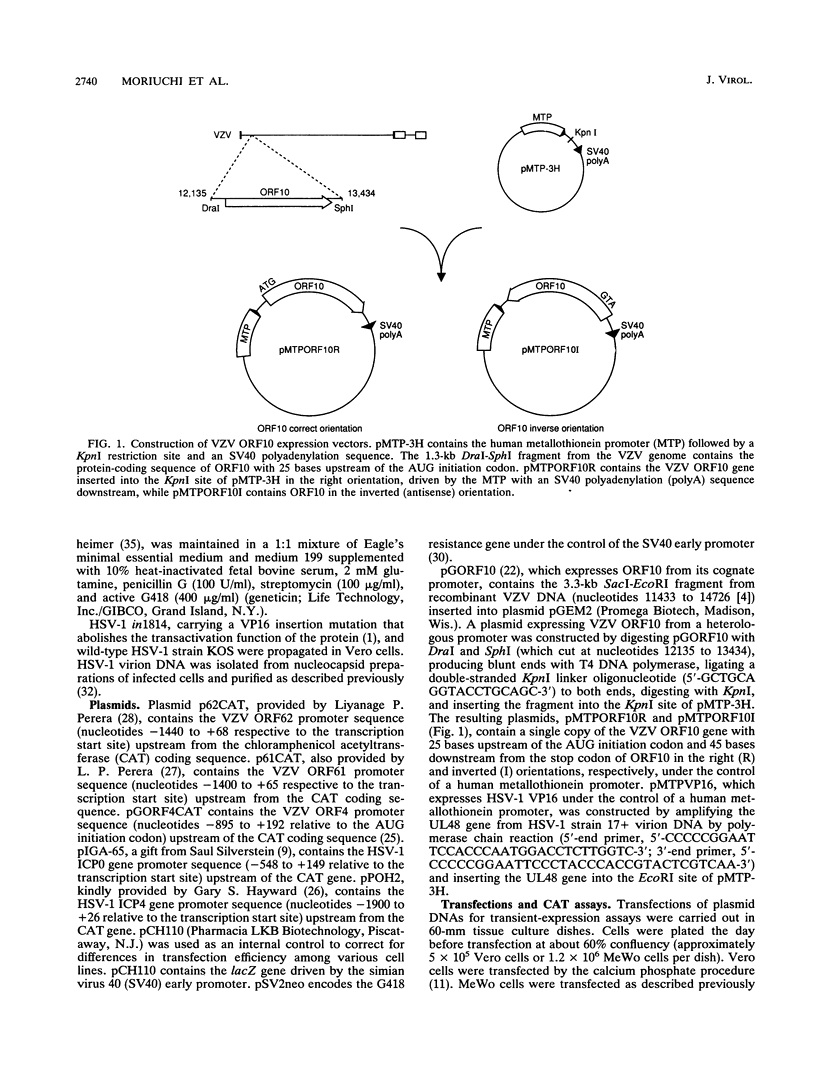
- Inchauspe G., Nagpal S., Ostrove J. M. Mapping of two varicella-zoster virus-encoded genes that activate the expression of viral early and late genes. Virology. 1989 Dec;173(2):700–709. doi: 10.1016/0042-6822(89)90583-7. [DOI] [PubMed] [Google Scholar]
- Jackers P., Defechereux P., Baudoux L., Lambert C., Massaer M., Merville-Louis M. P., Rentier B., Piette J. Characterization of regulatory functions of the varicella-zoster virus gene 63-encoded protein. J Virol. 1992 Jun;66(6):3899–3903. doi: 10.1128/jvi.66.6.3899-3903.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinchington P. R., Hougland J. K., Arvin A. M., Ruyechan W. T., Hay J. The varicella-zoster virus immediate-early protein IE62 is a major component of virus particles. J Virol. 1992 Jan;66(1):359–366. doi: 10.1128/jvi.66.1.359-366.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackem S., Roizman B. Structural features of the herpes simplex virus alpha gene 4, 0, and 27 promoter-regulatory sequences which confer alpha regulation on chimeric thymidine kinase genes. J Virol. 1982 Dec;44(3):939–949. doi: 10.1128/jvi.44.3.939-949.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeoch D. J., Dalrymple M. A., Davison A. J., Dolan A., Frame M. C., McNab D., Perry L. J., Scott J. E., Taylor P. The complete DNA sequence of the long unique region in the genome of herpes simplex virus type 1. J Gen Virol. 1988 Jul;69(Pt 7):1531–1574. doi: 10.1099/0022-1317-69-7-1531. [DOI] [PubMed] [Google Scholar]
- McKee T. A., Disney G. H., Everett R. D., Preston C. M. Control of expression of the varicella-zoster virus major immediate early gene. J Gen Virol. 1990 Apr;71(Pt 4):897–906. doi: 10.1099/0022-1317-71-4-897. [DOI] [PubMed] [Google Scholar]
- Meier J. L., Holman R. P., Croen K. D., Smialek J. E., Straus S. E. Varicella-zoster virus transcription in human trigeminal ganglia. Virology. 1993 Mar;193(1):193–200. doi: 10.1006/viro.1993.1115. [DOI] [PubMed] [Google Scholar]
- Moriuchi H., Moriuchi M., Smith H. A., Straus S. E., Cohen J. I. Varicella-zoster virus open reading frame 61 protein is functionally homologous to herpes simplex virus type 1 ICP0. J Virol. 1992 Dec;66(12):7303–7308. doi: 10.1128/jvi.66.12.7303-7308.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagpal S., Ostrove J. M. Characterization of a potent varicella-zoster virus-encoded trans-repressor. J Virol. 1991 Oct;65(10):5289–5296. doi: 10.1128/jvi.65.10.5289-5296.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hare P., Hayward G. S. Comparison of upstream sequence requirements for positive and negative regulation of a herpes simplex virus immediate-early gene by three virus-encoded trans-acting factors. J Virol. 1987 Jan;61(1):190–199. doi: 10.1128/jvi.61.1.190-199.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera L. P., Mosca J. D., Ruyechan W. T., Hay J. Regulation of varicella-zoster virus gene expression in human T lymphocytes. J Virol. 1992 Sep;66(9):5298–5304. doi: 10.1128/jvi.66.9.5298-5304.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera L. P., Mosca J. D., Sadeghi-Zadeh M., Ruyechan W. T., Hay J. The varicella-zoster virus immediate early protein, IE62, can positively regulate its cognate promoter. Virology. 1992 Nov;191(1):346–354. doi: 10.1016/0042-6822(92)90197-w. [DOI] [PubMed] [Google Scholar]
- Purewal A. S., Smallwood A. V., Kaushal A., Adegboye D., Edington N. Identification and control of the cis-acting elements of the immediate early gene of equid herpesvirus type 1. J Gen Virol. 1992 Mar;73(Pt 3):513–519. doi: 10.1099/0022-1317-73-3-513. [DOI] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Stern S., Tanaka M., Herr W. The Oct-1 homoeodomain directs formation of a multiprotein-DNA complex with the HSV transactivator VP16. Nature. 1989 Oct 19;341(6243):624–630. doi: 10.1038/341624a0. [DOI] [PubMed] [Google Scholar]
- Straus S. E., Aulakh H. S., Ruyechan W. T., Hay J., Casey T. A., Vande Woude G. F., Owens J., Smith H. A. Structure of varicella-zoster virus DNA. J Virol. 1981 Nov;40(2):516–525. doi: 10.1128/jvi.40.2.516-525.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telford E. A., Watson M. S., McBride K., Davison A. J. The DNA sequence of equine herpesvirus-1. Virology. 1992 Jul;189(1):304–316. doi: 10.1016/0042-6822(92)90706-u. [DOI] [PubMed] [Google Scholar]
- Triezenberg S. J., Kingsbury R. C., McKnight S. L. Functional dissection of VP16, the trans-activator of herpes simplex virus immediate early gene expression. Genes Dev. 1988 Jun;2(6):718–729. doi: 10.1101/gad.2.6.718. [DOI] [PubMed] [Google Scholar]
- Weinheimer S. P., Boyd B. A., Durham S. K., Resnick J. L., O'Boyle D. R., 2nd Deletion of the VP16 open reading frame of herpes simplex virus type 1. J Virol. 1992 Jan;66(1):258–269. doi: 10.1128/jvi.66.1.258-269.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werstuck G., Bilan P., Capone J. P. Enhanced infectivity of herpes simplex virus type 1 viral DNA in a cell line expressing the trans-inducing factor Vmw65. J Virol. 1990 Mar;64(3):984–991. doi: 10.1128/jvi.64.3.984-991.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]