Abstract
Genetic or epigenetic inactivation of the pathway formed by the Fanconi anemia (FA) and BRCA1 proteins occurs in several cancer types, making the affected tumors potentially hypersensitive to DNA cross-linkers and other chemotherapeutic agents. It has been proposed that the inability of FA/BRCA-defective cells to form subnuclear foci of effector proteins, such as FANCD2, can be used as a biomarker to aid individualization of chemotherapy. We show that FANCD2 inactivation not only renders cells sensitive to cross-links, but also oxidative stress, a common effect of cancer therapeutics. Oxidative stress sensitivity does not correlate with FANCD2 or RAD51 foci formation, but associates with increased γH2AX foci levels and apoptosis. Therefore, FANCD2 may protect cells against cross-links and oxidative stress through distinct mechanisms, consistent with the growing notion that the pathway is not linear. Our data emphasize the need for multiple biomarkers, such as γH2AX, FANCD2, and RAD51, to capture all pathway activities.
1. INTRODUCTION
Fanconi anemia (FA) is a heterogeneous clinical syndrome characterized by bone marrow failure, congenital defects, and cancer predisposition [1–5]. Cells derived from FA patients exhibit multiple abnormalities including chromosomal instability and hypersensitivity to genotoxic agents, particularly drugs that cause DNA interstrand cross-links (ICLs), such as mitomycin C (MMC). FA is caused by mutations in any of the known 13 FANC genes, FANCA through FANCN [2, 5–7]. The FANC proteins together with BRCA1 cooperate in a common biochemical pathway, the FA/BRCA pathway, which is believed to function mainly in the detection, stabilization, and repair of stalled DNA replication forks [2, 4]. A multiprotein nuclear core complex is required for baseline and damage-induced monoubiquitination of the downstream effectors FANCD2 and FANCI [7]. In response to DNA damage such as replication fork-blocking ICLs, monoubiquitinated FANCD2 relocates into chromatin and colocalizes with BRCA2/FANCD1, RAD51, and other DNA damage response proteins; and these protein accumulations can be visualized as subnuclear foci [8–13]. Disruption of the nuclear core complex or mutation of FANCD2's monoubiquitination site at K561 impairs repair processes, including homologous recombination, that are active at stalled forks [8, 14]. These repair defects cause chromosomal aberrations, particularly chromatid-type breaks and exchanges, and cell death following ICL induction.
Inactivation of the FA/BRCA pathway by genetic or epigenetic mechanisms, which are frequently found in cancer, can be detected by the inability of the affected cells to form FANCD2 foci in response to DNA damage [15, 16]. There is currently great interest in using FANCD2 foci formation as a functional biomarker to predict the sensitivity of cancer cells to cross-linking drugs such as cisplatin [16].
In addition to ICL hypersensitivity, it has been proposed that FA cells suffer from a prooxidant state that is associated with overproduction or impaired detoxification of reactive oxygen species [17]. Cells with defects in the FA pathway may demonstrate increased apoptosis, hypersensitivity to oxygen, excessive oxidative DNA damage after treatment with hydrogen peroxide (H2O2), improved growth when maintained at low oxygen tension, or reduction of chromosomal aberrations after treatment with antioxidants [18–21]. At least for FANCC, molecular mechanisms have been identified that support a role in redox metabolism and apoptosis [22–24]. Mutational analysis dissociated FANCC's participation in apoptotic signaling pathways from its role in the response to MMC [25]. Other FA proteins for which an involvement in oxidative stress responses have been reported include FANCG, which not only participates in redox-regulated nuclear complex formation [26], but also locates to mitochondria where it interacts with peroxidase to prevent oxidative stress-induced apoptosis [27].
Importantly, the oxidative stress sensitivity caused by FA/BRCA defects likely results in or contributes to cellular chemosensitivity for various agents [28]. However, it is unknown whether this sensitivity can be detected by abrogated FANCD2 or RAD51 foci formation. Here, we use a human model cell line to describe a dual function of FANCD2 that protects against ICLs and oxidative damage through distinct mechanisms, which cannot be encompassed by a single protein biomarker.
2. MATERIAL AND METHODS
2.1. Cell lines
SV40-transformed fibroblasts derived from patients with FA group D2 (PD20 cells) or A (PD220) and their retrovirally complemented counterparts expressing wild-type protein were obtained from the OHSU Fanconi anemia cell repository [29]. Cells were maintained in αMEM with 2 mM glutamine and 15% fetal bovine serum (Sigma-Aldrich, Saint Louis, USA). All cell lines tested free of mycoplasma.
2.2. Cytotoxicity assays
Exponentially growing cells were incubated with 0–50 μM H2O2 at room temperature for 2 hours or 0–0.5 μg/mL MMC for 1 hour (both Sigma-Aldrich). Cells were plated for colony formation and stained after 14 days with methylene blue. Apoptosis was measured 24–48 hours after treatment. For scoring of apoptotic nuclei, cells were stained with DAPI (10 μg/mL). 500 nuclei per sample were examined with a fluorescence microscope (Olympus BX51) and assessed for cell morphology and apoptotic bodies. For detection of the sub-G1 DNA fraction, cells were stained with 0.1 mg/mL PI, containing 0.5 mg/mL RNase and 0.1% NP40 detergent, and assayed by flow cytometry (FACSCalibur, Becton Dickinson, Franklin Lakes, USA). 30,000 cells were analyzed using CellQuest software. Sub-G1 populations were determined by histogram gating.
2.3. Detection of subnuclear protein foci
Cells were seeded in chamber slides or onto cover slides and treated with H2O2 (25 μM) or MMC (0.25 μg/mL). After 0.5–5 hours, cells were fixed for 15 minutes with 4% paraformaldehyde at room temperature and permeabilized for 10 minutes with 0.5% Triton X-100 (Sigma-Aldrich) in phosphate-buffered saline. Following blocking with 10% serum for 1 hour at room temperature, cells were incubated for 2 hours at 37°C with anti-γ-H2AX (1 : 100 dilution, #4411-PC-100 from Trevigen, Gaithersburg, USA), anti-Rad51 (1 : 200, Ab-1 from Calbiochem, EMD Chemicals, San Diego, USA), or anti-FANCD2 (1 : 400, NB100-182 from Novus, Novus Biological, Colorado, USA) antibody. This was followed by incubation with species-specific fluorescein- or Alexa-488-conjugated secondary antibody (Pierce #31583 or Molecular Probes #A-21441). All slides were counterstained with DAPI and visualized by fluorescence microscopy. Only cells with nuclei containing more than five foci were scored. At least 300 nuclei were examined for each data point.
2.4. Cell cycle analysis
Cell cycle distributions with and without H2O2 treatment (25 μM for 2 hours) were assessed using PI staining. To measure DNA synthesis after treatment with H2O2 or ionizing radiation (IR) (Siemens Stabilipan 2 X-ray generator, 250 KVp, Siemens Medical Systems, Malvern, USA), cells were pulse labeled with 100 μM (+)-5-bromo-2′-deoxyuridine (BrdU, Sigma Aldrich) for 30 minutes at 37°C, blocked with 0.5% Tween 20, and incubated with anti-BrdU antibody (Becton Dickinson) for 1 hour at 37°C. Subsequently, cells were incubated with FITC-conjugated rabbit antimouse antibody (Dako, Carpinteria, USA) at 37°C for 45 minutes. BrdU-positive nuclei were scored using fluorescence microscopy and at least 300 nuclei were counted for each data point.
2.5. Cytogenetic studies
Analysis of chromosomal damage was performed as described [30]. Lethal G1 aberrations were scored including terminal and interstitial deletions as well as dicentric chromosomes. For G2 aberrations, chromatid-type aberrations such as chromatid fragments, isochromatid fragments, translocations as well as tri- and quadriradials were expressed as breaks per cell.
3. RESULTS
3.1. FANCD2 foci only partially reflect protein function
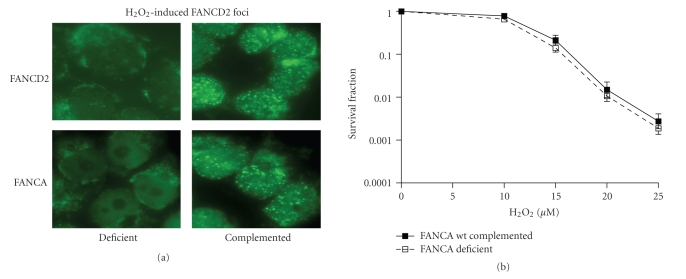
The ability of the FA pathway to remove replication fork blocking ICLs is dependent upon intact FANCD2 function. Mutation of FANCD2 in immortalized human fibroblasts obtained from a patient with FA group D2 (PD20) leads to ICL sensitivity (Figure 1(a)) and abrogated FANCD2 foci formation (not shown), while the wild-type complemented derivative cell line (PD20-wtD2) was normal in this regard. Accumulating evidence also links components of the FA pathway to the cellular response to oxidative stress [1, 17, 31, 32], but whether this process depends on foci formation as well was unknown. To address this question, cells were exposed to low concentrations of H2O2, which do not cause measurable DNA double-strand breaks (DSBs). We discovered that PD20 cells were significantly more sensitive to H2O2 than the PD20-wtD2 cells expressing wild-type FANCD2 (Figure 1(b)), which has not been described previously. Surprisingly, while H2O2 was a potent inducer of FANCD2 foci in cells with a functional FA pathway (Figure 2(a)), FANCA-mutant PD220 fibroblasts were not hypersensitive to H2O2 despite an inability of these cells to form FANCD2 foci (Figure 2(b)). This suggested that the aspect of FANCD2 function that depends on foci formation is not required for mediating resistance to oxidative DNA damage.
Figure 1.
Hypersensitivity of FANCD2-deficient human fibroblasts to DNA damage. (a) Clonogenic survival of cells with or without wild-type FANCD2 (PD20-wtD2 or PD20, resp.) after treatment with varying concentrations of MMC for one hour. (b) Analogously, clonogenic survival after exposure to hydrogen peroxide (H2O2) for two hours. Data represent logarithmic means +/− standard error based on four independent repeats.
Figure 2.
Resistance of FANCA-mutant cells with defective FANCD2 function to hydrogen peroxide (H2O2). (a) Representative images illustrating staining for subnuclear FANCD2 foci in isogenic fibroblast pairs, either deficient for FANCD2 (PD20) or FANCA (PD220) and their respective wild-type (wt) complemented counterparts. Foci were visualized three hours after treatment with H2O2 (25 μM for 2 hours). (b) Clonogenic survival of cells with or without wild-type FANCA after treatment with varying concentrations of H2O2. Data represent logarithmic means +/− standard error from 15-repeat experiments.
3.2. FANCD2 mediates resistance to oxidative damage independently of its function in the replication-associated DNA damage response
RAD51 subnuclear foci are thought to reflect sites of homologous recombination, which is required for the repair and restart of stalled replication forks [2]. Accordingly, PD20 cells, which are hypersensitive to the cross-linker MMC, were unable to mount an RAD51 foci response following MMC exposure (Figures 3(a) and 3(b)). In contrast, PD20 cells were clearly able to form RAD51 foci in response to H2O2, which was comparable to the foci formation seen in PD20-wtD2 cells exposed to the same H2O2 concentration. Next, we asked whether the oxidative damage-induced S-phase checkpoint also remained intact in PD20 cells. As reported previously [33], PD20 cells continued to incorporate BrdU following IR, indicating a defective S-phase checkpoint (radioresistant DNA synthesis) (Figure 3(c)). However, in response to H2O2, PD20 cells demonstrated a decrease in DNA synthesis indicating intact checkpoint function in response to oxidative damage. We also analyzed cell cycle distributions in response to H2O2 (Figure 3(d)) or IR (data not shown) to ensure that our observations were not biased by major imbalances in the position of cells in the cell cycle. We found that the cell cycle profiles of PD20 and PD20-wtD2 cells, treated or untreated, were almost identical.
Figure 3.
Normal DNA damage responses in FANCD2-deficient cells treated with hydrogen peroxide (H2O2). (a) Illustration of RAD51 foci formation in PD20 and PD20-wtD2 cells 5 hours after exposure to MMC (0.25 μg/mL for 1 hour) or H2O2 (25 μM for 2 hours). (b) Induction of RAD51 foci formation above background levels by MMC or H2O2. Because equal drug concentrations were used, rather than isoeffective concentrations with regard to cell survival, the extent of foci induction between FANCD-deficient and -complemented cells is not directly comparable. Data represent means with upper standard error based on three independent repeats. (c) DNA synthesis measured by BrdU pulse labeling in cells treated with ionizing radiation (IR, 8 Gy) or H2O2 (25 μM). Data represent means with upper standard error based on two independent experiments. (d) Cell cycle distribution of propidium-iodide cell populations by flow cytometry. A representative experiment is shown. Percentages of cells in the G1, S, and G2 (and M) phases of the cell cycle are indicated.
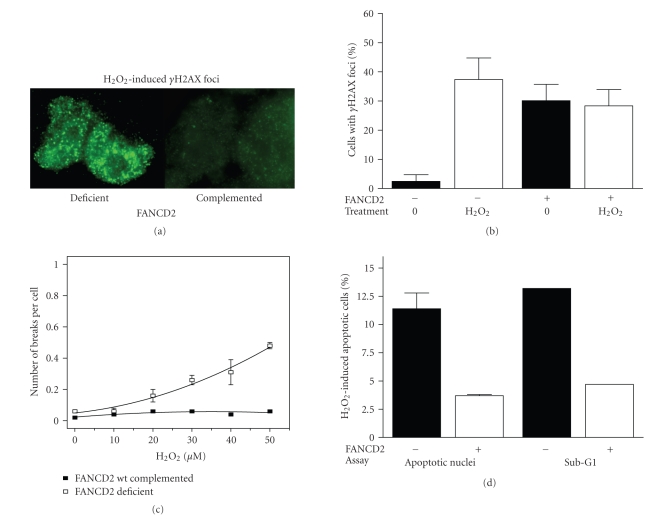
3.3. Increased cytotoxicity in H2O2-treated PD20 cells is not associated with induced chromosomal aberrations but increased apoptosis
Activation of the S-phase checkpoint by H2O2 (Figure 3(c)) suggested the presence of damaged DNA. We therefore studied γH2AX foci, which are typically used as markers of DSBs, but may also reflect stalled replication forks or oxidative damage [34, 35]. We discovered a substantial increase in the formation of γH2AX foci in PD20, but not PD20-wtD2 cells (Figures 4(a) and 4(b)). Next, we asked whether the increase in DNA damage as marked by γH2AX foci led to the occurrence of chromosomal aberrations in H2O2-treated PD20 cells. Treatment of FA cells with cross-linkers such as MMC or diepoxybutane generally results in a markedly elevated frequency of chromatid-type aberrations likely due to a failure to maintain and repair replication forks stalling at interstrand cross-links [2, 4] Typical radial structures are found in more than 20% of cells and the number of breaks per cell may range from 1 to 20 [3, 36]. In contrast, exposure of PD20 cells to H2O2 did not induce any radial formation up to a concentration of 40 μM of H2O2 despite the significant difference in clonogenic survival at doses up to 25 μM. We only recorded a mild increase in chromatid aberrations up to 0.5 break per cell at 50 μM of H2O2 (Figure 4(c)), which is clearly distinct from the hallmark chromosomal instability seen with cross-linkers yet consistent with the normal RAD51 and S-phase checkpoint responses to H2O2 (Figure 3). The observed lack of significant chromosomal instability in response to H2O2 is also consistent with previous observations [37]. To identify the mechanism of cell death, we turned our attention to apoptosis endpoints. Using two different assays, we found that H2O2-treated PD20 cells exhibited elevated levels of apoptosis compared to the wild-type complemented cells (Figure 4(d)). In contrast, MMC treatment does not typically induce increased apoptosis in FANC-deficient cells [38, 39]. Altogether, our data suggest that the mode of cell death following oxidative damage is not related to chromosomal aberrations that may arise due to failed repair of stalled replication forks.
Figure 4.
DNA damage and cell survival as a function of FANCD2 status. (a) Representative images of the formation of γH2AX foci in PD20 versus wild-type complemented cells 30 minutes after completion of H2O2 treatment. (b) Quantification of γH2AX foci response. Data represent means with upper standard error based on two independent experiments. (c) G2-type chromosomal aberrations are expressed as breaks per cell as a function of increasing H2O2 concentration in FANCD2-deficient and wild-type complemented PD20 cells. Data represent means with SEM based on at least three repeat experiments. (d) Apoptosis induction by H2O2 (50 μM) in cells with or without wild-type FANCD2 using fluorescence microscopy to assess apoptotic morphology by DAPI staining and flow cytometric analysis for sub-G1 DNA content. Representative experiments based on the apoptotic response at 24 hours are shown (similar results were obtained at 48 hours and with 25 μM H2O2).
4. DISCUSSION
The formation of subnuclear FANCD2 foci in response to damaged DNA has been regarded as a functional biomarker for the activity of the FA pathway [16, 40]. Here, we report for the first time that the ability of cells to form FANCD2 foci only partially reflects the activity of the FANCD2-dependent cytotoxic stress response. Specifically, FANCA-mutant cells were not found to be sensitive to DNA damage caused by H2O2 despite an inability to form FANCD2 foci (Figure 2). In addition, we found that the MMC hypersensitivity, but not the H2O2 hypersensitivity, of FANCD2-deficient fibroblasts was associated with an abrogation of RAD51 foci formation (Figure 3) [38], suggesting different cellular responses to the two agents. Of note, previous data on the ability of FA cells to form damage-induced RAD51 foci have been somewhat inconsistent, possibly a reflection of the cell type under study and the particular assay conditions [9–13]. Together, our data suggest that neither FANCD2 nor RAD51 foci formation adequately captures all activities of this pathway.
The mechanisms underlying the ICL and oxidative stress hypersensitivity of FA cells have been controversial [17, 41]. The susceptibility to oxidative stress has long been recognized as a general and uniform phenotype of primary cells from FA patients, while cross-linker sensitivity appears to be variable across complementation groups [1, 17, 21]. Saito et al. [21] found that the oxygen hypersensitivity of primary human fibroblast cultures was lost upon transformation with SV40 large T-Antigen, but the MMC hypersensitivity remained. It was thus argued that oxygen hypersensitivity represents a “secondary” defect of FA cells. Yet, our data indicate that SV40-transformed FANCD2-deficient PD20 fibroblasts have retained their susceptibility to oxygen (assuming that oxygen and H2O2 hypersensitivity are correlated). It is conceivable that differences in cell culture conditions used for establishing PD20 cells versus the cell lines reported by Saito et al. have had a differential impact on oxygen sensitivities. Alternatively, FANCD2 may play a more important role in the response to oxidative stress than other FA proteins such as FANCA. We acknowledge that because our cells are SV40-transformed, the p53 response is compromised; which could affect apoptosis, cell cycle profiles, and chromosomal aberrations. It will therefore be important to expand our findings to primary cell cultures.
The ICL resistance of cells is dependent upon the integrity of the nuclear FANC core complex, monoubiquitination, and subsequent chromatin localization of FANCD2 [8]. However, this model of the pathway appears to be incomplete. For example, a phosphomutant of FANCE restored FANCD2 monoubiquitination but not cross-linker sensitivity of FANCE-deficient lymphoblasts [42], and in a chicken cell system components of the FANC core complex mediated cross-linker resistance partly independent of FANCD2 monoubiquitination and chromatin targeting [43]. These observations suggest that the FANC core complex targets additional proteins required for cross-linker resistance and that nonubiquitinated FANCD2 may possess additional cellular functions. The latter notion is supported by the more severe phenotype of FANCD2−/− mice compared to FANCA or FANCC knockouts [10]. Part of the phenotype of FANCD2-deficient cells may also reflect an impaired function of FANCI, which forms a complex with FANCD2 [7].
We favor the view that FANC proteins are multifunctional proteins that form different subcomplexes with specific functions [1, 44]. In particular, FANCD2 appears to have a dual role in distinct cellular pathways that respond to replication fork damage and oxidative stress. Alternatively, the mechanisms underlying the observed H2O2 and ICL toxicity in FA cells may be overlapping, as discussed by Pagano et al. [28].
Of note, the observation that H2O2 induces the formation of FANCD2 foci (Figure 2) [41], which presumably locate to replication forks, is consistent with a dual model of function: various genotoxic stresses, including H2O2, hydroxyurea, or UV radiation, interfere with replication and thereby recruit FANCD2 to replication forks. However, only some types of DNA damage such as ICLs are severe enough to subsequently require the repair-promoting function of monoubiquitinated FANCD2, while cells exposed to UV or H2O2 do not appear to depend on this mechanism for their survival [11].
What are the molecular mechanisms by which FANCD2 protects against oxidative stress and apoptosis? Several interactions of FANCC with prosurvival and redox pathways have been reported [1, 22–24] and it is likely that FANCD2 possesses similar properties. Increased levels of γH2AX in FANCD2-deficient cells (Figure 4(b)) are consistent with an impaired ability to protect the DNA against reactive oxygen species, although we note that γH2AX foci may also reflect apoptosis, senescence, or changes in chromatin conformation that do not necessarily reflect DNA strand breakage [34, 45, 46]. The former is relevant to our observation that these cells may also be more prone to undergo apoptosis in the presence of high levels of oxidative DNA damage (Figure 4(d)). Interestingly, in a recent review article it has been hypothesized that H2O2 induces the formation of a complex containing FANCD2, FANCC, and STAT5 [1]. The complex may be required for optimal phosphorylation of STAT proteins, which function as nuclear transcription factors to promote cell survival.
In conclusion, the FA/BRCA pathway appears increasingly multifunctional and heterogeneous. Elucidating the precise roles of FANCD2 and other pathway members in the response to diverse cytotoxic stresses will be important for a better understanding of the chemosensitivity of cancers, which frequently harbor defects in this pathway. Multiple predictive biomarkers, including γH2AX, FANCD2, and RAD51 foci, are required for accurately identifying pre-existing FA/BRCA defects in tumors and thus aiding individualization of therapy.
ACKNOWLEDGMENTS
This work was supported in part by a grant from the Susan G. Komen for The Cure Breast Cancer Foundation to LAK, by NIH Grant no. P01 CA095227 to KDH, and by a grant from the Roggenbuck Foundation, Germany, to KB. The excellent technical assistance of Ms. Agnieszka Wrona is acknowledged.
References
- 1.Bagby GC, Alter BP. Fanconi anemia. Seminars in Hematology. 2006;43(3):147–156. doi: 10.1053/j.seminhematol.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 2.Kennedy RD, D'Andrea AD. The Fanconi anemia/BRCA pathway: new faces in the crowd. Genes & Development. 2005;19(24):2925–2940. doi: 10.1101/gad.1370505. [DOI] [PubMed] [Google Scholar]
- 3.Joenje H, Patel KJ. The emerging genetic and molecular basis of Fanconi anaemia. Nature Reviews Genetics. 2001;2(6):446–457. doi: 10.1038/35076590. [DOI] [PubMed] [Google Scholar]
- 4.Niedernhofer LJ, Lalai AS, Hoeijmakers JHJ. Fanconi anemia (cross)linked to DNA repair. Cell. 2005;123(7):1191–1198. doi: 10.1016/j.cell.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 5.Wang W. Emergence of a DNA-damage response network consisting of Fanconi anaemia and BRCA proteins. Nature Reviews Genetics. 2007;8(10):735–748. doi: 10.1038/nrg2159. [DOI] [PubMed] [Google Scholar]
- 6.Patel KJ. Fanconi anemia and breast cancer susceptibility. Nature Genetics. 2007;39(2):142–143. doi: 10.1038/ng0207-142. [DOI] [PubMed] [Google Scholar]
- 7.Smogorzewska A, Matsuoka S, Vinciguerra P, et al. Identification of the FANCI protein, a monoubiquitinated FANCD2 paralog required for DNA repair. Cell. 2007;129(2):289–301. doi: 10.1016/j.cell.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garcia-Higuera I, Taniguchi T, Ganesan S, et al. Interaction of the Fanconi anemia proteins and BRCA1 in a common pathway. Molecular Cell. 2001;7(2):249–262. doi: 10.1016/s1097-2765(01)00173-3. [DOI] [PubMed] [Google Scholar]
- 9.Godthelp BC, Wiegant WW, Waisfisz Q, et al. Inducibility of nuclear Rad51 foci after DNA damage distinguishes all Fanconi anemia complementation groups from D1/BRCA2. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis. 2006;594(1-2):39–48. doi: 10.1016/j.mrfmmm.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 10.Houghtaling S, Timmers C, Noll M, et al. Epithelial cancer in Fanconi anemia complementation group D2 (Fancd2) knockout mice. Genes & Development. 2003;17(16):2021–2035. doi: 10.1101/gad.1103403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tebbs RS, Hinz JM, Yamada NA, et al. New insights into the Fanconi anemia pathway from an isogenic FancG hamster CHO mutant. DNA Repair. 2005;4(1):11–22. doi: 10.1016/j.dnarep.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 12.Wang X, Andreassen PR, D'Andrea AD. Functional interaction of monoubiquitinated FANCD2 and BRCA2/FANCD1 in chromatin. Molecular and Cellular Biology. 2004;24(13):5850–5862. doi: 10.1128/MCB.24.13.5850-5862.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Digweed M, Rothe S, Demuth I, et al. Attenuation of the formation of DNA-repair foci containing RAD51 in Fanconi anaemia. Carcinogenesis. 2002;23(7):1121–1126. doi: 10.1093/carcin/23.7.1121. [DOI] [PubMed] [Google Scholar]
- 14.Nakanishi K, Yang Y-G, Pierce AJ, et al. Human Fanconi anemia monoubiquitination pathway promotes homologous DNA repair. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(4):1110–1115. doi: 10.1073/pnas.0407796102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lyakhovich A, Surralles J. Disruption of the Fanconi anemia/BRCA pathway in sporadic cancer. Cancer Letters. 2006;232(1):99–106. doi: 10.1016/j.canlet.2005.07.038. [DOI] [PubMed] [Google Scholar]
- 16.Kennedy RD, D'Andrea AD. DNA repair pathways in clinical practice: lessons from pediatric cancer susceptibility syndromes. Journal of Clinical Oncology. 2006;24(23):3799–3808. doi: 10.1200/JCO.2005.05.4171. [DOI] [PubMed] [Google Scholar]
- 17.Pagano G, Youssoufian H. Fanconi anaemia proteins: major roles in cell protection against oxidative damage. BioEssays. 2003;25(6):589–595. doi: 10.1002/bies.10283. [DOI] [PubMed] [Google Scholar]
- 18.Takeuchi T, Morimoto K. Increased formation of 8-hydroxydeoxyguanosine, an oxidative DNA damage, in lymphoblasts from Fanconi's anemia patients due to possible catalase deficiency. Carcinogenesis. 1993;14(6):1115–1120. doi: 10.1093/carcin/14.6.1115. [DOI] [PubMed] [Google Scholar]
- 19.Joenje H, Arwert F, Eriksson AW, de Koning H, Oostra AB. Oxygen-dependence of chromosomal aberrations in Fanconi's anaemia. Nature. 1981;290(5802):142–143. doi: 10.1038/290142a0. [DOI] [PubMed] [Google Scholar]
- 20.Dallapicola B, Porfirio B, Mokini V, Alimena G, Isacchi G, Gandini E. Effect of oxidants and antioxidants on chromosomal breakage in Fanconi anemia lymphocytes. Human Genetics. 1985;69(1):62–65. doi: 10.1007/BF00295530. [DOI] [PubMed] [Google Scholar]
- 21.Saito H, Hammond AT, Moses RE. Hypersensitivity to oxygen is a uniform and secondary defect in Fanconi anemia cells. Mutation Research/DNA Repair. 1993;294(3):255–262. doi: 10.1016/0921-8777(93)90008-5. [DOI] [PubMed] [Google Scholar]
- 22.Cumming RC, Lightfoot J, Beard K, Youssoufian H, O'brien PJ, Buchwald M. Fanconi anemia group C protein prevents apoptosis in hematopoietic cells through redox regulation of GSTP1. Nature Medicine. 2001;7(7):814–820. doi: 10.1038/89937. [DOI] [PubMed] [Google Scholar]
- 23.Fagerlie SR, Koretsky T, Torok-Storb B, Bagby GC. Impaired type I IFN-induced Jak/STAT signaling in FA-C cells and abnormal CD4+ Th cell subsets in Fancc−/− mice. Journal of Immunology. 2004;173(6):3863–3870. doi: 10.4049/jimmunol.173.6.3863. [DOI] [PubMed] [Google Scholar]
- 24.Saadatzadeh MR, Bijangi-Vishehsaraei K, Hong P, Bergmann H, Haneline LS. Oxidant hypersensitivity of Fanconi anemia type C-deficient cells is dependent on a redox-regulated apoptotic pathway. Journal of Biological Chemistry. 2004;279(16):16805–16812. doi: 10.1074/jbc.M313721200. [DOI] [PubMed] [Google Scholar]
- 25.Pang Q, Christianson TA, Keeble W, et al. The Fanconi anemia complementation group C gene product: structural evidence of multifunctionality. Blood. 2001;98(5):1392–1401. doi: 10.1182/blood.v98.5.1392. [DOI] [PubMed] [Google Scholar]
- 26.Park S-J, Ciccone SLM, Beck BD, et al. Oxidative stress/damage induces multimerization and interaction of Fanconi anemia proteins. Journal of Biological Chemistry. 2004;279(29):30053–30059. doi: 10.1074/jbc.M403527200. [DOI] [PubMed] [Google Scholar]
- 27.Mukhopadhyay SS, Leung KS, Hicks MJ, Hastings PJ, Youssoufian H, Plon SE. Defective mitochondrial peroxiredoxin-3 results in sensitivity to oxidative stress in Fanconi anemia. Journal of Cell Biology. 2006;175(2):225–235. doi: 10.1083/jcb.200607061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pagano G, Degan P, D'Ischia M, et al. Oxidative stress as a multiple effector in Fanconi anaemia clinical phenotype. European Journal of Haematology. 2005;75(2):93–100. doi: 10.1111/j.1600-0609.2005.00507.x. [DOI] [PubMed] [Google Scholar]
- 29.Jakobs PM, Sahaayaruban P, Saito H, et al. Immortalization of four new Fanconi anemia fibroblast cell lines by an improved procedure. Somatic Cell and Molecular Genetics. 1996;22(2):151–157. doi: 10.1007/BF02369905. [DOI] [PubMed] [Google Scholar]
- 30.Borgmann K, Röper B, El-Awady RA, et al. Indicators of late normal tissue response after radiotherapy for head and neck cancer: fibroblasts, lymphocytes, genetics, DNA repair, and chromosome aberrations. Radiotherapy and Oncology. 2002;64(2):141–152. doi: 10.1016/s0167-8140(02)00167-6. [DOI] [PubMed] [Google Scholar]
- 31.Bae I, Fan S, Meng Q, et al. BRCA1 induces antioxidant gene expression and resistance to oxidative stress. Cancer Research. 2004;64(21):7893–7909. doi: 10.1158/0008-5472.CAN-04-1119. [DOI] [PubMed] [Google Scholar]
- 32.Barzilai A, Rotman G, Shiloh Y. ATM deficiency and oxidative stress: a new dimension of defective response to DNA damage. DNA Repair. 2002;1(1):3–25. doi: 10.1016/s1568-7864(01)00007-6. [DOI] [PubMed] [Google Scholar]
- 33.Taniguchi T, Garcia-Higuera I, Xu B, et al. Convergence of the Fanconi anemia and ataxia telangiectasia signaling pathways. Cell. 2002;109(4):459–472. doi: 10.1016/s0092-8674(02)00747-x. [DOI] [PubMed] [Google Scholar]
- 34.Ward IM, Chen J. Histone H2AX is phosphorylated in an ATR-dependent manner in response to replicational stress. Journal of Biological Chemistry. 2001;276(51):47759–47762. doi: 10.1074/jbc.C100569200. [DOI] [PubMed] [Google Scholar]
- 35.Tanaka T, Halicka HD, Huang X, Traganos F, Darzynkiewicz Z. Constitutive histone H2AX phosphorylation and ATM activation, the reporters of DNA damage by endogenous oxidants. Cell Cycle. 2006;5(17):1940–1945. doi: 10.4161/cc.5.17.3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Timmers C, Taniguchi T, Hejna J, et al. Positional cloning of a novel Fanconi anemia gene, FANCD2. Molecular Cell. 2001;7(2):241–248. doi: 10.1016/s1097-2765(01)00172-1. [DOI] [PubMed] [Google Scholar]
- 37.Johnstone P, Reifsteck C, Kohler S, Worland P, Olson S, Moses RE. Fanconi anemia group A and D cell lines respond normally to inhibitors of cell cycle regulation. Somatic Cell and Molecular Genetics. 1997;23(6):371–377. doi: 10.1007/BF02673747. [DOI] [PubMed] [Google Scholar]
- 38.Kuhnert V, Kachnic L, Li L, et al. The role of FANCD2 in determining cellular resistance to ionizing radiation. International Journal of Radiation Oncology Biology Physics. 2007;69(3, supplement 1):S594–S595. [Google Scholar]
- 39.Ridet A, Guillouf C, Duchaud E, et al. Deregulated apoptosis is a hallmark of the Fanconi anemia syndrome. Cancer Research. 1997;57(9):1722–1730. [PubMed] [Google Scholar]
- 40.Burkitt K, Ljungman M. Compromised Fanconi anemia response due to BRCA1 deficiency in cisplatin-sensitive head and neck cancer cell lines. Cancer Letters. 2007;253(1):131–137. doi: 10.1016/j.canlet.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 41.D'Andrea AD. Cellular function of the Fanconi anemia pathway. Nature Medicine. 2001;7:1259–1260. doi: 10.1038/nm1201-1259a. [DOI] [PubMed] [Google Scholar]
- 42.Wang X, Kennedy RD, Ray K, Stuckert P, Ellenberger T, D'Andrea AD. Chk1-mediated phosphorylation of FANCE is required for the Fanconi anemia/BRCA pathway. Molecular and Cellular Biology. 2007;27(8):3098–3108. doi: 10.1128/MCB.02357-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matsushita N, Kitao H, Ishiai M, et al. A FancD2-monoubiquitin fusion reveals hidden functions of Fanconi anemia core complex in DNA repair. Molecular Cell. 2005;19(6):841–847. doi: 10.1016/j.molcel.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 44.Medhurst AL, Laghmani ElH, Steltenpool J, et al. Evidence for subcomplexes in the Fanconi anemia pathway. Blood. 2006;108(6):2072–2080. doi: 10.1182/blood-2005-11-008151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rogakou EP, Nieves-Neira W, Boon C, Pommier Y, Bonner WM. Initiation of DNA fragmentation during apoptosis induces phosphorylation of H2AX histone at serine 139. Journal of Biological Chemistry. 2000;275(13):9390–9395. doi: 10.1074/jbc.275.13.9390. [DOI] [PubMed] [Google Scholar]
- 46.Sedelnikova OA, Horikawa I, Redon C, et al. Delayed kinetics of DNA double-strand break processing in normal and pathological aging. Aging Cell. 2008;7(1):89–100. doi: 10.1111/j.1474-9726.2007.00354.x. [DOI] [PubMed] [Google Scholar]