Abstract
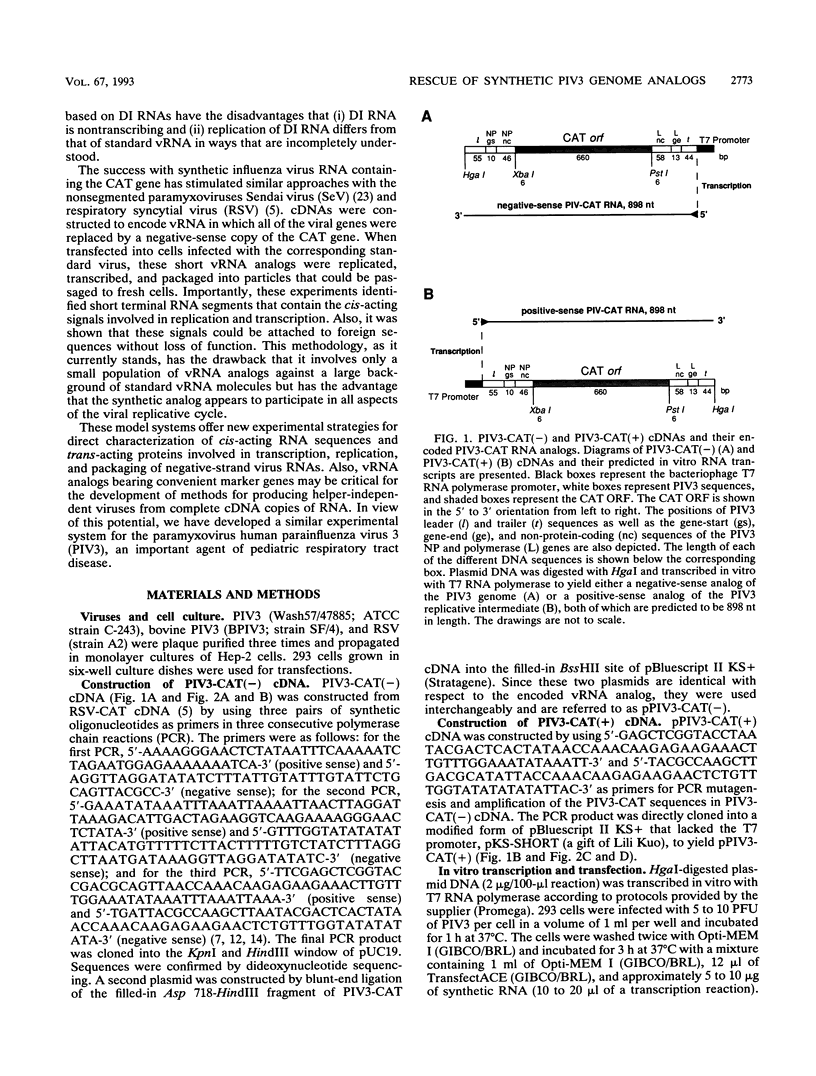
The genome of human parainfluenza virus type 3 (PIV3) is a single negative-sense RNA strand (vRNA) that is 15,463 nucleotides in length. A cDNA was constructed to encode an 898-nucleotide, internally deleted version of PIV3 vRNA, PIV3-CAT vRNA, in which the viral genes were replaced with the bacterial chloramphenicol acetyltransferase (CAT) reporter gene. The CAT gene was flanked in turn by sequences representing (i) nontranslated sequences of the first and last genes in the PIV3 genome, (ii) PIV3 gene-start and gene-end sequences, which are presumed to be transcription signals, and (iii) 3' extracistronic (leader) and 5' extracistronic (trailer) terminal regions of PIV3 vRNA. A second cDNA was constructed to encode the exact complement of PIV3-CAT vRNA; this positive-sense RNA, PIV3-CAT vcRNA, would correspond to the predicted replicative intermediate of PIV3-CAT vRNA. When synthesized in vitro by runoff transcription with T7 RNA polymerase and transfected separately into PIV3-infected cells, both PIV3-CAT vRNA and vcRNA were rescued with similar efficiencies; that is, they were expressed to yield CAT and were packaged into particles that could be used to infect fresh cells. Rescue of PIV3-CAT vRNA was strictly dependent on complementation by PIV3; PIV3 could not be replaced by respiratory syncytial virus or, unexpectedly, by a bovine strain of PIV3. Passage was blocked by prior incubation with neutralizing monoclonal antibodies specific to the PIV3 attachment protein. Also, during nine serial passages, the expression of CAT by PIV3-CAT vRNA increased more than 3,000-fold. These results indicated that the 3'-terminal 111 nucleotides and the 5'-terminal 115 nucleotides of PIV3 vRNA, which are present in PIV3-CAT vRNA, contained all of the cis-acting RNA sequences required for replication, gene expression, and transmission.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beeler J. A., van Wyke Coelingh K. Neutralization epitopes of the F glycoprotein of respiratory syncytial virus: effect of mutation upon fusion function. J Virol. 1989 Jul;63(7):2941–2950. doi: 10.1128/jvi.63.7.2941-2950.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castrucci M. R., Bilsel P., Kawaoka Y. Attenuation of influenza A virus by insertion of a foreign epitope into the neuraminidase. J Virol. 1992 Aug;66(8):4647–4653. doi: 10.1128/jvi.66.8.4647-4653.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins P. L., Mink M. A., Stec D. S. Rescue of synthetic analogs of respiratory syncytial virus genomic RNA and effect of truncations and mutations on the expression of a foreign reporter gene. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9663–9667. doi: 10.1073/pnas.88.21.9663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran J. A., Kolakofsky D. Rescue of a Sendai virus DI genome by other parainfluenza viruses: implications for genome replication. Virology. 1991 May;182(1):168–176. doi: 10.1016/0042-6822(91)90660-4. [DOI] [PubMed] [Google Scholar]
- Dimock K., Rud E. W., Kang C. Y. 3'-Terminal sequence of human parainfluenza virus 3 genomic RNA. Nucleic Acids Res. 1986 Jun 11;14(11):4694–4694. doi: 10.1093/nar/14.11.4694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duesberg P. H. Distinct subunits of the ribonucleoprotein of influenza virus. J Mol Biol. 1969 Jun 28;42(3):485–499. doi: 10.1016/0022-2836(69)90237-x. [DOI] [PubMed] [Google Scholar]
- Enami M., Luytjes W., Krystal M., Palese P. Introduction of site-specific mutations into the genome of influenza virus. Proc Natl Acad Sci U S A. 1990 May;87(10):3802–3805. doi: 10.1073/pnas.87.10.3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enami M., Palese P. High-efficiency formation of influenza virus transfectants. J Virol. 1991 May;65(5):2711–2713. doi: 10.1128/jvi.65.5.2711-2713.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galinski M. S., Mink M. A., Pons M. W. Molecular cloning and sequence analysis of the human parainfluenza 3 virus gene encoding the L protein. Virology. 1988 Aug;165(2):499–510. doi: 10.1016/0042-6822(88)90594-6. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jambou R. C., Elango N., Venkatesan S., Collins P. L. Complete sequence of the major nucleocapsid protein gene of human parainfluenza type 3 virus: comparison with other negative strand viruses. J Gen Virol. 1986 Nov;67(Pt 11):2543–2548. doi: 10.1099/0022-1317-67-11-2543. [DOI] [PubMed] [Google Scholar]
- Kingsbury D. W., Webster R. G. Some properties of influenza virus nucleocapsids. J Virol. 1969 Sep;4(3):219–225. doi: 10.1128/jvi.4.3.219-225.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S. Q., Schulman J. L., Moran T., Bona C., Palese P. Influenza A virus transfectants with chimeric hemagglutinins containing epitopes from different subtypes. J Virol. 1992 Jan;66(1):399–404. doi: 10.1128/jvi.66.1.399-404.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo G. X., Luytjes W., Enami M., Palese P. The polyadenylation signal of influenza virus RNA involves a stretch of uridines followed by the RNA duplex of the panhandle structure. J Virol. 1991 Jun;65(6):2861–2867. doi: 10.1128/jvi.65.6.2861-2867.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luytjes W., Krystal M., Enami M., Parvin J. D., Palese P. Amplification, expression, and packaging of foreign gene by influenza virus. Cell. 1989 Dec 22;59(6):1107–1113. doi: 10.1016/0092-8674(89)90766-6. [DOI] [PubMed] [Google Scholar]
- Mirakhur B., Peluso R. W. In vitro assembly of a functional nucleocapsid from the negative-stranded genome RNA of a defective interfering particle of vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7511–7515. doi: 10.1073/pnas.85.20.7511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscona A. Defective interfering particles of human parainfluenza virus type 3 are associated with persistent infection in cell culture. Virology. 1991 Aug;183(2):821–824. doi: 10.1016/0042-6822(91)91018-c. [DOI] [PubMed] [Google Scholar]
- Murphy D. G., Dimock K., Kang C. Y. Defective interfering particles of human parainfluenza virus 3. Virology. 1987 Jun;158(2):439–443. doi: 10.1016/0042-6822(87)90217-0. [DOI] [PubMed] [Google Scholar]
- Muster T., Subbarao E. K., Enami M., Murphy B. R., Palese P. An influenza A virus containing influenza B virus 5' and 3' noncoding regions on the neuraminidase gene is attenuated in mice. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5177–5181. doi: 10.1073/pnas.88.12.5177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park K. H., Huang T., Correia F. F., Krystal M. Rescue of a foreign gene by Sendai virus. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5537–5541. doi: 10.1073/pnas.88.13.5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattnaik A. K., Ball L. A., LeGrone A. W., Wertz G. W. Infectious defective interfering particles of VSV from transcripts of a cDNA clone. Cell. 1992 Jun 12;69(6):1011–1020. doi: 10.1016/0092-8674(92)90619-n. [DOI] [PubMed] [Google Scholar]
- Re G. G., Kingsbury D. W. Nucleotide sequences that affect replicative and transcriptional efficiencies of Sendai virus deletion mutants. J Virol. 1986 May;58(2):578–582. doi: 10.1128/jvi.58.2.578-582.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Re G. G., Kingsbury D. W. Paradoxical effects of Sendai virus DI RNA size on survival: inefficient envelopment of small nucleocapsids. Virology. 1988 Aug;165(2):331–337. doi: 10.1016/0042-6822(88)90577-6. [DOI] [PubMed] [Google Scholar]
- Seong B. L., Brownlee G. G. A new method for reconstituting influenza polymerase and RNA in vitro: a study of the promoter elements for cRNA and vRNA synthesis in vitro and viral rescue in vivo. Virology. 1992 Jan;186(1):247–260. doi: 10.1016/0042-6822(92)90079-5. [DOI] [PubMed] [Google Scholar]
- Yamanaka K., Ogasawara N., Yoshikawa H., Ishihama A., Nagata K. In vivo analysis of the promoter structure of the influenza virus RNA genome using a transfection system with an engineered RNA. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5369–5373. doi: 10.1073/pnas.88.12.5369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wyke Coelingh K. L., Winter C., Murphy B. R. Antigenic variation in the hemagglutinin-neuraminidase protein of human parainfluenza type 3 virus. Virology. 1985 Jun;143(2):569–582. doi: 10.1016/0042-6822(85)90395-2. [DOI] [PubMed] [Google Scholar]