Abstract
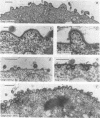
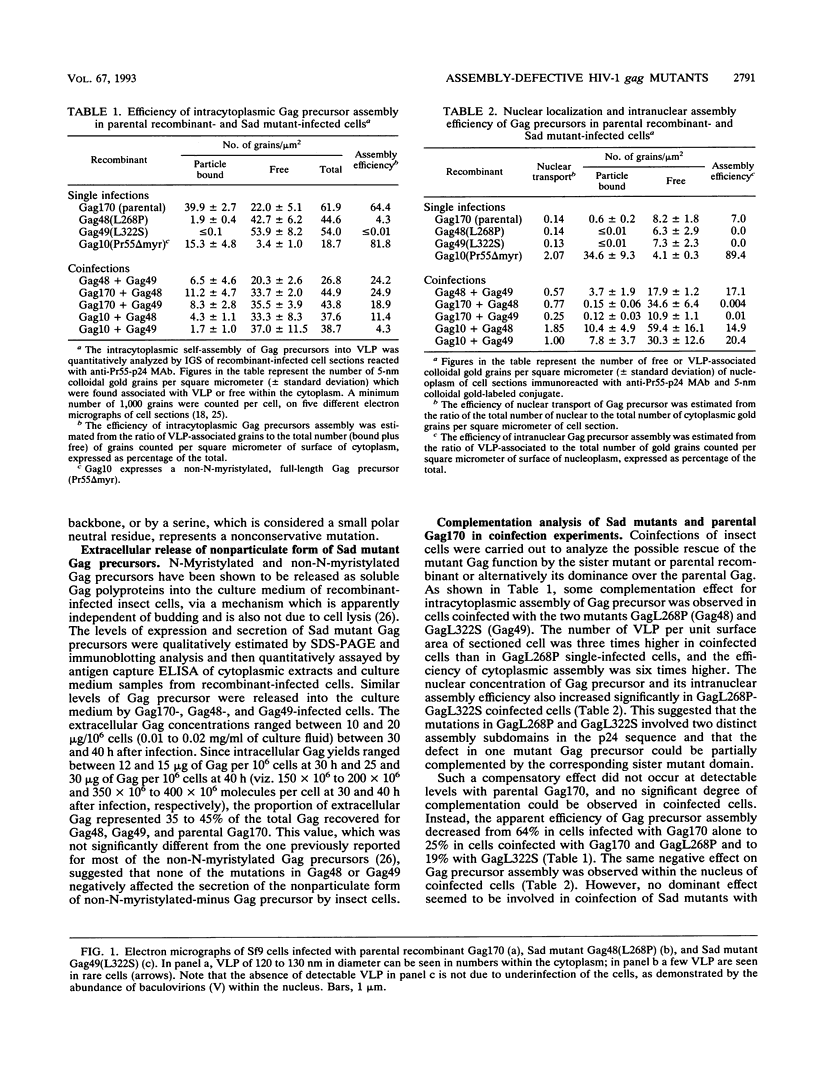
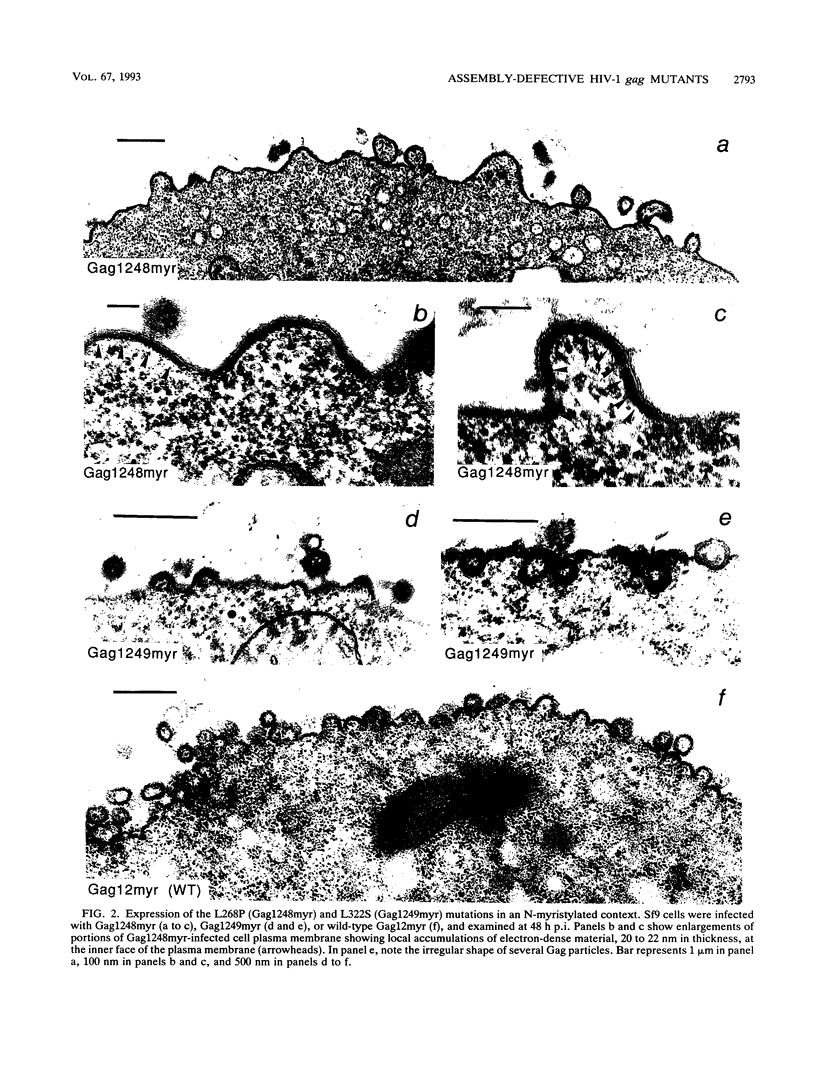
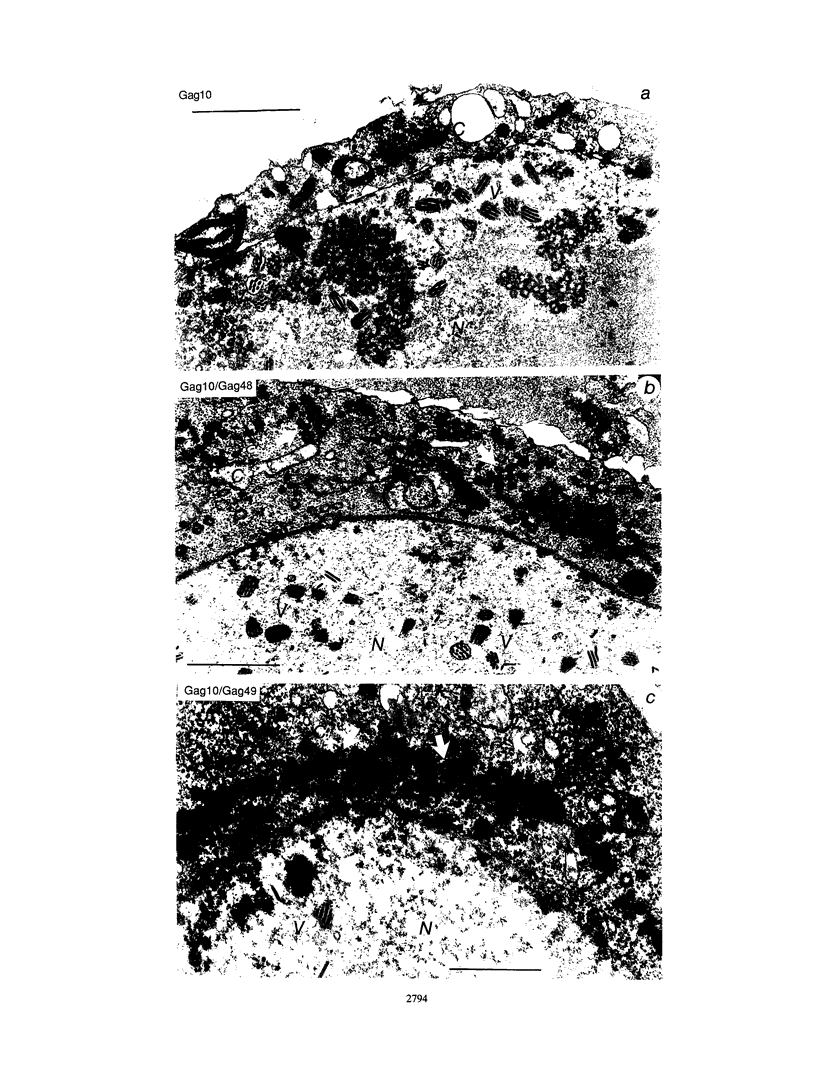
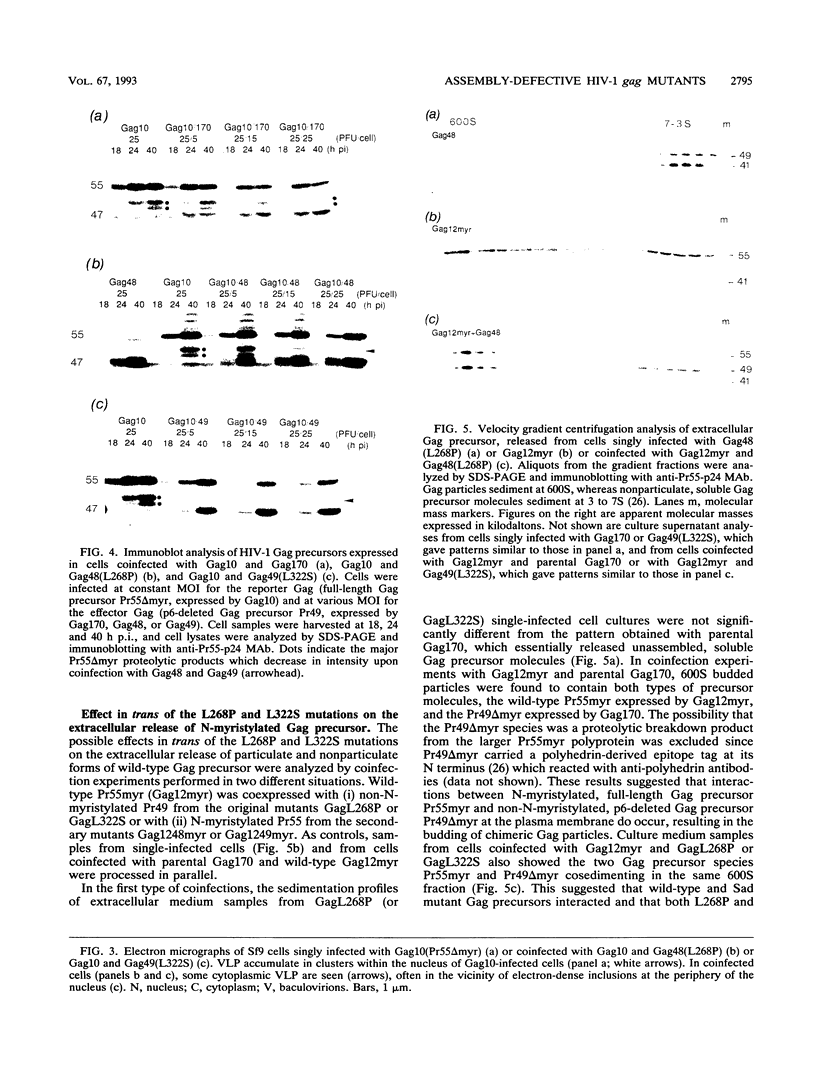
Two substitution mutants of the human immunodeficiency virus type 1 gag gene product were isolated after nitrous acid mutagenesis of a recombinant baculovirus expressing a non-N-myristylated, p6-deleted Gag precursor (Pr49). Both mutants failed to assemble intracellular Gag virus-like particles, as does the parental recombinant, and therefore expressed a self-assembly defective (Sad) phenotype in insect cells. The mutations consisted of nonconservative changes involving highly conserved hydrophobic residues in the p24 domain, Leu to Pro at position 268 (L268P) and Leu to Ser at amino acid 322 (L322S). Experimental data suggested that the two mutated residues belonged to functionally different regions of the Gag precursor. (i) A partial complementation effect between the two mutants for Gag precursor assembly was observed in coinfection experiments. (ii) The two mutations showed different phenotypes when placed in the N-myristylated context, of which only the L268P mutation abolished extracellular budding and release of Gag particles at the plasma membrane. Both L268P and L322S mutants had a trans-dominant negative effect on the intracellular assembly of a non-N-myristylated, full-length (Pr55) Gag precursor expressed by a coinfecting recombinant. None of the mutants, however, showed any detectable effect in trans on membrane targeting and budding of the coexpressed N-myristylated wild-type Gag precursor.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
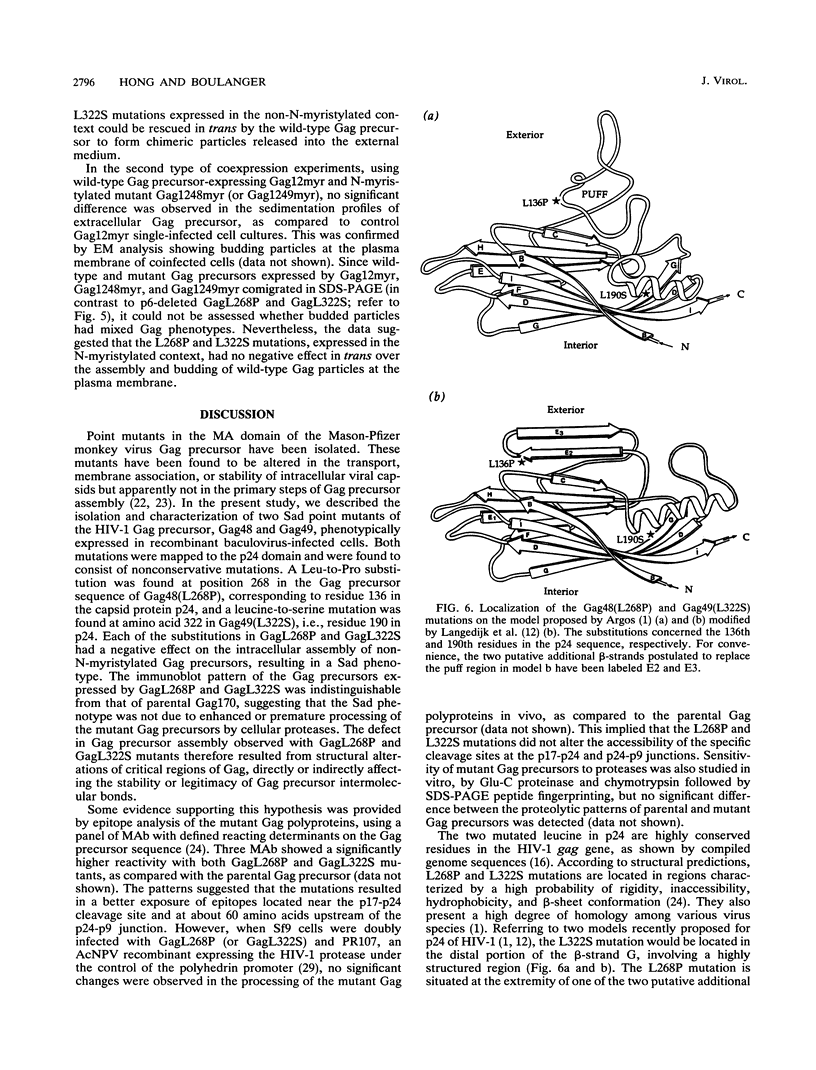
- Argos P. A possible homology between immunodeficiency virus p24 core protein and picornaviral VP2 coat protein: prediction of HIV p24 antigenic sites. EMBO J. 1989 Mar;8(3):779–785. doi: 10.1002/j.1460-2075.1989.tb03438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cann A. J., Karn J. Molecular biology of HIV: new insights into the virus life-cycle. AIDS. 1989;3 (Suppl 1):S19–S34. [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Delchambre M., Gheysen D., Thines D., Thiriart C., Jacobs E., Verdin E., Horth M., Burny A., Bex F. The GAG precursor of simian immunodeficiency virus assembles into virus-like particles. EMBO J. 1989 Sep;8(9):2653–2660. doi: 10.1002/j.1460-2075.1989.tb08405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelderblom H. R. Assembly and morphology of HIV: potential effect of structure on viral function. AIDS. 1991 Jun;5(6):617–637. [PubMed] [Google Scholar]
- Gheysen D., Jacobs E., de Foresta F., Thiriart C., Francotte M., Thines D., De Wilde M. Assembly and release of HIV-1 precursor Pr55gag virus-like particles from recombinant baculovirus-infected insect cells. Cell. 1989 Oct 6;59(1):103–112. doi: 10.1016/0092-8674(89)90873-8. [DOI] [PubMed] [Google Scholar]
- Hoshikawa N., Kojima A., Yasuda A., Takayashiki E., Masuko S., Chiba J., Sata T., Kurata T. Role of the gag and pol genes of human immunodeficiency virus in the morphogenesis and maturation of retrovirus-like particles expressed by recombinant vaccinia virus: an ultrastructural study. J Gen Virol. 1991 Oct;72(Pt 10):2509–2517. doi: 10.1099/0022-1317-72-10-2509. [DOI] [PubMed] [Google Scholar]
- Hunkapiller T., Kaiser R. J., Koop B. F., Hood L. Large-scale and automated DNA sequence determination. Science. 1991 Oct 4;254(5028):59–67. doi: 10.1126/science.1925562. [DOI] [PubMed] [Google Scholar]
- Jowett J. B., Hockley D. J., Nermut M. V., Jones I. M. Distinct signals in human immunodeficiency virus type 1 Pr55 necessary for RNA binding and particle formation. J Gen Virol. 1992 Dec;73(Pt 12):3079–3086. doi: 10.1099/0022-1317-73-12-3079. [DOI] [PubMed] [Google Scholar]
- Kristensen T., Voss H., Schwager C., Stegemann J., Sproat B., Ansorge W. T7 DNA polymerase in automated dideoxy sequencing. Nucleic Acids Res. 1988 Apr 25;16(8):3487–3496. doi: 10.1093/nar/16.8.3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Langedijk J. P., Schalken J. J., Tersmette M., Huisman J. G., Meloen R. H. Location of epitopes on the major core protein p24 of human immunodeficiency virus. J Gen Virol. 1990 Nov;71(Pt 11):2609–2614. doi: 10.1099/0022-1317-71-11-2609. [DOI] [PubMed] [Google Scholar]
- Layne S. P., Merges M. J., Dembo M., Spouge J. L., Conley S. R., Moore J. P., Raina J. L., Renz H., Gelderblom H. R., Nara P. L. Factors underlying spontaneous inactivation and susceptibility to neutralization of human immunodeficiency virus. Virology. 1992 Aug;189(2):695–714. doi: 10.1016/0042-6822(92)90593-e. [DOI] [PubMed] [Google Scholar]
- Martin G. R., Warocquier R., Cousin C., D'Halluin J. C., Boulanger P. A. Isolation and phenotypic characterization of human adenovirus type 2 temperature-sensitive mutants. J Gen Virol. 1978 Nov;41(2):303–314. doi: 10.1099/0022-1317-41-2-303. [DOI] [PubMed] [Google Scholar]
- Mervis R. J., Ahmad N., Lillehoj E. P., Raum M. G., Salazar F. H., Chan H. W., Venkatesan S. The gag gene products of human immunodeficiency virus type 1: alignment within the gag open reading frame, identification of posttranslational modifications, and evidence for alternative gag precursors. J Virol. 1988 Nov;62(11):3993–4002. doi: 10.1128/jvi.62.11.3993-4002.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novelli A., Boulanger P. A. Assembly of adenovirus type 2 fiber synthesized in cell-free translation system. J Biol Chem. 1991 May 15;266(14):9299–9303. [PubMed] [Google Scholar]
- Novelli A., Boulanger P. A. Deletion analysis of functional domains in baculovirus-expressed adenovirus type 2 fiber. Virology. 1991 Nov;185(1):365–376. doi: 10.1016/0042-6822(91)90784-9. [DOI] [PubMed] [Google Scholar]
- Overton H. A., Fujii Y., Price I. R., Jones I. M. The protease and gag gene products of the human immunodeficiency virus: authentic cleavage and post-translational modification in an insect cell expression system. Virology. 1989 May;170(1):107–116. doi: 10.1016/0042-6822(89)90357-7. [DOI] [PubMed] [Google Scholar]
- Overton H. A., McMillan D. J., Gridley S. J., Brenner J., Redshaw S., Mills J. S. Effect of two novel inhibitors of the human immunodeficiency virus protease on the maturation of the HIV gag and gag-pol polyproteins. Virology. 1990 Nov;179(1):508–511. doi: 10.1016/0042-6822(90)90326-m. [DOI] [PubMed] [Google Scholar]
- Rasmussen L., Battles J. K., Ennis W. H., Nagashima K., Gonda M. A. Characterization of virus-like particles produced by a recombinant baculovirus containing the gag gene of the bovine immunodeficiency-like virus. Virology. 1990 Oct;178(2):435–451. doi: 10.1016/0042-6822(90)90341-n. [DOI] [PubMed] [Google Scholar]
- Rhee S. S., Hunter E. A single amino acid substitution within the matrix protein of a type D retrovirus converts its morphogenesis to that of a type C retrovirus. Cell. 1990 Oct 5;63(1):77–86. doi: 10.1016/0092-8674(90)90289-q. [DOI] [PubMed] [Google Scholar]
- Rhee S. S., Hunter E. Amino acid substitutions within the matrix protein of type D retroviruses affect assembly, transport and membrane association of a capsid. EMBO J. 1991 Mar;10(3):535–546. doi: 10.1002/j.1460-2075.1991.tb07980.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert-Hebmann V., Emiliani S., Jean F., Resnicoff M., Traincard F., Devaux C. Clonal analysis of murine B cell response to the human immunodeficiency virus type 1 (HIV1)-gag p17 and p25 antigens. Mol Immunol. 1992 Jun;29(6):729–738. doi: 10.1016/0161-5890(92)90183-x. [DOI] [PubMed] [Google Scholar]
- Royer M., Cerutti M., Gay B., Hong S. S., Devauchelle G., Boulanger P. Functional domains of HIV-1 gag-polyprotein expressed in baculovirus-infected cells. Virology. 1991 Sep;184(1):417–422. doi: 10.1016/0042-6822(91)90861-5. [DOI] [PubMed] [Google Scholar]
- Royer M., Hong S. S., Gay B., Cerutti M., Boulanger P. Expression and extracellular release of human immunodeficiency virus type 1 Gag precursors by recombinant baculovirus-infected cells. J Virol. 1992 May;66(5):3230–3235. doi: 10.1128/jvi.66.5.3230-3235.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsumi M., Jean F., Robert V., Chermann J. C., Devaux C. Use of monoclonal antibodies for the detection and quantitation of HIV1 core protein p25: comparative evaluation of in vitro HIV1 infection by immunofluorescence, antigen capture ELISA and reverse transcriptase assays. Res Virol. 1990 Nov-Dec;141(6):649–661. doi: 10.1016/0923-2516(90)90037-j. [DOI] [PubMed] [Google Scholar]
- Trono D., Feinberg M. B., Baltimore D. HIV-1 Gag mutants can dominantly interfere with the replication of the wild-type virus. Cell. 1989 Oct 6;59(1):113–120. doi: 10.1016/0092-8674(89)90874-x. [DOI] [PubMed] [Google Scholar]
- Valverde V., Lemay P., Masson J. M., Gay B., Boulanger P. Autoprocessing of the human immunodeficiency virus type 1 protease precursor expressed in Escherichia coli from a synthetic gene. J Gen Virol. 1992 Mar;73(Pt 3):639–651. doi: 10.1099/0022-1317-73-3-639. [DOI] [PubMed] [Google Scholar]
- Wain-Hobson S., Sonigo P., Danos O., Cole S., Alizon M. Nucleotide sequence of the AIDS virus, LAV. Cell. 1985 Jan;40(1):9–17. doi: 10.1016/0092-8674(85)90303-4. [DOI] [PubMed] [Google Scholar]
- Wills J. W., Craven R. C. Form, function, and use of retroviral gag proteins. AIDS. 1991 Jun;5(6):639–654. doi: 10.1097/00002030-199106000-00002. [DOI] [PubMed] [Google Scholar]