Abstract
The avian brainstem serves as a useful model to answer the question of how afferent activity influences the viability of target neurons. Approximately 20–30% of neurons in the avian cochlear nucleus, nucleus magnocellularis (NM) die following deafferentation (i.e., deafness produced by cochlea removal). Interestingly, Bcl-2 mRNA (but not protein) is upregulated in 20–30% of NM neurons following deafferentation. We have recently shown that chronic treatments of lithium upregulates the neuroprotective protein Bcl-2 and increases neuronal survival following deafferentation. The pathways leading to the upregulation of Bcl-2 expression following these two manipulations are unknown. The present experiments examine changes in glycogen synthase kinase-3 beta (Gsk-3β), and transcription factors nuclear factor κ B (NFκB), β-catenin, and pCreb following lithium administration and following deafferentation. These molecules are known to be influenced by lithium and to regulate Bcl-2 expression in other model systems. Lithium decreased immunolabeling for Gsk-3β and increased expression for all three transcription factors. Deafferentation, however, did not alter Gsk-3β or NFκB, resulted in lower β-catenin expression, but did increase pCreb immunoreactivity. While it is possible that pCreb is a common link in the regulation of Bcl-2 following these two manipulations, the timing and distribution of pCreb labeling suggests that it is not the sole determinant of Bcl-2 upregulation following deafferentation. It is likely that the regulation of Bcl-2 gene expression by lithium and by deafferentation involve different molecular pathways.
Keywords: auditory system, nucleus magnocellularis, cell death, neuroprotection, Bcl-2
1. Introduction
Cell death in the nervous system can be induced by many events including injury, disease states and absence or loss of normal afferent sensory inputs. In the developing auditory system deafferentation, produced by cochlea removal, can result in death of cochlear nucleus neurons (Born and Rubel, 1985; Hashisaki and Rubel, 1989; Tierney et al., 1997). The brainstem auditory system of the chick has proved to be a useful model for understanding this form of cell death (Rubel et al., 1990). In this system, the ipsilateral eighth (VIIIth) nerve synapses on a homogenous population of auditory neurons in nucleus magnocellularis (NM) and provides NM neurons with their sole excitatory input (Lippe et al., 1980; Rubel et al., 1990; Born et al., 1991). Consequently, unilateral cochlea removal deafferents the ipsilateral NM, but leaves innervation to the contralateral NM unaffected. This allows for within-subject comparisons between deafferented and intact neurons. Approximately 20–30% of deafferented NM neurons die within a few days. The remaining neurons survive, albeit at a lower metabolic state and with a reduction in soma size (Parks and Rubel, 1978; Born and Rubel, 1985; Born and Rubel, 1988).
Deafferentation-induced changes in NM neurons are observed across the entire population of neurons as early as one hour after cochlea removal. At this early time there is a reduction in protein synthesis, RNA synthesis, and an increase in intracellular calcium (Steward and Rubel, 1985; Garden et al., 1995; Zirpel et al., 1995). At later time points (6–12 hours post cochlea removal) the NM neurons appear to divide into two discrete populations: those that die and those that will survive. Cells that are destined to die show a breakdown of polyribosomes (Rubel et al., 1991) and an apparent total cessation of protein synthesis (Steward and Rubel, 1985).
The deafferentation-induced cell death of NM neurons includes some features of typical apoptosis such as shrinkage in neuronal size and lack of cytoplasmic membrane rupture (Born and Rubel, 1985). Yet, these neurons do not typically show other characteristics of apoptosis, such as nuclear condensation and cytoplasmic blebbing. At the molecular level of analysis, some molecules that are commonly involved in controlling apoptosis have also been shown to be altered by deafferentation (Wilkinson et al., 2003). One particularly intriguing set of experiments involves the cytoprotective protein Bcl-2. Bcl-2 is located in the outer mitochondrial membrane and prevents the toxic release of cytochrome c into the cytosol (Festjens et al., 2006). Mostafapour et al. (2002) showed that overexpression of Bcl-2 in mice protects cochlear nucleus neurons from deafferentation-induced cell death. Wilkinson et al. (2002) observed a dramatic and transient upregulation of mRNA for Bcl-2 in the deafferented NM neurons of the chick. Unexpectedly, however, the upregulation was observed in 20–30% of the neurons, suggesting that this cytoprotective gene was upregulated in the dying subpopulation of NM neurons. They suggested that cellular signaling mechanisms are engaged in the dying population of cells that upregulate expression of the potentially cytoprotective Bcl-2 message, but by the time the message is upregulated, the cellular protein synthesis machinery is broken down. Consequently the message is left untranslated and cannot have its protective influence.
Bush and Hyson (2006) suggested that the upregulation of Bcl-2 message was a clue that Bcl-2 protein could protect NM neurons from deafferentation-induced cell death if the protein was produced prior to cochlea removal. They used a pharmacological treatment, chronic administration of lithium, which has been shown to upregulate Bcl-2 protein in other systems (Nonaka and Chuang, 1998; Chuang and Chen, 1999; Wei et al., 2001). They found that chronic lithium administration did upregulate Bcl-2 protein in NM neurons and did protect neurons from deafferentation-induced cell death.
Lithium is a commonly used treatment for Bipolar Disorder, although its mechanism of action is unclear. It is clear, however, that lithium can alter gene expression in neurons and that it can lead to neuroprotection (Chen et al., 1999). A first step in lithium’s action, in many cases, is the inhibition of glycogen synthase kinase-3 beta (Gsk-3β) (Grimes and Jope, 2001). This kinase has been implicated in certain psychiatric disorders and neurodegenerative diseases (Chen et al., 2004). Unlike many protein kinases, Gsk-3β is highly active in resting cells and is primarily regulated by inactivation (Grimes and Jope, 2001). Gsk-3β, in turn, regulates a variety of transcription factors including, but not limited to, nuclear factor kappa B (NFκB), and β-catenin, and pCreb (Grimes and Jope, 2001). Inhibition of Gsk-3β by lithium can lead to increased activity of all of these transcription factors, which can subsequently increase the expression of neuroprotective proteins such as Bcl-2.
The experiments by Wilkinson et al. (2002) and Bush and Hyson (2006) clearly demonstrates that Bcl-2 expression in NM neurons can be upregulated by two different conditions: 1.) deafferentation leads to a transient upregulation of mRNA in a subpopulation of cells, and 2.) chronic administration of lithium leads to an upregulation of Bcl-2 protein across the entire population of cells. There are several possible transcription factors that regulate Bcl-2 expression and it is not known which of these are involved in the regulation of expression observed in NM neurons. In addition it is possible that the transcription factors involved in the regulation of Bcl-2 by lithium are different than those involved in the regulation observed after deafferentation. The present experiments examine both lithium- and deafferentation-induced changes in concentration of the active forms of the kinase Gsk-3β and the transcription factors pCreb, β-catenin, and NFκB in attempt to identify the possible sequence of events leading to the upregulation of Bcl-2 expression in NM neurons
2. Results
2.1. Lithium control of protein expression
Chronic lithium altered the expression of the proteins of interest in NM, as can be seen in Figure 1. Gsk-3β labeling (Figure 1A and E) was observed in the cytoplasm of NM neurons and subjects treated with lithium had lighter staining than those treated with saline. Lithium administration increased immunolabeling for NFκB, pCreb, and β-catenin. Examples of these effects can be seen in Figures 1 B–H. NFκB (Figures 1B and F) labeling was observed in the cytoplasm, while β-catenin (Figures 1C and G) and pCreb (Figures 1D and H) labeling was mainly in the nuclei. A comparison of labeling on the intact sides of both saline- and lithium-treated groups show greater labeling in lithium treated subjects for all three transcription factors. Objective analysis of labeling density confirmed these visual impressions. Figure 2 displays the average gray scale density measurements of NM neurons in lithium- and saline-treated subjects on each side of the brain at the various survival times. To avoid the possible confounding effects of cochlea removal, statistical analyses of the effects of lithium were restricted to comparisons of labeling on the intact side of the brain. Tissue sections from pairs of lithium- and saline-treated subjects were processed simultaneously to avoid variance due to processing variables, and a percentage difference in gray scale density measurements was calculated for each lithium-saline pair of subjects. Figure 2 displays the mean percentage difference in labeling. There was a reliable difference between lithium and saline treated subjects for all proteins of interest (t(10) = 16.792, p < 0.01, t (10) = 17.211, p<0.01, t(10)=36.955, p<0.001, t(10)=9.101, p<0.05) for Gsk-3β, NFκB, β-catenin, and pCreb, respectively. In all cases, the labeling density appeared to show a normal distribution. Lithium administration appeared to produce a uniform shift in the distribution of labeling densities.
Figure 1. Representative photomicrographs of intact sides of saline (top)- and lithium (bottom)- treated pairs.
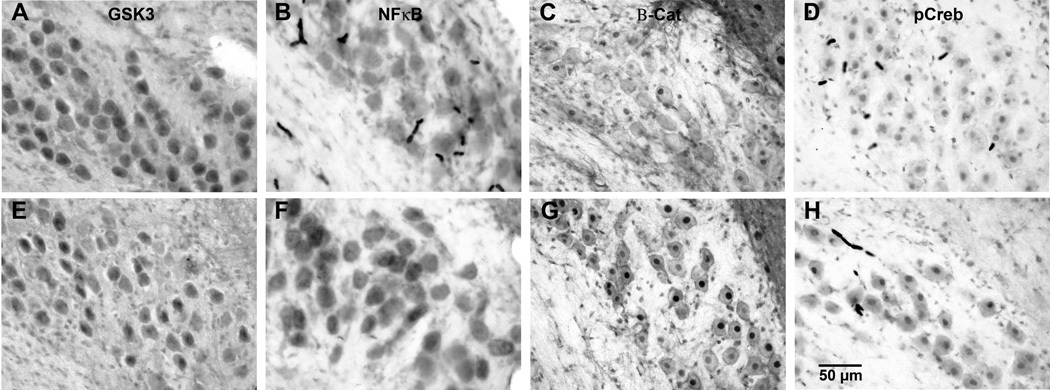
Tissue sections were immunolabeled with antibodies recognizing Gsk-3β (A,E), NFκB (B,F), β-catenin (C,G), and pCreb (D,H). Lithium-treated subjects showed lighter labeling for Gsk-3β, but darker labeling for NFκB, Beta-catenin, and pCreb. These examples are from the intact sides of subjects 3 hr following cochlea removal except for pCreb, which was from a subject 1 hr following cochlea removal.
Figure 2. Percent difference in immunolabeling between lithium- and saline-treated subjects.

((Lithium-Saline)/Saline *100). Positive numbers indicate that lithium subjects stained more heavily for the antigen. Statistically reliable differences were observed for all four antigens (* indicates p < 0.05). Error bars represent standard error of the mean. Since this analysis compared the intact sides of the brains in each group, subjects were pooled across survival times for each antigen.
2.2. Deafferentation-induced differences
Deafferentation did not result in consistent changes in immunolabeling for either Gsk-3β or NFκB at any of the time points examined, and statistical analyses did not reveal an effect of cochlea removal (p = 0.875 and 0.507 respectively). Labeling for the transcription factors, β-catenin and pCreb, however (Figures 3), did show differences between the intact and deafferented sides of the section for both treatment groups. There was lower labeling for β-catenin on the deafferented side of the section in both lithium- and saline-treated subjects, and this difference in labeling appeared most robust at 6 hours after cochlea removal (Figure 3A and B). To compare the effects of cochlea removal between groups, scores were converted to average percentage difference between intact and deafferented sides of the same brain (Figure 4). A two-way (Time × Drug) ANOVA on these percent difference scores revealed a reliable effect of Time after cochlea removal (F (2, 21) = 3.1, p< 0.05) but no reliable effect of Drug (F (1, 21), p <1) or Drug × Time interaction (F (2, 21) = 1.98, p = 0.16). Post-hoc pairwise comparisons (Newman-Keuls) revealed that the magnitude of the effect at 6 hours following cochlea removal was greater than that at 1 or 3 hours after surgery.
Figure 3. Representative photomicrographs of displaying deafferentation-induced differences in labeling for β-catenin (A, B) and pCreb (C,D).
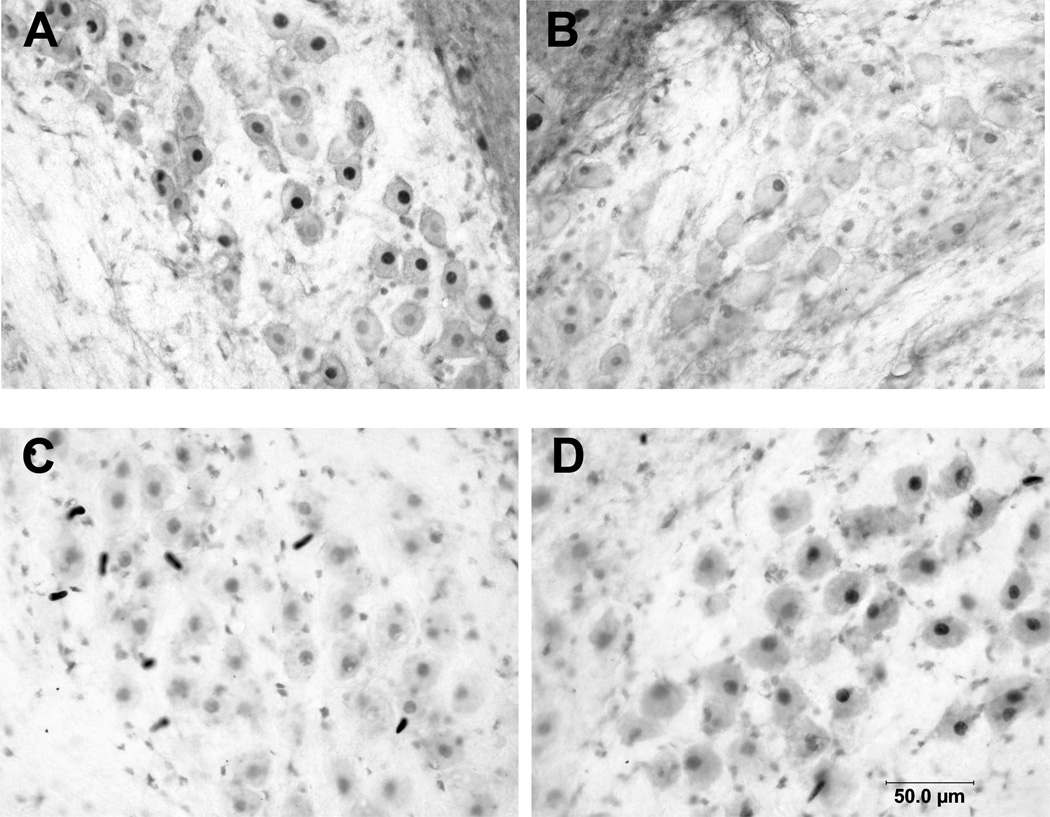
Lighter labeling for Beta-catenin was observed on the deafferented side (B) compared to the intact side of the same tissue section (A) (example from subject 6 hr following cochlea removal). Darker labeling for pCreb, however, was observed on the deafferented (D) side of the brain compared to the intact side of the same tissue section (C) (1 hr following cochlea removal).
Figure 4. Percent difference between deafferented and intact sides of the brain for the various antigens at different survival times following cochlea removal.
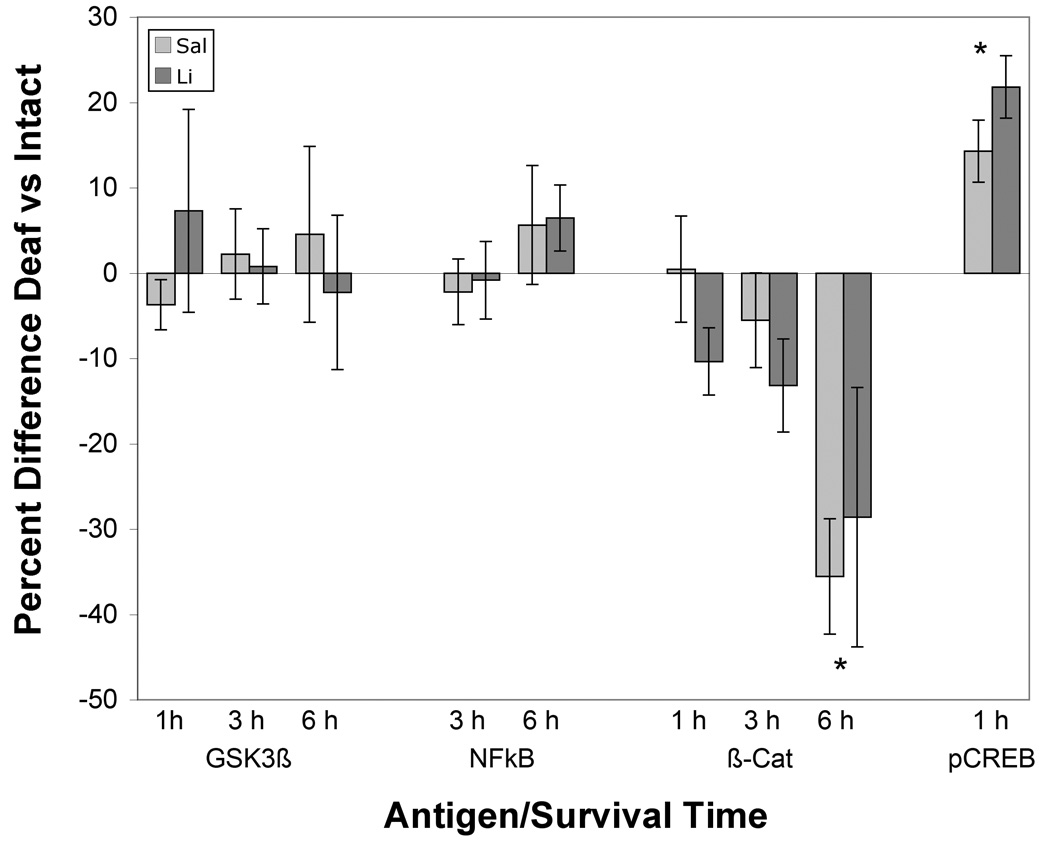
((Deafferented-intact))/intact *100). No reliable differences between sides were observed when labeling of Gsk-3β or NFκB. There was a statistically reliable decrease in labeling for β-catenin by 6 hours following cochlea removal. Labeling for pCreb was darker on the deafferented side of both treatment groups at 1 hour post cochlea removal. Asterisks indicate times at which there statistically reliable differences (p < 0.05) between deafferented and intact sides of the brain. No reliable differences were observed between lithium and saline treatment groups at any survival time for any antigen. Error bars represent standard error in the mean.
Deafferentation resulted in an increase in immunoreactivity for pCreb one hour following cochlea removal in both lithium- and saline-treated subjects (Figure 3C and D and Figure 4). A two-way mixed ANOVA using Drug as the between-subjects variable and Side as the within-subjects variable revealed that there was a reliable effect of Side (F (1, 10) = 52.4, p < 0.001) and, as noted above, a reliable effect of Drug (F(1,10) = 9.1, p < 0.05), but no reliable Drug × Side interaction (F(1, 10) = 3.1, p = 0.11). Converting scores to percent difference between sides confirmed that both drug treatment groups had a reliable difference between sides (t (6) = 3.9 (saline) and t(4) = 5.9 (lithium), p < 0.05) (Figure 4). There was no statistically reliable difference in the magnitude of the effect between saline and lithium treated subjects (between-subjects t-test on the percent difference scores (t (10) = 1.4, p = 0.19).
In all cases, it appeared that labeling density was normally distributed across the population of NM neurons. Cochlea removal resulted in a reliable difference in the mean labeling for the two transcription factors, but there still appeared to be a normal distribution of labeling density across the population.
3. Discussion
Cochlea removal results in neuronal cell death and atrophy in the avian cochlear nucleus, nucleus magnocellularis (NM) (Born and Rubel, 1985). Previous studies have shown that chronic administration of lithium increases immunolabeling for the neuroprotective molecule Bcl-2 in NM neurons and protects NM neurons from deafferentation-induced cell death (Bush and Hyson, 2006). Cochlea removal in the absence of drug treatment also leads to an upregulation of Bcl-2 mRNA (although not Bcl-2 protein) in NM neurons. The present set of experiments examined candidate molecules that might contribute to the upregulation of Bcl-2 in these two different conditions. Lithium administration decreases Gsk-3β expression, but increases the expression of the transcription factors NFκB, β-catenin and pCreb. Cochlea removal, on the other hand, did not appear to alter the expression of either Gsk-3β or NFκB and decreased the expression of β-catenin. As reported previously (Zirpel et al., 2000), however, cochlea removal did upregulate expression of pCreb.
3.1. Technical Considerations
The objective densitometric analysis of immunolabeled tissue is, at best, semi-quantitative. Conclusions can be made about relative changes in expression but there is no information about absolute concentration of the proteins of interest. In addition, processing variables, such as incubation time, can dramatically influence the overall darkness of immunolabeling. To limit the influence of these processing variables, pairs of lithium- and saline-treated brains were processed simultaneously. Marks (hole punches) on the ventral surface of the brainstem were used to identify individual brains after the sections were mounted onto slides. Effects of lithium were evaluated by comparing these pairs simultaneously processed tissue sections. Although some variables, such as the quality of fixation, could not be completely controlled, the consistency of the data suggests that the matched lithium-saline comparisons used in the analyses are valid. Statistical analyses lead to identical conclusions whether the matched lithium- and saline-treatment was analyzed as a between-subject variable or if they were treated as a within-subject variable. The influence of processing variables is even less problematic for the analyses of the effect of cochlea removal. These effects are evaluated by comparing labeling on opposite sides of the same tissue section.
3.2. Lithium control of protein expression
There are several ways by which lithium could lead to upregulation of neuroprotective proteins, such as Bcl-2, and the most commonly proposed pathway involves inhibition of Gsk-3β. The present experiments confirmed that lithium reduces levels of Gsk-3β in NM neurons. This is in accordance with others (De Sarno et al., 2002; Grimes and Jope 2001; Gould et al., 2003) who have shown that chronic lithium pretreatment down-regulates the constitutively active Gsk-3β found in neurons. It has been relatively well established that Gsk-3β is a primary target of lithium and is one of the mostly highly regulated molecules by lithium pretreatment. Gsk-3β normally degrades or blocks the activation of the transcription factors examined in this report (Grimes and Jope, 2001; Hoeflich et al., 2000, Tamatani et al., 1999; Yost et al., 1996; Kopinksy et al., 2003). By inhibiting Gsk-3β’s activity with lithium, the target transcription factors can have enhanced activity. β-catenin is phosphorylated by Gsk-3β, and it appears that there is an increase in β-catenin when lithium inhibits Gsk-3β (Yost et al., 1996). Inhibition of Gsk-3β also results in increased expression of pCreb in other systems (Chuang et al., 2002), and increased expression of Gsk-3β resulted in decreased phosphorylation of the substrate Creb among others (Ilouz et al., 2006; Kopinsky et al., 2003; Einat et al., 2003). Some studies (Hoeflich et al., 2000), however, have shown that NFκB could be positively or negatively correlated to Gsk-3β inhibition. The present studies show that the transcription factors NFκB, β-catenin, and pCreb are all increased in NM neurons following chronic lithium administration. Any of these transcription factors could lead to increases in Bcl-2 (Tamatani et al., 1999; Pahl, 1999; Hammonds et al., 2007; Li et al., 2007) or other neuroprotective molecules, and ultimately account for the neuroprotection observed following chronic lithium administration.
3.3. Deafferentation control of expression
Only two of the candidate molecules, β-catenin and pCreb, show a deafferentation-induced difference in immunolabeling. β-catenin was reduced by 6 hours following cochlea removal. This is in line with the overall reduction in protein synthesis that is observed in NM neurons following deafferentation (Steward and Rubel, 1985). The transcription factor pCreb, however, was increased one hour after cochlea removal, and there were statistically reliable differences between the intact and deafferented sides in both lithium- and saline-treated groups. This result suggests that although lithium increases the overall levels of pCreb immunoreactivity in NM neurons, it cannot prevent the further increase in pCreb observed following deafferentation. pCreb labeling was only evaluated one hour following cochlea removal since previous studies (Zirpel et al., 2000) showed that no pCreb labeling was observed at later time points. The present studies confirmed this transient expression (data not presented) and further show that this transient increase in pCreb is also observed in lithium-treated subjects. The previous study examining pCreb expression (Zirpel et al., 2000) reported upregulation in a subpopulation (70%) of deafferented NM neurons. In contrast, the present study found that the labeling densities for both transcription factors maintained a normal distribution across the population of deafferented NM neurons. It is not clear why these results differ, but it is possible that the variation in the proportion of the cell population affected by cochlea removal is because different breeds of chicks were used in the two studies. Alternatively, the results may be different because of subtle differences in the antigen retrieval steps used in the immunolabeling protocols.
3.4. Synthesis
Current knowledge supports the hypothesis that lithium is neuroprotective in different animal models of disease states and hypoxia/stroke paradigms. This protection requires long term pretreatment, which suggests that changes in gene expression are necessary (Chuang et al., 2000; Silverstone and Romans, 1996). One possible neuroprotective molecule that is upregulated by lithium is Bcl-2 (Bush and Hyson, 2006). Bcl-2 mRNA is also robustly upregulated in a subpopulation (approximately 20–30%) of NM neurons following cochlea removal (Wilkinson et al., 2002). This is an intriguing finding because 20–30% of NM neurons go on to die after deafferentation (Born and Rubel, 1985). It was hypothesized that the upregulation of Bcl-2mRNA in a dying subpopulation of neurons was an indicator of cells that were destined to die, and that the potentially neuroprotective Bcl-2 message was unable to be translated into protein in these neurons because protein synthesis machinery was compromised (Steward and Rubel, 1985) by the time the message had increased. Bush and Hyson (2006) showed that chronic lithium pretreatment increased immunolabeling for Bcl-2 protein in NM neurons and reduced deafferentation-induced cell death by approximately 50%.
The present studies suggest that the upregulation of Bcl-2 expression by lithium may involve a different sequence of events than the upregulation of Bcl-2 mRNA in NM neurons following cochlea removal. Lithium, but not cochlea removal, reduces immunolabeling for Gsk-3β. Consequently, there was an increase in expression of all three of the transcription factors examined (NFκB, β-catenin, and pCreb). Cochlea removal did not alter NFκB and reduced levels of β-catenin. Only pCreb showed an increase in expression following cochlea removal (see also, Zirpel et al., 2000). This suggests that, of the possible promoters examined to date, pCreb is the most likely candidate for upregulating Bcl-2 mRNA in NM neurons following deafferentation. One difficulty with this conclusion, however, is that it appears that pCreb is upregulated in the majority of deafferented neurons, but Bcl-2 mRNA s upregulated in only 20–30% of the neurons. Additionally, pCreb upregulation is observed only very soon after cochlea removal (one hour) whereas Bcl-2 mRNA upregulation is observed 6–12 hours post cochlea removal. It is possible that pCreb is responsible for the upregulation of Bcl-2mRNA following deafferentation, but there is a delay between the time at which pCreb promotes gene transcription and the time at which the fully transcribed and edited mRNA is present and able to be detected by the oligonucleotide probe. If this were the case, then it would still be mysterious as to why the mRNA is only observed in a subpopulation of neurons. One could speculate that there is some threshold of pCreb activation that must be achieved or there is a competing (repressive) influence that has not yet been identified in those cells that do not increase Bcl-2 expression. Alternatively, it is possible that the apparent upregulation of Bcl-2 mRNA in NM neurons is attributable to reduced degradation of the mRNA rather than increased production.
4. Experimental Procedures
4.1 Animals and Surgical Procedures
The subjects were Ross × Ross chicks hatched at Florida State University from eggs obtained from a local supplier. Post-hatch chicks, of either sex, received daily injections of either lithium chloride (LiCl) or saline for 17 days prior to unilateral cochlea removal. Protein expression in NM neurons was examined at various time points after CR using immunocytochemistry. All procedures were in accordance with Animal Care and Use Committee guidelines.
4.2. Daily injections
Post-hatch chicks (P0) were started on a lithium chloride or saline injection regime identical to that used by Bush and Hyson (2006). Both groups of animals received a daily subcutaneous injection for a total of 17 days. The dose of LiCl was gradually increased across age, beginning at 1.5 mEq/kg for the first four days, followed by 2.3 mEq/kg for seven days, and finally, 3.0 mEq/kg for the last six days. This schedule was adapted from the protocols of both Wei et al. (2001) and Nonaka and Chuang (1998). The volume of each injection was 0.01 ml/kg. Control subjects received daily 0.01 ml/kg injections of saline. This injection schedule produced plasma lithium levels that were proportional to the injection concentration (Bush and Hyson 2006). No lithium is detectable in serum 24 hours after the last injection suggesting that lithium was rapidly excreted from the subject (Bush and Hyson, 2006).
4.3. Cochlea Removal Surgery
Subjects received unilateral cochlea removal surgery under halothane anesthesia, one hour after the last daily injection on P16. A small incision was made to widen the ear canal and the tympanic membrane was punctured. The columella was removed, followed by the extraction of the basilar papilla (cochlea) through the oval window using forceps. The extracted tissue was viewed under a dissection microscope to ensure complete cochlea removal. The middle ear cavity was packed with Gelfoam and the external incision sealed with surgical adhesive.
4.4. Tissue preparation
Subjects were allowed to survive for various times (1, 3, or 6 hours) following cochlea removal prior to tissue preparation for immunocytochemistry. Subjects were deeply anesthetized with pentobarbital and perfused with 0.9% saline followed by ice cold 4% paraformaldehyde. Brainstems were blocked and post-fixed in 4% paraformaldehyde for 1–2 h followed by overnight cryoprotection at 4°C in phosphate buffered saline (PBS) containing 20% sucrose. The tissue was rapidly frozen in 2-methylbutane on dry ice and embedded in TBS tissue freezing medium for cryosectioning using a Leica CM 1850 cryostat. Sections were cut at 25 µm and were collected into ice cold PBS for later mounting. Every section containing NM was collected.
4.5. Immunocytochemistry
Pairs of subjects, one treated with LiCl and one treated with saline, were processed simultaneously (n = 9, three pairs per time point). Puncture marks were made in the ventral portion of the brainstem to identify treatment condition following the simultaneous processing. Cryosections containing NM were placed in a vial containing PBS. Alternate sections of the same subject were processed using different primary antibodies. For NFκB, tissue was microwaved for 20 seconds on low power to expose the epitope. Sections were then washed 2 X ten minutes in PBS and endogenous peroxidase activity was quenched by incubating in 0.03% H2O2 in methanol for ten minutes. Following three 10 minute rinses in PBS, sections were placed in a blocking solution containing 2% Bovine Serum Albumin (BSA), 1% Normal Goat Serum (NGS) (Gsk-3β and β-catenin) or Normal Horse Serum (NHS) (NFκB and pCreb), 0.1% Triton-X100 (Gsk-3β, β-catenin, NFκB) or 0.4% Saponin (pCreb) in PBS for an hour. Sections were transferred to various concentrations of the primary antibodies, Gsk-3β (h-76) (1:500, Santa Cruz, sc-9166), pCreb (ser 133) (1:1000, Upstate Signaling, 06–519), NFκB (p65 subunit) (1:250, Chemicon, mab3026), and β-catenin (cat-5h10) (1:1000, Zymed, 18-0226) in blocking solution and incubated on a rotator overnight at 4°C. The specificity of the reaction was assured by incubating control sections in the absence of primary antibodies. The following day, the sections were removed from 4°C and allowed to incubate at room temperature for two hours. Sections were washed 3 × 10 minutes in PBS. Sections were then incubated in a 1:200 concentration of goat anti-rabbit or horse anti-mouse secondary antibodies in blocking solution for one hour. Following 3 ten-minute washes with PBS, sections were incubated in avidin-biotin- peroxidase complex (ABC; Vector Laboratories, Burlingame, CA, USA) for one hour. After another round of washes, with PBS, sections were reacted with diaminobenzidine (DAB) tetrahydrochloride and 0.01% H2O2 for visualization. Following a final round of washes, sections were mounted on slides and allowed to dry overnight. The following day, slides were dehydrated in a graded series of ethanol and cleared in xylenes. Slides were coverslipped in DPX mounting medium and allowed to dry overnight.
4.6. Objective analysis of anatomical tissue
Immunolabeling of NM neurons was objectively analyzed using densitometry using NIH Image J. When the important comparison is between lithium- and saline-treated subjects, the tissue was processed simultaneously in the same reagents to prevent processing variables from affecting the results. For examining the effects of cochlea removal, the staining densities on the intact side of the brainstem were compared to those on the deafferented side of the same tissue section. In most cases, the sections that were analyzed were obtained from the middle third of the anterior-posterior extent of NM. The light levels and contrast settings were the same for all tissue sections analyzed. On average, 50 neurons were measured in each nucleus, on each side of the section. All neurons with a clear, intact visible cell membrane were included in the analysis. Neurons were measured starting from the most medial edge of the nucleus and proceeding laterally until either all NM neurons in that section were measured or the criterion of 50 cells was reached. Approximately 3 sections were analyzed per brain. The data were analyzed using Microsoft EXCEL and SPSS.
Acknowledgements
The authors would like to thank Stan King and Katie McConnell for technical assistance. This work was supported by NIDCD grant DC00858.
Abbreviations
- ABC
avidin biotin complex
- ANOVA
Analysis of variance
- BSA
bovine serum albumin
- DAB
diaminobenzidine
- Gsk-3β
glycogen synthase kinase-3 beta
- NFκB
nuclear factor kappa B
- NGS
normal goat serum
- NHS
normal horse serum
- NM
nucleus magnocellularis
- PBS
phosphate buffered saline
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Born DE, Rubel EW. Afferent influences on brain stem auditory nuclei of the chicken, neuron number and size following cochlea removal. J. Comp. Neurol. 1985;231:435–445. doi: 10.1002/cne.902310403. [DOI] [PubMed] [Google Scholar]
- Born DE, Rubel EW. Afferent influences on brain stem auditory nuclei of the chicken: presynaptic action potentials regulate protein synthesis in nucleus magnocellularis neurons. J. Neuroscience. 1988;8:901–919. doi: 10.1523/JNEUROSCI.08-03-00901.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Born DE, Durham D, Rubel EW. Afferent influences on brainstem auditory nuclei of the chick: nucleus magnocellularis neuronal activity following cochlea removal. Brain Res. 1991;557:37–47. doi: 10.1016/0006-8993(91)90113-a. [DOI] [PubMed] [Google Scholar]
- Bush AL, Hyson RL. Lithium increases Bcl-2 expression in chick cochlear nucleus and protects against deafferentation-induced cell death. Neuroscience. 2006;138:1341–1349. doi: 10.1016/j.neuroscience.2005.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang D, Chen R. Long term lithium treatment suppresses p53 and bax expression but increases bcl-2 expression. J. Biol. Chem. 1999;274:6039–6042. doi: 10.1074/jbc.274.10.6039. [DOI] [PubMed] [Google Scholar]
- Chuang D, Chen R, Chalecka-Franaszek E, Ren M, Hashimoto R, Senatorov V, Kanai H, Hough C, Hiroi T, Leeds P. Neuroprotective effects of lithium in cultured cells and animal models of diseases. Bipolar Disorders. 2002;4:129–136. doi: 10.1034/j.1399-5618.2002.01179.x. [DOI] [PubMed] [Google Scholar]
- Chen G, Zeng WZ, Yuan PX, Huang LD, Jiang YM, Zhao ZH, Manji HK. The mood stabilizing agents lithium and valproate robustly increases the levels of the neuroprotective protein Bcl-2 in the CNS. J. Neurochem. 1999;72:879–882. doi: 10.1046/j.1471-4159.1999.720879.x. [DOI] [PubMed] [Google Scholar]
- Chen G, Bower K, Cuiling MA, Fang S, Thiele CJ, Luo J. Glycogen synthase kinase 3beta (Gsk3β) mediates 6-hydroxydopamine-induced neuronal death. FASEB Journal. 2004;10:1162–1164. doi: 10.1096/fj.04-1551fje. [DOI] [PubMed] [Google Scholar]
- De Sarno P, Li X, Jope RS. Regulation of AKT and glycogen synthase kinase-3beta phosphorylation by sodium valproate and lithium. Neuropharmacology. 2002;43:1158–1164. doi: 10.1016/s0028-3908(02)00215-0. [DOI] [PubMed] [Google Scholar]
- Einat H, Yuan P, Gould TD, Li J, Du JH, Zhang L, Manji H, Chen G. The role of the extracellular signal-regulated kinase signaling pathway in mood modulation. J. Neurosci. 2003;23:7311–7316. doi: 10.1523/JNEUROSCI.23-19-07311.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Festjens N, Cornelis S, Lamkanfi M, Vandenabeele P. Caspase-containing complexes in the regulation of cell death and inflammation. Biol. Chem. 2006;387:1005–1016. doi: 10.1515/BC.2006.124. [DOI] [PubMed] [Google Scholar]
- Garden GA, Redeker-DeWulf V, Rubel EW. Afferent influences on brainstem auditory nuclei of the chicken: regulation of transcriptional activity following cochlea removal. J. Comp. Neurol. 1995;359:412–423. doi: 10.1002/cne.903590305. [DOI] [PubMed] [Google Scholar]
- Grimes CA, Jope RS. The multifaceted roles of glycogen synthase kinase 3beta in cellular signaling. Prog. Neurobiol. 2001;65:391–426. doi: 10.1016/s0301-0082(01)00011-9. [DOI] [PubMed] [Google Scholar]
- Gould TD, Chen G, Manji HK. In vivo evidence in the brain for lithium inhibition of glycogen synthase kinase-3. Neuropsychopharmacology. 2003;29:32–38. doi: 10.1038/sj.npp.1300283. [DOI] [PubMed] [Google Scholar]
- Hammonds MD, Shim SS, Feng P, Calabrese JR. Effects of subchronic lithium treatment on levels of BDNF, Bcl-2 and phosphor-CREB in the rat hippocampus. Basic Clin. Pharmacol, Toxicol. 2007;100:356–359. doi: 10.1111/j.1742-7843.2007.00058.x. [DOI] [PubMed] [Google Scholar]
- Hashisaki GT, Rubel EW. Effects of unilateral cochlea removal on anteroventral cochlear nucleus neurons in developing gerbils. J. Comp. Neurol. 1989;283:65–73. doi: 10.1002/cne.902830402. [DOI] [PubMed] [Google Scholar]
- Hoeflich KP, Luo J, Rubie EA, Tsao MS, Jin O, Woodgett JR. Requirement for glycogen synthase kinase-3beta in cell survival and NFκB activation. Nature. 2000;406:86–90. doi: 10.1038/35017574. [DOI] [PubMed] [Google Scholar]
- Ilouz R, Kowalsman N, Eisentein M, Eldar-Finkelan H. Identification of novel glycogen synthase kinase-3beta substrate-interacting residues suggests a common mechanism for substrate recognition. J. Biol. Chem. 2006;41:30621–30630. doi: 10.1074/jbc.M604633200. [DOI] [PubMed] [Google Scholar]
- Kopinsky KL, Chalecka-Franaszek E, Gonzalez-Zulueta M, Chuang DM. Chronic lithium treatment antagonizes glutamate-induced decrease of phosphorylated Creb in neurons via reducing protein phophatase 1 and increasing MEK activities. Neuroscience. 2003;116:425–435. doi: 10.1016/s0306-4522(02)00573-0. [DOI] [PubMed] [Google Scholar]
- Li Q, Dashwood WM, Zhong X, Nakagama H, Dashwood RH. Bcl-2 overexpression in PhIP-induced colon tumors: cloning of rat Bcl-2 promoter and characterization of a pathway involving beta-catenin, c-Myc and E2F1. Oncogene. 2007;26:6194–6202. doi: 10.1038/sj.onc.1210438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippe WR, Steward O, Rubel EW. The effect of unilateral basiliar papilla removal upon nuclei laminaris and magnocellularis of the chick examined with [3H]2- deoxy-D-glucose autoradiography. Brain Res. 1980;916:239–248. doi: 10.1016/0006-8993(80)90715-5. [DOI] [PubMed] [Google Scholar]
- Mostafapour SP, Mae del Puerto N, Rubel EW. Bcl-2 Overexpression eliminates deprivation-induced cell death of brainstem auditory neurons. J. Neurosci. 2002;22:4670–4674. doi: 10.1523/JNEUROSCI.22-11-04670.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonaka S, Chuang DM. Neuroprotective effects of chronic lithium on focal cerebral ischemia in rats. Neuroreport. 1998;9:2081–2084. doi: 10.1097/00001756-199806220-00031. [DOI] [PubMed] [Google Scholar]
- Pardo R, Andreolotti AG, Ramos B, Picatoste F, Claro E. Opposed effects of lithium on the MEK-ERK pathway in neural cells: Inhibition in astrocytes and stimulation in neurons by GSK3 independent mechanisms. J. Neurochem. 2003;87:417–426. doi: 10.1046/j.1471-4159.2003.02015.x. [DOI] [PubMed] [Google Scholar]
- Parks TN, Rubel EW. Organization and development of the brain stem auditory nuclei of the chicken: primary afferent projections. J. Comp. Neurol. 1978;180:439–448. doi: 10.1002/cne.901800303. [DOI] [PubMed] [Google Scholar]
- Pahl HL. Activators and target genes of REL/ NFκB transcription factors. Oncogene. 1999;18:6853–6866. doi: 10.1038/sj.onc.1203239. [DOI] [PubMed] [Google Scholar]
- Rowe MK, Chuang DM. Lithium neuroprotection: molecular mechanisms and clinical implications. Exper. Rev. Mol. Med. 2004;21:1–18. doi: 10.1017/S1462399404008385. [DOI] [PubMed] [Google Scholar]
- Rubel EW, Hyson RL, Durham D. Afferent regulation of neurons in the brain stem auditory system. J. Neurobiol. 1990;21:169–196. doi: 10.1002/neu.480210112. [DOI] [PubMed] [Google Scholar]
- Silverstone T, Romans S. Long term treatment of Bipolar Disorder. Drugs. 1996;5:367–382. doi: 10.2165/00003495-199651030-00003. [DOI] [PubMed] [Google Scholar]
- Steward O, Rubel EW. Afferent influences on brain stem auditory nuclei of the chicken: presynaptic action potentials regulate protein synthesis in nucleus magnocellularis neurons. J. Neurosci. 1985;231:385–395. doi: 10.1523/JNEUROSCI.08-03-00901.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamatani M, Che Y, Matsukai H, Ogawa S, Okado H, Miyake S, Mizuno T, Tohyama M. Tumor necrosis factor induces bcl-2 and bcl-x expression through NFκB activation in primary hippocampal neurons. J. Biol. Chem. 1999;274:8531–8538. doi: 10.1074/jbc.274.13.8531. [DOI] [PubMed] [Google Scholar]
- Tierney TS, Russell FA, Moore DR. Susceptibility of developing cochlear nucleus neurons to deafferentation-induced death abruptly ends just before the onset of hearing. J. Comp. Neurol. 1997;378:295–306. doi: 10.1002/(sici)1096-9861(19970210)378:2<295::aid-cne11>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Verma YK, Gangenahalli GU, Singh VK, Gupta P, Chandra R, Sharma RK, Raj HJ. Cell Death regulation by B-cell lymphoma protein. Apoptosis. 2006;11:459–471. doi: 10.1007/s10495-006-5702-1. [DOI] [PubMed] [Google Scholar]
- Wei H, Qin ZH, Senatorov VV, Wei W, Wang Y, Qian Y, Chuang DM. Lithium supresses excitotoxicity-induced striatal lesions in a rat model of Huntington’s disease. Neuroscience. 2001;106:603–612. doi: 10.1016/s0306-4522(01)00311-6. [DOI] [PubMed] [Google Scholar]
- Wilkinson BL, Sadler KA, Hyson RL. Rapid deafferentation-induced upregulation of Bcl-2 mRNA in the chick cochlear nucleus. Mol. Brian Res. 2002;99:67–74. doi: 10.1016/s0169-328x(02)00113-4. [DOI] [PubMed] [Google Scholar]
- Wilkinson BL, Elam JS, Fadool DA, Hyson RL. Afferent regulation of cytochrome c and active caspase-9 in the avian cochlear nucleus. Neuroscience. 2003;120:1071–1079. doi: 10.1016/S0306-4522(03)00387-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yost C, Torres M, Miller JR, Huang E, Kimelman D, Moon RT. The axis-inducing activity, stability, and subcellular distribution of beta-catenin is regulated in Xenopus embryos by glycogen synthase kinase 3. Genes Dev. 1996;15:1443–1454. doi: 10.1101/gad.10.12.1443. [DOI] [PubMed] [Google Scholar]
- Zirpel L, Lachicha EA, Lippe WR. Deafferentation increases the intracellular calcium of cochlear nucleus neurons in the embryonic chick. J. Neurophysiol. 1995;74:1355–1357. doi: 10.1152/jn.1995.74.3.1355. [DOI] [PubMed] [Google Scholar]
- Zirpel L, Janowiak MA, Veltri CA, Parks TN. AMPA receptor-mediated, calcium-dependent CREB phosphorylation in a subpopulation of auditory neurons surviving activity deprivation. J. Neurosci. 2000;16:6267–6275. doi: 10.1523/JNEUROSCI.20-16-06267.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]


