Abstract
Chronic graft-versus-host-disease (cGVHD) is a major barrier of successful allogeneic hematopoietic stem cell transplantation (allo-HSCT), with highly variable clinical presentations. The pathophysiology of cGVHD remains relatively poorly understood. The utilization of murine models to study cGVHD encompasses experimental challenges distinct from those that have been successfully used to study acute GVHD (aGVHD). Nevertheless, despite these challenges, murine models of cGVHD have contributed to the understanding of cGVHD, and highlight its mechanistic complexity. In this article, insights into the pathophysiology of cGVHD obtained from murine studies are summarized in the context of their relevancy to clinical cGVHD. Despite experimental limitations, current and future models of murine cGVHD will continue to provide insights into the understanding of clinical cGVHD and provide information for new therapeutic interventions.
Introduction
Chronic graft-versus-host disease (cGVHD) remains a significant barrier to successful allogeneic hematopoietic stem cell transplantation (allo-HSCT). The incidence of cGVHD following allo-HSCT ranges from 25-80% and is associated with significant morbidity and mortality1, despite the fact that cGVHD is also associated with a lower relapse rate presumably due to graft versus tumor effects2. Clinical manifestations of cGVHD are highly variable with respect to organ involvement and extent, and further complicated by different methodologies of clinical scoring to define disease severity3. These challenges in cGVHD have been addressed in a series of efforts in the clinical community3-8. It is now recognized that cGHVD is a distinct clinical entity from acute GVHD (aGVHD) and not merely an temporal extension of the latter9. Pathophysiologically, in aGVHD, necrotic changes to target organs (skin, liver, and gastrointestinal tract) predominate the pathologic phenotype. In contrast, fibrosis and chronic inflammation of target organs, often including the same target organs in aGVHD, are the pathologic hallmarks of cGVHD1. These differences in the phenotypic outcomes, which largely parallel manifestations in humans, delineate murine models of aGVHD and cGVHD (Table 1). Not surprisingly, the immune mechanisms that are implicated in the induction and propagation of cGVHD have been shown to be distinct from those of aGVHD.
Table 1.
Experimental Readouts in Murine Models of Acute and Chronic GVHD
| Acute GVHD | Chronic GVHD |
|---|---|
|
|
cGVHD evolves as a consequence of dysregulated alloreactive reactions between donor-derived immune cells and host cell populations. In contrast to aGVHD, the immune mechanisms leading to the development of cGVHD remain more incompletely understood. There are a number of factors that account for this. First, the clinical features of cGVHD until recently, with the establishment of the National Institutes of Health Consensus Project on cGVHD3, have not been defined in a systematic and objective fashion so that assessments of cGVHD have varied from institution to institution. Second, the clinical features of cGVHD themselves are highly variable and mimic, but not completely replicate, a variety of autoimmune and immunodeficient diseases, each with distinct pathophysiologic mechanisms. Third, the delayed onset of cGVHD is in many cases complicated by comorbidities of allo-HSCT, such as immune deficiency due to chronic immunosuppressive therapy, infections, end-organ damage, and disease relapse, which serve to alter the natural history of cGVHD.
Importantly, defining the pathophysiology of cGVHD has been complicated by the absence of animal models that completely recapitulate the disease or its clinical setting, in contrast to aGVHD where murine models of major and minor histocompatability (MHC) mismatched HSCT have provided a relatively comprehensive picture of its pathophysiology as a clinical disease10. Several factors contribute to the difficulty of studying an animal model of cGVHD. To date, no animal model described encompasses all of the features observed in clinical cGVHD. Furthermore, the clinical relevance of animal models of cGVHD based on preparative regimens, composition of the donor graft, genetic backgrounds of donor and host animals, post-transplant immune suppression, and post-transplant events has been frequently called into question. Despite these limitations, the study of available models of cGVHD has provided insights with respect to the pathogenesis of clinical cGVHD that correlates with clinical observations. Furthermore, observations derived from studies in these murine models have identified potential therapeutic strategies in the management of clinical cGVHD.
The purpose of this review is to describe murine models that have been used in the study of cGVHD, the immunologic mechanisms that underlie each of the graft-versus-host reactions (GVHR) that lead to the cGVHD phenotypes, and their relevancy to clinical cGVHD. These models are divided into three broad classifications (Table 2), based on phenotype and immunologic mechanism, which encompass the majority of murine cGVHD models that have been described to date. For each, descriptions on how the model is established, their salient phenotypes and pathophysiologic mechanisms, and their relevancy to clinical cGVHD are discussed.
Table 2.
Establishment of Selected Murine Models of cGVHD
| cGVHD Model | Donor -> Recipient Strains | Cells (Cell Dose) | Radiation Dose (cGy) | Clinical Phenotypea | References |
|---|---|---|---|---|---|
|
| |||||
| SLE-cGVHD | B6 -> (B6xDBA2)F1 | Spl (8x107-1x108) only | None | Auto Ab/ICG | 18,22-38 |
| B6 -> (B6xDBA2)F1 | TCD BM (1x107) + Spl (5x106) | 900 | IPS | 16,17 | |
| B6 -> (B6xBALB/c)F1 | Spl (6x107) only | None | aGVHD -> cGVHD | 23 | |
| bm12b -> B6 | Spl (1x108) only | None | Auto Ab/nephritis | 11-13 | |
| DBA2 -> BALB/c | Spl (5x107) only | 650 | Auto Ab/Scl | 53 | |
|
| |||||
| Scl-cGVHD | B10.D2 -> BALB/c | ±TCD BM (1x106-1x107) + Spl (6x106-1x108) | 700-1000 | Scl/fibrosisc | 47,48,54,58-63,66,69,75,76,78,80,82,84 |
| B10.D2 -> BALB/c | Spl (2.5x107-1x108) only | 600 | Scl/fibrosis | 49 | |
| B10.D2 -> BALB/c | Spl (2.5x107) only | 850 | Parotid Dysfxn. | 52 | |
| [C3H.SW ->B6]CD4 -> B6 | TCD C3H.SW BM (5 x 106) + CD4 (3 x 105)d | 1000 | Scl | 64 | |
| B6 -> CB6F1 | TCD BM (5 x 106) + Spl (3 x 106) | 1100 | Scl | 82 | |
| DBA2 -> BALB/c | Spl (5x107) only | 650 | Auto Ab/Scl | 53 | |
|
| |||||
| cGVHD Due to Thymus Dysfunction | B6-H2-Ab1-/--> C3H/HeN | TCD BM (5x106) | 1300 | Scl/fibrosis | 111 |
Abbreviations: Spl = splenocytes; TCD BM = T-cell depleted bone marrow; Auto Ab = autoantibodies; ICG = Immune complex glomerulonephritis; IPS = Idiopathic Pneumonitis Syndrome; Scl = sclerodermatous skin changes.
Common strain combinations, preparative regimens, observed phenotypes and cited references for SLE-cGVHD and Scl-cGVHD models are indicated in bold type. Models and preparative regimens resulting in unique phenotypes are listed individually with references.
bm12 = mutant form of I-Aβ β-chain of B6 TCR
Fibrosis in this model includes fibrotic changes in lung, liver, salivary glands, and/or eye
cGHVD induced by adoptively transferring CD4+ cells from B6 mice that were recipients of TCD BM plus naïve CD8+ cells from C3H.SW donors into lethally irradiated secondary B6 hosts along with TCD BM from C3H.SW mice
CD4 Stimulated B-Cells and Autoantibody Production in SLE-cGVHD
Biology
One model that has been extensively utilized in the study of cGVHD in mice involves adoptive transfer of immune cells from MHC antigen disparate donors. While the use of MHC-mismatched cell transfers in most cases result in a phenotype resembling lethal acute GVHD, in a number of models the transfer of MHC-mismatched cells resulted in a phenotype that resembles clinical systemic lupus erythematosus (SLE), hereafter termed SLE-cGVHD. Most involve parent-into-F1 combinations, resulting in mismatches in both class I and class II MHC, whereby unfractionated peripheral immune cells are adoptively transferred into non-irradiated host mice. Another model resulting in a similar phenotype as the parent-into-F1 model utilizes coisogenic mice that differ only in the class II MHC molecule as a result of a mutant form of the class II I-A locus in MHC11-13. The phenotype that arises from these models is predominated by the generation of autoantibodies directed against dsDNA, ssDNA, and chromatin, and immune-complex glomerulonephritis14,15. Progressive idiopathic pneumonia syndrome (IPS) has also been reported in a parent-into-F1 model of GVHD16,17.
The immunologic mechanisms that result in the SLE-like phenotype in this model have been characterized and shown to be distinct from the mechanisms resulting in aGVHD in several ways (Figure 1). First, the common characteristic of the mouse strain combinations used in the SLE cGVHD models involve disparities in class II MHC, indicating that stimulation of CD4 T-cells are important in SLE-cGVHD18. This was exemplified by experiments involving strain combinations involving mutants in class I and class II MHC. Lymph node cells and splenocytes from B6 mice were administered into non-irradiated (B6 x bm1)F1 hosts containing a mutated allele in class I MHC or (B6 x bm12)F1 hosts containing a mutated allele in class II MHC. Whereas the B6 into (B6 x bm1)F1 transplant resulted in mild cGHVD, the B6 into (B6 x bm12)F1 transplant resulted in a significant cGVHD characterized by autoantibody production, increased splenic weights, and higher numbers of antibody producing cells. In contrast, disparities in both MHC class I and class II, i.e. B6 into (bm1 x bm12)F1, resulted in an aGVHD phenotype19. Depletion of the donor inoculum of CD8 T-cells but not CD4 T-cells resulted in autoantibody formation and immune-complex glomerulonephritis, while both CD4 and CD8 T-cells were required for aGVHD20,21.
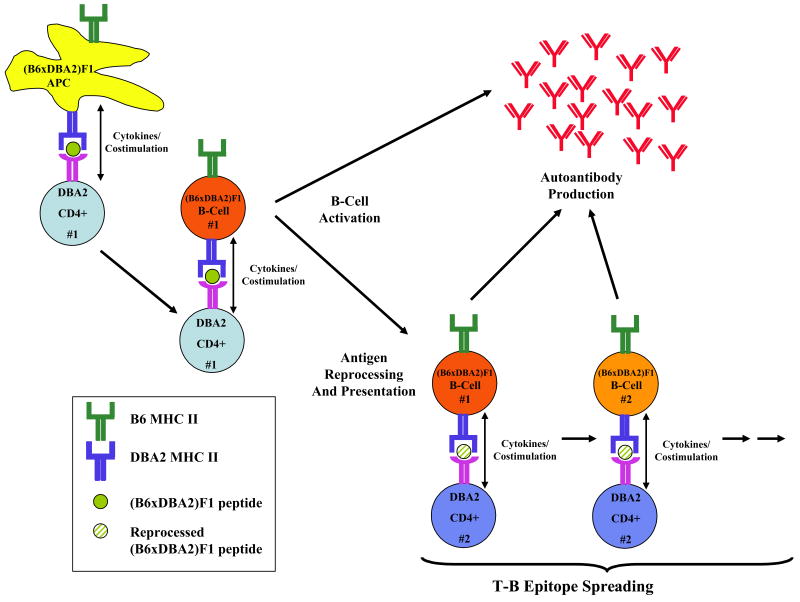
Figure 1. Murine SLE-cGVHD.
Illustrated are the events presumed to occur in the DBA2 into (B6xDBA2)F1 model of SLE-cGVHD. DBA2 CD4 T-cells are stimulated by host (B6xDBA2)F1 APC through interactions between peptide presented in the context of host class II MHC. Activation of CD4 T-cells in turn stimulate host B-cells to produce autoantibodies. In addition, B-cells could stimulate additional donor CD4 T-cells through antigen reprocessing and presentation in the context of its class II MHC. In this way, the generation of autoantibodies against a progressively wider range of epitopes is perpetuated.
Although CD4 T-cells have been shown to be critical in the induction of SLE-cGVHD, CD8 T-cells appear to play an immunomodulatory role in that the balance between CD4 and CD8 determines the ultimate GVHD phenotype in this model. In support of this, the most commonly studied strain combination resulting in SLE-cGVHD involves the administration of DBA2 (H-2d haplotype) splenocytes into (B6 x DBA2)F1 hosts (H-2bd haplotype) despite the fact that there is complete mismatch at both MHC class I and class II alleles. Paradoxically, adoptive transfer experiments in the reciprocal direction, i.e. B6 splenocytes into (B6 x DBA2)F1 hosts, results in an aGVHD phenotype18. One factor that may account for this is the lower frequency of precursor cytotoxic T-lymphocytes (CTL) in the DBA2 compared to the B6 inoculum22. The association of low precursor CTL numbers with cGVHD and high precursor CTL numbers with aGVHD has been demonstrated in other parent into F1 models, although the course and severity of the GVHD phenotype is variable23. In addition to the notion that a paucity of host alloreactive CD8 T-cells helps determine the GVH phenotype, DBA2 derived CD8 T-cells generate relatively weak in vitro allogeneic responses, and more recent studies suggest that induction of CD8 anergy results in the shift from an aGVHD phenotype to an SLE-cGVHD phenotype24,25. Finally, shifting the cytokine balance in SLE-cGVHD from Th2 predominant to Th1 predominant using systemic administration of IL-12 at the time of adoptive transfer resulted in the suppression of autoantibody production, normalization of host splenic B and T cells, restoration of donor anti-host alloreactivity26, and decreased severity of immune-complex glomerulonephritis27.
CD4 T-cell activation and their interactions with antibody producing B-cells has been a major focus of investigation in describing the pathophysiology of SLE-cGVHD. Distinct from aGVHD that is predominated by the activation and proliferation of type 1 helper T-cells (Th1), producing IL-2 and IFN-γ, CD4 T-cell activation in SLE-cGVHD results in the production of type 2 helper T-cells (Th2) producing the cytokines IL-4 and IL-1028,29 that contribute to polyclonal B-cell activation (Table 1). The importance of B-cell activity in SLE-cGVHD is supported by a number of studies where B-cell activation is disrupted, including blockade of CD40 ligand30, blockade of T-cell co-stimulation by CTLA4Ig31, stimulation of the tumor necrosis factor receptor superfamily member 4-1BB32, and the aforementioned skewing towards a Th1 predominant phenotype with administration of IL-12. In these studies, inhibition of T-cell dependent antibody production resulted in reversal of the SLE-cGVHD phenotype. Similarly, promoting host B-cell persistence by transferring perforin deficient T-cells in the aGVHD model of B6 into (B6 x DBA2)F1 hosts resulted in a shift to a GVHD phenotype resembling SLE-cGVHD33.
Progression of SLE-cGVHD can occur by a number of mechanisms. First, B-cells, being efficient antigen presenting cells (APC), present multiple epitopes of an individual antigen in the context of its class II MHC, and can activate multiple clones of helper T-cells. These epitopes include reprocessed antigens or peptides derived from immunoglobulins, both of which are cross-reactive with the original epitope. Activation of these T-cells, in turn, can further promote B-cell activation and autoantibody production against a progressively broader range of host-derived epitopes. In this way, the generation of humoral responses against host antigens is continuously perpetuated34-37. Another proposed mechanism of SLE progression involves the inability to completely clear apoptotic cells following GVHR from secondary immune organs, thus providing an additional source of autoantigens and further driving autoantibody production38.
Correlations to Clinical cGVHD
The relevance of the parent-into-F1 SLE-cGVHD model to clinical cGVHD has been called into questions for a number of reasons. First, the absence of bone marrow derived stem cells in the donor inoculum and the absence of any host immunodepletion prior to cell transfer is inconsistent with the setting of clinical allo-HSCT. Second, while some features of cGVHD following allo-HSCT mimic SLE, the similarities are not absolute. For example, the profile of autoantibody expression in patients with cGVHD are highly heterogeneous, and includes autoantibodies associated with other collagen vascular diseases39,40. Moreover, the reported incidence of renal complications attributable to cGVHD following allo-HSCT is relatively low1,41. Third, the phenotype in the SLE-cGVHD model arises from interactions between donor-derived CD4 T-cells and host-derived B-cells, whereas donor-derived B-cells do not appear to be involved in the pathogenesis13. Similar interactions between T-cells and B-cells have not been defined in the clinical setting of mixed chimerism, and have not been consistently observed in other models of murine cGVHD (see below).
Despite these limitations, the SLE-cGVHD model has contributed to the understanding of clinical cGVHD in a number of ways. First, the model highlights the importance of B-cells in contributing to the SLE-cGVHD phenotype. In addition to the demonstration of autoantibodies in clinical cGVHD39,40, patients with extensive cGVHD were more likely to have faster B-cell recovery and detectable autoantibodies following allo-HSCT42. Treatment of patients with refractory cGVHD with anti-CD20 chimeric monoclonal antibody (rituximab) resulted in objective improvements in cGVHD41. Finally, the SLE-cGVHD model could provide insights into interactions between host and donor immune cells that occur during immune recovery from allo-HSCT following reduced intensity conditioning (RIC) where transient or chronic states of mixed chimerism are common43-45. For example, in a murine model of MHC mismatched allo-HSCT utilizing RIC, there was an association between the establishment of mixed chimerism, high levels of autoantibody production, and persistence of host B-cells46. To date, however, clinical correlates of these observations have not been firmly established.
Pro-Fibrotic Pathways in Sclerodermatous (Scl)-cGVHD
Biology
Another model that has been extensively used in the study of cGVHD in mice involves the adoptive transfer of donor immune cells, usually unfractionated splenocytes, into sublethally irradiated host mice that are MHC matched but mismatched at loci encoding minor histocompatibility antigens (miHA). The most common strain combination utilizes unfractionated splenocyte or purified T-cell populations from B10.D2 (H-2d) into BALB/c (H-2d) hosts. Significant experimental model differences between the B10.D2 into BALB/c transplants and the parent into F1 SLE-cGVHD model exist (Table 2). First, transplant conditions differ in that the B10.D2 into BALB/c model involves irradiation of the host and the co-administration of donor bone marrow in addition to splenocyte populations, resulting in full donor lymphoid chimerism. Thus, the effector arms of GVHD in the Slc-cGVHD model are predominantly of donor origin in contrast to the mixed chimerism that is established in the SLE-cGVHD models. Moreover, with pre-transplant radiation, tissue damage and local inflammation in target organs of cGVHD may play a role in the onset and severity of the cGVHD phenotype. Second, the resulting observed phenotypes are substantially different from SLE-cGVHD. In contrast to autoantibody production observed in the SLE-cGVHD model, the B10.D2 into BALB/c model results in a phenotype that encompasses many features of autoimmune scleroderma. (For this reason, miHA mismatched transplants resulting in a sclerodermatous phenotype hereafter will be referred to as the Scl-cGVHD model.) Fibrotic changes of the skin and other organs including the gastrointestinal tract and the liver are the phenotypic hallmarks in Slc-cGVHD, which are detectable approximately 21 days following transplant, and are characterized by loss of dermal fat, hair follicle destruction, mononuclear cell infiltration, and increased collagen deposition47. Additional manifestations include weight loss proportional to the donor cell innoculum47 and fibrosis of the lung48, liver and bile duct49-51, and parotid salivary gland52. Autoantibody production does not appear to play a prominent role in the Scl-cGVHD model, although immunoglobulin deposition at the dermo-epidermal junction and in the kidney has been observed47,53. Significant mortality is observed as early as six weeks following transplant depending on the pre-transplant radiation dose54.
The known pathophysiologic mechanisms involved in the Scl-cGVHD model are illustrated in Figure 2. Not surprisingly, given the distinct phenotypes between the Slc-cGVHD and SLE-cGVHD models and the differences in scientific approach, their pathophysiologic mechanisms differ as well. Initially following transplantation, occurring as early as seven days post-transplant and about 14 days prior to the onset of skin lesions, inflammatory signals are released from damaged tissue, including the chemokines MCP-1, MIP-1α, and RANTES. As a result, infiltration of donor-derived mononuclear infiltration cells, consisting of monocytes and activated macrophages as well as T-cells, occurs. Activated macrophages isolated from sclerodermatous skin lesions are notable for increased expression of scavenger receptors such as ScR-A and MARCO, which in addition to their functions in phagocytosis can also participate in antigen presentation55,56. The elevation of chemokine expression in situ is accompanied by changes in the expression of adhesion molecules in target organs and their ligands on inflammatory cells57. For example, compared with syngeneic transplant controls, expression of the cellular adhesion molecules VCAM-1 and ICAM-1 is increased in cGVHD target organs, including the skin, liver, and gastrointestinal tract. Concomitantly, expression of the ligands for these VCAM-1 and ICAM-1, α4 integrin (as part of VLA-4) and LFA-1 respectively, which are expressed by activated lymphocytes, are also increased58,59. The importance of the role of adhesion molecules in initiating cGVHD was further demonstrated by the systemic administration of monoclonal antibodies directed against VCAM-1 prior to and following transplant. Mice treated with the antibody had significantly reduced mortality and morbidity due to Scl-cGVHD compared to untreated mice60.
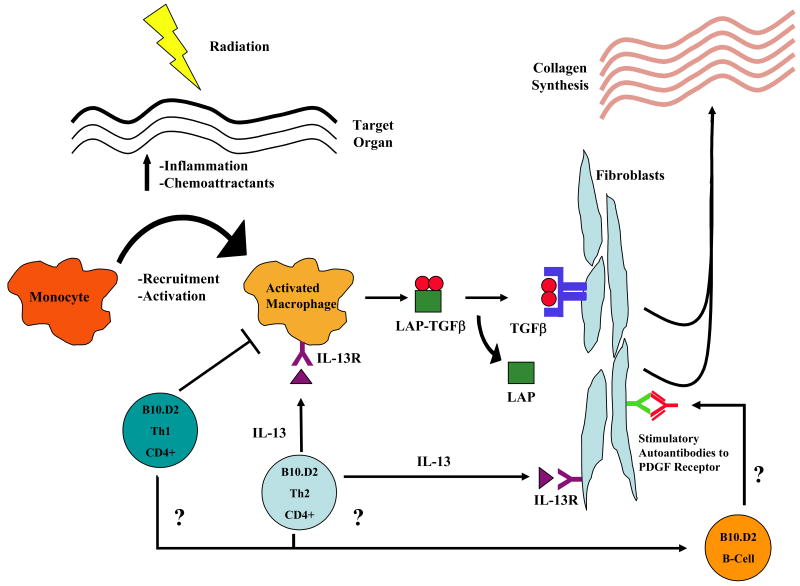
Figure 2. Murine Scl-cGVHD.
Illustrated are the events presumed to occur in the B10.D2 into BALB/c miHA mismatched transplant model. Tissue damage results in the upregulation in inflammatory mediators and chemoattractants in target tissue and in adhesion molecules in monocytes and donor-derived T-cells. Upon activation by donor and/or host APC (not shown in the figure), CD4 T-cells are recruited into target tissue and signal activated macrophages to produce TGF-β by way of IL-13 signaling. TGF-β, in turn, binds to its receptor in fibroblasts resulting in increased collagen synthesis, resulting in fibrosis. In addition to stimulation of collagen by fibroblasts through TGF-β signaling, CD4 T-cells can directly stimulate collagen synthesis by fibroblasts through IL-13 signaling. Clinical data demonstrating the role of stimulatory autoantibodies against the platelet derived growth factor (PDGF) receptor are also shown, but their role in murine cGVHD has not yet been demonstrated.
Investigation into the role of antigen presenting cells (APC) and their interactions with T-cells in Scl-cGVHD suggests a complex interplay between donor derived and recipient derived cell populations. With respect to T-cells, donor-derived naïve CD4 T-cells alone are sufficient and necessary to generate Scl-cGVHD, consistent with the SLE-cGVHD murine model and clinical observations that CD4 T-cells play an important role in cGVHD; CD8 T-cells, as well as the effector/memory fraction of CD4 T-cells are insufficient to induce cGVHD61-63. The role of CD4 T-cells in cGVHD is further supported in a murine model of cGVHD progressing from aGVHD. Alloreactive CD4 T-cells generated in the setting of aGVHD, when transferred into lethally irradiated secondary hosts, resulted in a cGVHD phenotype64. With respect to APC, in contrast to miHA mismatched models of murine aGVHD, which are primarily dependent on host APC for its initiation65, both donor and host APC are capable of eliciting cGVHD in the Scl-cGVHD model. Moreover, using donor-host strain combinations deficient in costimulatory molecules on APC, the requirements for APC function in Scl-cGVHD differ with respect to target organ. While both donor and recipient APC were sufficient to induce skin cGVHD, donor APC were found to be the primary inducers of gut cGVHD. Costimulatory requirements of T-cells were also different between organs in that both CD80/86 and CD40 costimulation of allospecific T-cells was required for initiation of gut cGVHD, whereas CD80/86 alone was sufficient to induce skin cGVHD66. From these studies, it is apparent that differential patterns of inflammation at the various target tissues and subsequent T-cell activation in draining lymph nodes play an important role in defining the pattern of host and donor derived APC and T-cell recruitment, and that this process appears to be tightly regulated67,68.
Genetic factors that define the presentation and recognition of miHA appear to be critical determinants in Scl-cGVHD manifestations. Transplants involving donor-recipient strain combinations that differ with respect to the pattern of immunodominant antigen presentation dictated by MHC haplotype can produce both aGVHD and cGHVD phenotypes. For example, transplant of different B10 donor strains into MHC-matched BALB recipient strains where the only difference among the transplant pairs was in MHC haplotype expression yielded distinct GVHD phenotypes. Specifically, B10 (H-2b) into BALB.B (H-2b), B10.BR (H-2k) into BALB.K (H-2k), and B10.D2 (H-2d) into BALB/c (H-2d) transplants differed in clinical outcome in that the first two transplant pairings resulted in systemic GVHD characterized by diarrhea and hunched posture and little in the way of sclerodermatous skin changes that is characteristic of the B10.D2 into BALB/C transplant pairing. Transplants involving donor B10 hybrids and recipient BALB hybrids, e.g. (B10 x B10.D2) F1 (H-2bd) transplanted into (BALB.B x BALB/c)F1 (H-2bd) recipients, resulted in a co-dominant expression of the haplotype specific GVHD phenotypes in that recipient mice had both systemic and Scl-cGVHD manifestations69. Analogously, patterns of T-cell receptor (TCR) expression can also potentially play a role in effecting the phenotype of GVHD. To illustrate this, the transfer of splenocytes derived from DBA2 mice into sublethally irradiated BALB/c recipients results in a cGVHD phenotype that includes both autoantibody production observed in the DBA2 into (B6 x DBA2)F1 model of SLE-cGVHD, but also the fibrotic changes characteristic of the B10.D2 into BALB/c model of Scl-cGVHD53. TCR repertoires between DBA2 mice and B10.D2 mice differ with respect to the expression of endogenous mouse mammary tumor virus superantigens resulting in deletion of TCR Vβ gene segments, which in turn confer different patterns of immune responses depending on the target antigen70,71. The diversity in TCR-MHC:peptide combinations in these models likely play a major role in the diversity of clinical cGVHD. Moreover, expression of genes other than those involved in antigen presentation and recognition may play a role in affecting the phenotype of cGVHD72,73.
Fibrosis constitutes the ultimate outcome of the allogeneic immune response in Scl-cGVHD. TGF-β plays a critical role in the generation of fibrotic changes in the skin, and shares commonality with other models of fibrosis attributed to TGF-β signaling. TGF-β is secreted by activated macrophages in an inactive form non-covalently bound to latency-associated protein (LAP). Following cleavage from LAP, TGF-β binds to its receptor on fibroblasts, and, via signaling mediated by SMAD proteins, modulates collagen synthesis that leads to fibrosis74. In murine Scl-cGVHD, disruption of the TGF-β signaling pathway using anti-TGF-β antibodies or by systemic administration of LAP significantly reduces the severity of skin disease48,75,76. Additionally, TGF-β mediated fibrosis is controlled by Th2 CD4 T-cells and is counter-regulated by Th1 CD4 T-cells. The production of TGF-β by activated macrophages may explain the exacerbation of Scl-cGVHD in mice receiving splenocytes from mice receiving G-CSF as a model of allogeneic peripheral blood stem cell transplant (allo-PBSCT), where the severity of skin fibrosis is influenced by the myelomonocytic fraction of the donor graft and is correlated with the degree of cutaneous CD11b+ cell infiltration77,78. While the role of TGF-β in mediating the skin changes Scl-cGVHD has been well-characterized, other cytokines secreted by Th2 CD4 T-cells may have important roles in modulating fibrosis. For example, IL-13 has been shown to be a major mediator of fibrosis, either by directly stimulating fibroblasts to produce collagen, or by indirectly stimulating macrophages through the IL-13 receptor to produce TGF-β74. Consistent with this, based on sequential gene expression analysis in the skin of Scl-cGVHD mice57, IL-13 expression is elevated in the early phases of cGHVD, although the precise nature of IL-13's functional role in fibrosis in this model remains to be defined.
In addition to the interactions between T-cells and monocytes/macrophages that regulate fibrosis, there is evidence generated from the Scl-cGVHD model that mast cells and eosinophils may play a role in the induction of collagen synthesis leading to fibrosis. In vitro studies suggest that both cell types can effect fibroblast proliferation and collagen production79. In the skin of Scl-cGVHD mice, mast cell infiltration and degranulation that temporally correlated with the onset of skin fibrosis was observed 47,80. Eosinophilic infiltration of the liver was also reported in this model81. Furthermore, treatment with the mast cell stabilizer nedocromil sodium prior to and following B10.D2 into BALB/c transplant resulted in amelioration of skin cGVHD82. While the mechanisms involving interactions among mast cells, eosinophils, T-cells, and fibroblasts in Scl-cGVHD remain undefined, the murine Scl-cGVHD model provides an experimental platform to investigate these interactions and identify additional targets for the treatment of fibrosis.
In addition to the pro-fibrotic pathways as a major manifestation in murine Scl-cGVHD, defects in immune responses have also been observed. For example, in a parent into F1 model of cGVHD, immune responses to viruses were impaired due to impaired tissue-specific homing of antigen-specific T-cells, which may partly explain the increased incidence in opportunistic infections in patients with cGVHD83. CD4+ CD25+ regulatory T-cells may also play an important role in the progression of disease phenotype. Reconstitution of BALB/c RAG knockout recipients with donor B10.D2 CD4+ CD25+ depleted T-cells resulted in a more severe cGVHD phenotype than unfractionated CD4+ cells. Moreover, supplementation of the donor graft with either donor or host derived CD4+ CD25+ cells ameliorated the cGVHD phenotype84. However, the mechanisms by which regulatory T-cells modulate Scl-cGVHD remain undefined.
Correlations to Clinical cGVHD
The Scl-cGVHD model shares many phenotypic features with the sclerodermatous form of clinical cGVHD, which, in combination with poor performance status, thrombocytopenia, hepatic dysfunction, and progressive cGVHD onset from prior aGVHD, is an unfavorable prognostic factor for survival85,86. The incidence of sclerodermatous cGVHD among all long-term survivors of allo-HSCT is estimated to be 3-10%, although both its incidence and severity could be expected to rise with the increasing numbers unrelated donor transplants being performed and the increased use of mobilized peripheral blood as a stem cell source87-90. Sclerodermatous cGVHD has also been reported following donor leukocyte infusions (DLI) for relapsed disease91, consistent with the murine models Scl-cGVHD in which mature post-thymic T-cells are required for its pathogenesis.
Fibrosis is a feature frequently observed in multiple organs in clinical cGVHD other than skin92,93. There is also evidence that the mediators of fibrosis described in the murine Scl-cGVHD are similar to that observed in clinical cGVHD. In vitro stimulation of human mononuclear cells with allogeneic fibroblasts and IL-4, which, like IL-13 is a pro-fibrotic cytokine, results in increased collagen synthesis. Addition of IL-12, a potent inducer of Th1 activation, suppressed this production94. Serum levels of TGF-β are increased in patients with cGVHD following allo-HSCT95. Interestingly, in allo-PBSCT, in contrast to allo-BMT, which is associated with an increased incidence and severity of cGVHD attributed to the increased numbers of T-cells in the donor allograft89,96, the incidence of GVHD was correlated with the number of myelomonocytic-committed CD34+ progenitors97, potentially providing an increased source of TGF-β, although there was no distinction made between aGVHD and cGVHD. Elevations in IL-13 were observed in the bronchoalveolar lavage fluid of recipients of lung transplants with bronchiolitis obliterans98, further suggesting the importance of this cytokine in promoting fibrotic processes during cGVHD. These clinical observations serve to provide a rationale for cytokine-directed therapy in sclerodermatous cGVHD99. Finally, stimulatory autoantibodies to the platelet derived growth factor (PDGF) receptor have been found in patients with extensive sclerodermatous cGVHD as well as patients with systemic sclerosis that was associated with increased collagen gene expression100,101. Whether these stimulatory autoantibodies exist in murine Scl-cGVHD models remains to be determined.
One limitation to the murine Scl-cGVHD model is that it generally parallels a severe form of clinical cGVHD that is found in only a subset of allo-HSCT recipients with cGVHD. The clinical spectrum of clinical cGVHD is extremely broad with respect to severity and extent1,3,9, and cutaneous manifestations of cGVHD are likewise highly variable102. Moreover, the clinical manifestations of cGVHD may be influenced by iatrogenic factors including preparative regimen and stem cell source44,89,103,104. It would be of interest if the spectrum of cGVHD phenotypes can be recapitulated in murine cGVHD models that incorporate these clinically relevant variables. Finally, as was previously detailed, genetic heterogeneity in stem cell donors and recipients play a critical role in determining cGHVD phenotype. While this factor cannot be fully recapitulated in murine models, the use of inbred mouse trains in combinatorial donor-recipient transplant pairings provides insight into the influence of miHA mismatch on the clinical outcome of allo-HSCT with respect to GVHD, and may serve as a model to identify the nature of individual miHA determinants that are important in the development of cGVHD.
cGVHD Caused by Defects in Thymic Function
Biology
The thymus, in addition to being the primary site of T-cell development, is also a major site of tolerance induction to self-antigens through the negative selection of auto-reactive T-cell clones. Negative selection is regulated through tissue restricted antigen expression on thymic epithelial cells and thymic dendritic cells105. Because of its role in central tolerance, the thymus is viewed as an organ critical for the initiation and propagation of GVHD by the production of auto-reactive T-cells resulting from impaired negative selection following treatment-related or immune mediated damage. Evidence for this comes in part from the observation that mice thymectomized during the neonatal period spontaneously develop multi-organ autoimmune disease106.
While murine models of aGVHD have directly shown that the thymus is indeed structurally and functionally adversely affected by the presence of donor-derived alloreactive immune cells107-110, the effects of cGVHD on thymic function are less clear. While some clinical studies have associated cGVHD with impairment of thymic function111-113, others have attributed the low numbers of recent thymic emigrants seen in clinical cGVHD to their impaired survival in the peripheral immune system rather than decreased thymic function114. Yet another possibility is that bone marrow derived T-cell progenitors are unable to effectively home to the thymus in the setting of cGVHD83. Additionally, the role of thymic dysfunction itself on the pathogenesis of cGVHD in humans has not been clearly established.
Interestingly, the murine models described in this review thus far have not clearly defined a thymic role in the induction of cGVHD. In the DBA2 into (B6 x DBA2)F1 model of SLE-cGVHD, there were no apparent effects on thymic cytoarchitecture and T-cell development in contrast to the B6 into (B6 x DBA2)F1 model of aGVHD108. Similarly, in the miHA mismatched murine Scl-cGVHD models, thymic production of donor-derived T-cells was not necessary for induction of the phenotype. In the B10.D2 into BALB/c model, donor bone marrow cells alone without post-thymic T-cells did not result in cGVHD associated phenotypic changes66, while administration of DBA2 splenocytes and bone marrow cells into thymectomized BALB/c hosts did not change the incidence or severity of cGVHD when compared to thymus-intact mice. However, in these mice, thymic cellularity was adversely affected by cGVHD, although more specific parameters of thymic function such as the enumeration of recent thymic emigrants by T-cell receptor excision circle quantitation or evaluations of T-cell receptor repertoire diversity were not performed53.
A murine model of cGVHD attributed to thymic dysfunction has recently been described, whereby lethally irradiated C3H/HeN recipients received T-cell depleted bone marrow from MHC-mismatched B6 mice deficient in MHC class II antigens (B6 H-2 Ab1-/-). As a consequence of impaired thymic negative selection resulting from the absence of MHC class II on host-derived thymic dendritic cells (DC), many features clinical cGVHD were observed. These features included sclerodermatous skin changes, weight loss, bile duct loss and fibrosis, inflammation and mononuclear cell infiltration of the salivary glands, and increased mortality (Figure 3). Transplantation of B6 H-2 Ab1-/- bone marrow into thymectomized recipients did not result in cGVHD while transplantation of wild-type B6 bone marrow resulted in a less severe cGVHD phenotype. Furthermore, adoptive transfer of CD4 T-cells generated in the cGVHD mice and donor APC into secondary irradiated C3H/HeN recipients resulted in the cGVHD phenotype. Thymic regulatory T-cell production was not affected but was insufficient to inhibit cGVHD. Autoantibody production was not reported in this model115.
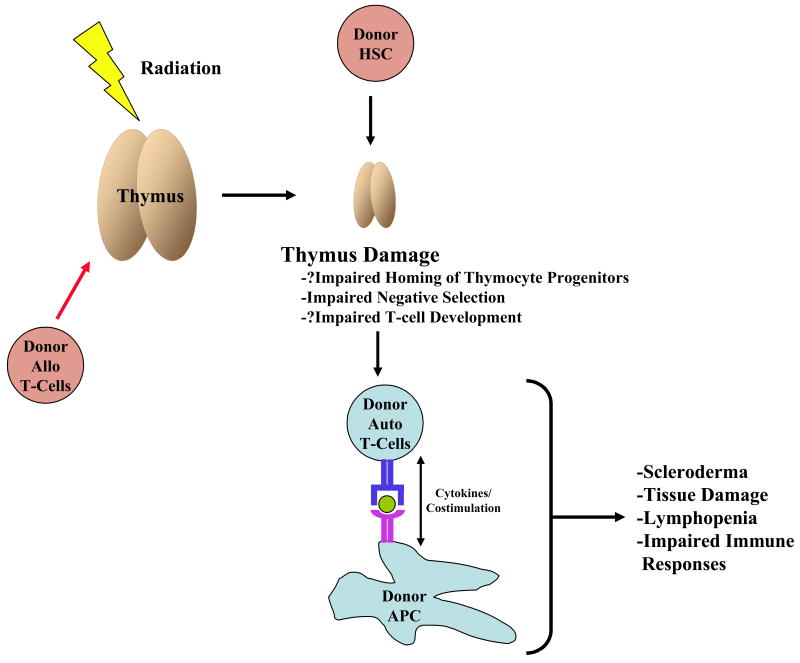
Figure 3. cGVHD Caused by Thymus Dysfunction.
The thymus is critical for central tolerance. Damage to the thymus by radiation and/or infiltration of donor alloreactive T-cells results in thymic damage that leads to impaired negative selection and T-cell development as modeled by transplantation of thymic DC lacking class II MHC. Impaired negative selection leads to the development of host derived donor reactive T-cells, which, upon activation with donor derived APC, leads to cGVHD.
To date, the B6 H-2 Ab1-/- into C3H/HeN transplant model is the only one described that directly links aberrant thymic function with induction of a cGVHD phenotype, and suggests a role for donor-derived APC as an important variable in modulating central tolerance and peripheral stimulation of donor-derived T-cells against host antigens following allo-HSCT, although the exact mechanism is not known. While the model demonstrates that the complete abrogation of negative selection of CD4 T-cells induces cGVHD, a number of caveats related to the role of the thymus in cGVHD are worth considering. First, none of the murine models of cGVHD involving genetically unmodified mice has provided evidence of impaired negative selection that would lead to cGVHD. It remains to be seen whether experimental conditions exist that result in observable thymic dysfunction contribute to or alter the cGVHD phenotype in these models. Second, the clinical relevance of the B6 H-2 Ab1-/- into C3H/HeN transplant model is limited by the fact that thymic function is perpetually impaired by the absence of MHC class II antigen on APC beyond the period when complete regeneration of thymic function occurs116. It is not clear, for example, whether there exists a temporal “window” of thymic damage followed by recovery during which deficits in negative selection are necessary and/or sufficient to induce cGVHD. Moreover, it is not known if the thymus in the B6 H-2 Ab1-/- into C3H/HeN transplant model is itself a target of GVHD, since negative selection is not only mediated by donor derived DC, but also host medullary thymic epithelium105. Finally, the clinical relevance of this model is unclear given that thymic function declines with age and is questionably present in older individuals undergoing allo-HSCT who develop cGVHD. Nevertheless, observations from this model suggest that enhancements in thymic function following allo-HSCT through the administration of positive thymic regulators may play a role in ameliorating clinical cGVHD64. Clinical studies involving administration of positive thymic regulators in the post-transplant setting will be able to more fully characterize the role of thymic dysfunction in the induction and/or progression of cGVHD.
Concluding Remarks
Clinical cGVHD is an extremely complex and diverse disease, which makes the establishment of murine models that recapitulate all features of the disease difficult to establish. Murine models of cGVHD have been useful in identifying many of the pathophysiologic mechanisms involved in autoantibody production and fibrotic changes, two features commonly found in cGVHD. However, important clinical variables attributed to the development of cGVHD in humans are difficult to accurately reproduce in mice and to date no murine model encompasses all of them. Additionally, methods in reduced intensity conditioning and the use of mobilized peripheral blood stem cells, which have been shown to influence the incidence and spectrum of clinical cGVHD, have yet to be recapitulated in murine models. Similarly, cGVHD evolved from aGVHD, which has potentially important implications in defining the role of thymic function in cGVHD, has also yet to be modeled in mice. As the clinical spectrum of cGVHD continues to be characterized in a systematic way with respect to clinical features, pathologic examination, and biomarkers, it is likely that novel murine transplant models will be successfully developed to recapitulate these features, and identify additional potential targets for therapeutic intervention in the management of clinical cGVHD.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Baird K, Pavletic SZ. Chronic graft versus host disease. Curr Opin Hematol. 2006;13:426–435. doi: 10.1097/01.moh.0000245689.47333.ff. [DOI] [PubMed] [Google Scholar]
- 2.Lee SJ, Klein JP, Barrett AJ, et al. Severity of chronic graft-versus-host disease: association with treatment-related mortality and relapse. Blood. 2002;100:406–414. doi: 10.1182/blood.v100.2.406. [DOI] [PubMed] [Google Scholar]
- 3.Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11:945–956. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 4.Couriel D, Carpenter PA, Cutler C, et al. Ancillary therapy and supportive care of chronic graft-versus-host disease: national institutes of health consensus development project on criteria for clinical trials in chronic Graft-versus-host disease: V. Ancillary Therapy and Supportive Care Working Group Report. Biol Blood Marrow Transplant. 2006;12:375–396. doi: 10.1016/j.bbmt.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 5.Martin PJ, Weisdorf D, Przepiorka D, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: VI. Design of Clinical Trials Working Group report. Biol Blood Marrow Transplant. 2006;12:491–505. doi: 10.1016/j.bbmt.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 6.Pavletic SZ, Martin P, Lee SJ, et al. Measuring therapeutic response in chronic graft-versus-host disease: National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: IV. Response Criteria Working Group report. Biol Blood Marrow Transplant. 2006;12:252–266. doi: 10.1016/j.bbmt.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 7.Schultz KR, Miklos DB, Fowler D, et al. Toward biomarkers for chronic graft-versus-host disease: National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: III. Biomarker Working Group Report. Biol Blood Marrow Transplant. 2006;12:126–137. doi: 10.1016/j.bbmt.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 8.Shulman HM, Kleiner D, Lee SJ, et al. Histopathologic diagnosis of chronic graft-versus-host disease: National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: II. Pathology Working Group Report. Biol Blood Marrow Transplant. 2006;12:31–47. doi: 10.1016/j.bbmt.2005.10.023. [DOI] [PubMed] [Google Scholar]
- 9.Lee SJ. New approaches for preventing and treating chronic graft-versus-host disease. Blood. 2005;105:4200–4206. doi: 10.1182/blood-2004-10-4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferrara JL, Levy R, Chao NJ. Pathophysiologic mechanisms of acute graft-vs.-host disease. Biol Blood Marrow Transplant. 1999;5:347–356. doi: 10.1016/s1083-8791(99)70011-x. [DOI] [PubMed] [Google Scholar]
- 11.Ma Z, Chen F, Madaio MP, Cohen PL, Eisenberg RA. Modulation of autoimmunity by TLR9 in the chronic graft-vs-host model of systemic lupus erythematosus. J Immunol. 2006;177:7444–7450. doi: 10.4049/jimmunol.177.10.7444. [DOI] [PubMed] [Google Scholar]
- 12.Morris SC, Cheek RL, Cohen PL, Eisenberg RA. Autoantibodies in chronic graft versus host result from cognate T-B interactions. J Exp Med. 1990;171:503–517. doi: 10.1084/jem.171.2.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morris SC, Cheek RL, Cohen PL, Eisenberg RA. Allotype-specific immunoregulation of autoantibody production by host B cells in chronic graft-versus host disease. J Immunol. 1990;144:916–922. [PubMed] [Google Scholar]
- 14.Bruijn JA, van Elven EH, Hogendoorn PC, Corver WE, Hoedemaeker PJ, Fleuren GJ. Murine chronic graft-versus-host disease as a model for lupus nephritis. Am J Pathol. 1988;130:639–641. [PMC free article] [PubMed] [Google Scholar]
- 15.Ka SM, Rifai A, Chen JH, et al. Glomerular crescent-related biomarkers in a murine model of chronic graft versus host disease. Nephrol Dial Transplant. 2006;21:288–298. doi: 10.1093/ndt/gfi229. [DOI] [PubMed] [Google Scholar]
- 16.Shankar G, Bryson JS, Jennings CD, Morris PE, Cohen DA. Idiopathic pneumonia syndrome in mice after allogeneic bone marrow transplantation. Am J Respir Cell Mol Biol. 1998;18:235–242. doi: 10.1165/ajrcmb.18.2.2988. [DOI] [PubMed] [Google Scholar]
- 17.Shankar G, Scott Bryson J, Darrell Jennings C, Kaplan AM, Cohen DA. Idiopathic pneumonia syndrome after allogeneic bone marrow transplantation in mice. Role of pretransplant radiation conditioning. Am J Respir Cell Mol Biol. 1999;20:1116–1124. doi: 10.1165/ajrcmb.20.6.3455. [DOI] [PubMed] [Google Scholar]
- 18.Via CS, Shearer GM. T-cell interactions in autoimmunity: insights from a murine model of graft-versus-host disease. Immunol Today. 1988;9:207–213. doi: 10.1016/0167-5699(88)91215-7. [DOI] [PubMed] [Google Scholar]
- 19.Rolink AG, Pals ST, Gleichmann E. Allosuppressor and allohelper T cells in acute and chronic graft-vs.-host disease. II. F1 recipients carrying mutations at H-2K and/or I-A. J Exp Med. 1983;157:755–771. doi: 10.1084/jem.157.2.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rolink AG, Gleichmann E. Allosuppressor- and allohelper-T cells in acute and chronic graft-vs.-host (GVH) disease. III. Different Lyt subsets of donor T cells induce different pathological syndromes. J Exp Med. 1983;158:546–558. doi: 10.1084/jem.158.2.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hakim FT, Sharrow SO, Payne S, Shearer GM. Repopulation of host lymphohematopoietic systems by donor cells during graft-versus-host reaction in unirradiated adult F1 mice injected with parental lymphocytes. J Immunol. 1991;146:2108–2115. [PubMed] [Google Scholar]
- 22.Via CS, Sharrow SO, Shearer GM. Role of cytotoxic T lymphocytes in the prevention of lupus-like disease occurring in a murine model of graft-vs-host disease. J Immunol. 1987;139:1840–1849. [PubMed] [Google Scholar]
- 23.Tschetter JR, Mozes E, Shearer GM. Progression from acute to chronic disease in a murine parent-into-F1 model of graft-versus-host disease. J Immunol. 2000;165:5987–5994. doi: 10.4049/jimmunol.165.10.5987. [DOI] [PubMed] [Google Scholar]
- 24.Kim J, Choi WS, Kang H, et al. Conversion of alloantigen-specific CD8+ T cell anergy to CD8+ T cell priming through in vivo ligation of glucocorticoid-induced TNF receptor. J Immunol. 2006;176:5223–5231. doi: 10.4049/jimmunol.176.9.5223. [DOI] [PubMed] [Google Scholar]
- 25.Shustov A, Nguyen P, Finkelman F, Elkon KB, Via CS. Differential expression of Fas and Fas ligand in acute and chronic graft-versus-host disease: up-regulation of Fas and Fas ligand requires CD8+ T cell activation and IFN-gamma production. J Immunol. 1998;161:2848–2855. [PubMed] [Google Scholar]
- 26.Via CS, Rus V, Gately MK, Finkelman FD. IL-12 stimulates the development of acute graft-versus-host disease in mice that normally would develop chronic, autoimmune graft-versus-host disease. J Immunol. 1994;153:4040–4047. [PubMed] [Google Scholar]
- 27.Okubo T, Hagiwara E, Ohno S, et al. Administration of an IL-12-encoding DNA plasmid prevents the development of chronic graft-versus-host disease (GVHD) J Immunol. 1999;162:4013–4017. [PubMed] [Google Scholar]
- 28.De Wit D, Van Mechelen M, Zanin C, et al. Preferential activation of Th2 cells in chronic graft-versus-host reaction. J Immunol. 1993;150:361–366. [PubMed] [Google Scholar]
- 29.Garlisi CG, Pennline KJ, Smith SR, Siegel MI, Umland SP. Cytokine gene expression in mice undergoing chronic graft-versus-host disease. Mol Immunol. 1993;30:669–677. doi: 10.1016/0161-5890(93)90078-p. [DOI] [PubMed] [Google Scholar]
- 30.Durie FH, Aruffo A, Ledbetter J, et al. Antibody to the ligand of CD40, gp39, blocks the occurrence of the acute and chronic forms of graft-vs-host disease. J Clin Invest. 1994;94:1333–1338. doi: 10.1172/JCI117453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Via CS, Rus V, Nguyen P, Linsley P, Gause WC. Differential effect of CTLA4Ig on murine graft-versus-host disease (GVHD) development: CTLA4Ig prevents both acute and chronic GVHD development but reverses only chronic GVHD. J Immunol. 1996;157:4258–4267. [PubMed] [Google Scholar]
- 32.Kim J, Choi WS, La S, et al. Stimulation with 4-1BB (CD137) inhibits chronic graft-versus-host disease by inducing activation-induced cell death of donor CD4+ T cells. Blood. 2005;105:2206–2213. doi: 10.1182/blood-2004-06-2080. [DOI] [PubMed] [Google Scholar]
- 33.Shustov A, Luzina I, Nguyen P, et al. Role of perforin in controlling B-cell hyperactivity and humoral autoimmunity. J Clin Invest. 2000;106:R39–47. doi: 10.1172/JCI8876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singh RR, Hahn BH. Reciprocal T-B determinant spreading develops spontaneously in murine lupus: implications for pathogenesis. Immunol Rev. 1998;164:201–208. doi: 10.1111/j.1600-065x.1998.tb01221.x. [DOI] [PubMed] [Google Scholar]
- 35.Singh RR, Ebling FM, Sercarz EE, Hahn BH. Immune tolerance to autoantibody-derived peptides delays development of autoimmunity in murine lupus. J Clin Invest. 1995;96:2990–2996. doi: 10.1172/JCI118371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mamula MJ, Janeway CA., Jr Do B cells drive the diversification of immune responses? Immunol Today. 1993;14:151–152. doi: 10.1016/0167-5699(93)90274-O. discussion 153-154. [DOI] [PubMed] [Google Scholar]
- 37.Yan J, Harvey BP, Gee RJ, Shlomchik MJ, Mamula MJ. B cells drive early T cell autoimmunity in vivo prior to dendritic cell-mediated autoantigen presentation. J Immunol. 2006;177:4481–4487. doi: 10.4049/jimmunol.177.7.4481. [DOI] [PubMed] [Google Scholar]
- 38.Grader-Beck T, Casciola-Rosen L, Lang TJ, Puliaev R, Rosen A, Via CS. Apoptotic splenocytes drive the autoimmune response to poly(ADP-ribose) polymerase 1 in a murine model of lupus. J Immunol. 2007;178:95–102. doi: 10.4049/jimmunol.178.1.95. [DOI] [PubMed] [Google Scholar]
- 39.Rouquette-Gally AM, Boyeldieu D, Gluckman E, Abuaf N, Combrisson A. Autoimmunity in 28 patients after allogeneic bone marrow transplantation: comparison with Sjogren syndrome and scleroderma. Br J Haematol. 1987;66:45–47. doi: 10.1111/j.1365-2141.1987.tb06888.x. [DOI] [PubMed] [Google Scholar]
- 40.Rouquette-Gally AM, Boyeldieu D, Prost AC, Gluckman E. Autoimmunity after allogeneic bone marrow transplantation. A study of 53 long-term-surviving patients. Transplantation. 1988;46:238–240. doi: 10.1097/00007890-198808000-00010. [DOI] [PubMed] [Google Scholar]
- 41.Ratanatharathorn V, Ayash L, Reynolds C, et al. Treatment of chronic graft-versus-host disease with anti-CD20 chimeric monoclonal antibody. Biol Blood Marrow Transplant. 2003;9:505–511. doi: 10.1016/s1083-8791(03)00216-7. [DOI] [PubMed] [Google Scholar]
- 42.Patriarca F, Skert C, Sperotto A, et al. The development of autoantibodies after allogeneic stem cell transplantation is related with chronic graft-vs-host disease and immune recovery. Exp Hematol. 2006;34:389–396. doi: 10.1016/j.exphem.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 43.Murphy WJ. Revisiting graft-versus-host disease models of autoimmunity: new insights in immune regulatory processes. J Clin Invest. 2000;106:745–747. doi: 10.1172/JCI11088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Subramaniam DS, Fowler DH, Pavletic SZ. Chronic graft-versus-host disease in the era of reduced-intensity conditioning. Leukemia. 2007;21:853–859. doi: 10.1038/sj.leu.2404642. [DOI] [PubMed] [Google Scholar]
- 45.Sykes M, Preffer F, McAfee S, et al. Mixed lymphohaemopoietic chimerism and graft-versus-lymphoma effects after non-myeloablative therapy and HLA-mismatched bone-marrow transplantation. Lancet. 1999;353:1755–1759. doi: 10.1016/S0140-6736(98)11135-2. [DOI] [PubMed] [Google Scholar]
- 46.Perruche S, Marandin A, Kleinclauss F, et al. Association of mixed hematopoietic chimerism with elevated circulating autoantibodies and chronic graft-versus-host disease occurrence. Transplantation. 2006;81:573–582. doi: 10.1097/01.tp.0000183878.53367.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Claman HN, Jaffee BD, Huff JC, Clark RA. Chronic graft-versus-host disease as a model for scleroderma. II. Mast cell depletion with deposition of immunoglobulins in the skin and fibrosis. Cell Immunol. 1985;94:73–84. doi: 10.1016/0008-8749(85)90086-3. [DOI] [PubMed] [Google Scholar]
- 48.McCormick LL, Zhang Y, Tootell E, Gilliam AC. Anti-TGF-beta treatment prevents skin and lung fibrosis in murine sclerodermatous graft-versus-host disease: a model for human scleroderma. J Immunol. 1999;163:5693–5699. [PubMed] [Google Scholar]
- 49.Howell CD, Yoder T, Claman HN, Vierling JM. Hepatic homing of mononuclear inflammatory cells isolated during murine chronic graft-vs-host disease. J Immunol. 1989;143:476–483. [PubMed] [Google Scholar]
- 50.Li J, Helm K, Howell CD. Contributions of donor CD4 and CD8 cells to liver injury during murine graft-versus-host disease. Transplantation. 1996;62:1621–1628. doi: 10.1097/00007890-199612150-00016. [DOI] [PubMed] [Google Scholar]
- 51.Ruzek MC, Jha S, Ledbetter S, Richards SM, Garman RD. A modified model of graft-versus-host-induced systemic sclerosis (scleroderma) exhibits all major aspects of the human disease. Arthritis Rheum. 2004;50:1319–1331. doi: 10.1002/art.20160. [DOI] [PubMed] [Google Scholar]
- 52.Levy S, Nagler A, Okon S, Marmary Y. Parotid salivary gland dysfunction in chronic graft-versus-host disease (cGVHD): a longitudinal study in a mouse model. Bone Marrow Transplant. 2000;25:1073–1078. doi: 10.1038/sj.bmt.1702383. [DOI] [PubMed] [Google Scholar]
- 53.Zhang C, Todorov I, Zhang Z, et al. Donor CD4+ T and B cells in transplants induce chronic graft-versus-host disease with autoimmune manifestations. Blood. 2006;107:2993–3001. doi: 10.1182/blood-2005-09-3623. [DOI] [PubMed] [Google Scholar]
- 54.Jaffee BD, Claman HN. Chronic graft-versus-host disease (GVHD) as a model for scleroderma. I. Description of model systems. Cell Immunol. 1983;77:1–12. doi: 10.1016/0008-8749(83)90001-1. [DOI] [PubMed] [Google Scholar]
- 55.Platt N, da Silva RP, Gordon S. Class A scavenger receptors and the phagocytosis of apoptotic cells. Immunol Lett. 1999;65:15–19. doi: 10.1016/s0165-2478(98)00118-7. [DOI] [PubMed] [Google Scholar]
- 56.Granucci F, Petralia F, Urbano M, et al. The scavenger receptor MARCO mediates cytoskeleton rearrangements in dendritic cells and microglia. Blood. 2003;102:2940–2947. doi: 10.1182/blood-2002-12-3651. [DOI] [PubMed] [Google Scholar]
- 57.Zhou L, Askew D, Wu C, Gilliam AC. Cutaneous gene expression by DNA microarray in murine sclerodermatous graft-versus-host disease, a model for human scleroderma. J Invest Dermatol. 2007;127:281–292. doi: 10.1038/sj.jid.5700517. [DOI] [PubMed] [Google Scholar]
- 58.Eyrich M, Burger G, Marquardt K, et al. Sequential expression of adhesion and costimulatory molecules in graft-versus-host disease target organs after murine bone marrow transplantation across minor histocompatibility antigen barriers. Biol Blood Marrow Transplant. 2005;11:371–382. doi: 10.1016/j.bbmt.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 59.Schiltz PM, Giorno RC, Claman HN. Increased ICAM-1 expression in the early stages of murine chronic graft-versus-host disease. Clin Immunol Immunopathol. 1994;71:136–141. doi: 10.1006/clin.1994.1063. [DOI] [PubMed] [Google Scholar]
- 60.Schlegel PG, Vaysburd M, Chen Y, Butcher EC, Chao NJ. Inhibition of T cell costimulation by VCAM-1 prevents murine graft-versus-host disease across minor histocompatibility barriers. J Immunol. 1995;155:3856–3865. [PubMed] [Google Scholar]
- 61.Hamilton BL. L3T4-positive T cells participate in the induction of graft-vs-host disease in response to minor histocompatibility antigens. J Immunol. 1987;139:2511–2515. [PubMed] [Google Scholar]
- 62.Korngold R, Sprent J. Variable capacity of L3T4+ T cells to cause lethal graft-versus-host disease across minor histocompatibility barriers in mice. J Exp Med. 1987;165:1552–1564. doi: 10.1084/jem.165.6.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Anderson BE, McNiff J, Yan J, et al. Memory CD4+ T cells do not induce graft-versus-host disease. J Clin Invest. 2003;112:101–108. doi: 10.1172/JCI17601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang Y, Hexner E, Frank D, Emerson SG. CD4+ T cells generated de novo from donor hemopoietic stem cells mediate the evolution from acute to chronic graft-versus-host disease. J Immunol. 2007;179:3305–3314. doi: 10.4049/jimmunol.179.5.3305. [DOI] [PubMed] [Google Scholar]
- 65.Shlomchik WD, Couzens MS, Tang CB, et al. Prevention of graft versus host disease by inactivation of host antigen-presenting cells. Science. 1999;285:412–415. doi: 10.1126/science.285.5426.412. [DOI] [PubMed] [Google Scholar]
- 66.Anderson BE, McNiff JM, Jain D, Blazar BR, Shlomchik WD, Shlomchik MJ. Distinct roles for donor- and host-derived antigen-presenting cells and costimulatory molecules in murine chronic graft-versus-host disease: requirements depend on target organ. Blood. 2005;105:2227–2234. doi: 10.1182/blood-2004-08-3032. [DOI] [PubMed] [Google Scholar]
- 67.Chakraverty R, Cote D, Buchli J, et al. An inflammatory checkpoint regulates recruitment of graft-versus-host reactive T cells to peripheral tissues. J Exp Med. 2006;203:2021–2031. doi: 10.1084/jem.20060376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Durakovic N, Radojcic V, Skarica M, et al. Factors governing the activation of adoptively transferred donor T cells infused after allogeneic bone marrow transplantation in the mouse. Blood. 2007;109:4564–4574. doi: 10.1182/blood-2006-09-048124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kaplan DH, Anderson BE, McNiff JM, Jain D, Shlomchik MJ, Shlomchik WD. Target antigens determine graft-versus-host disease phenotype. J Immunol. 2004;173:5467–5475. doi: 10.4049/jimmunol.173.9.5467. [DOI] [PubMed] [Google Scholar]
- 70.Tomonari K, Fairchild S, Rosenwasser OA. Influence of viral superantigens on V beta- and V alpha-specific positive and negative selection. Immunol Rev. 1993;131:131–168. doi: 10.1111/j.1600-065x.1993.tb01534.x. [DOI] [PubMed] [Google Scholar]
- 71.Schirrmacher V, Beutner U, Bucur M, Umansky V, Rocha M, von Hoegen P. Loss of endogenous mouse mammary tumor virus superantigen increases tumor resistance. J Immunol. 1998;161:563–570. [PubMed] [Google Scholar]
- 72.Allen RD, Slayback DL, Harper JM, Aguirre TL, Dobkins JA. A locus closely linked to Mtv7 on mouse chromosome 1 influences development of acute versus chronic graft-versus-host disease in a murine model. Clin Immunol. 2000;95:9–19. doi: 10.1006/clim.2000.4841. [DOI] [PubMed] [Google Scholar]
- 73.Slayback DL, Dobkins JA, Harper JM, Allen RD. Genetic factors influencing the development of chronic graft-versus-host disease in a murine model. Bone Marrow Transplant. 2000;26:931–938. doi: 10.1038/sj.bmt.1702661. [DOI] [PubMed] [Google Scholar]
- 74.Wynn TA. Fibrotic disease and the T(H)1/T(H)2 paradigm. Nat Rev Immunol. 2004;4:583–594. doi: 10.1038/nri1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang Y, McCormick LL, Desai SR, Wu C, Gilliam AC. Murine sclerodermatous graft-versus-host disease, a model for human scleroderma: cutaneous cytokines, chemokines, and immune cell activation. J Immunol. 2002;168:3088–3098. doi: 10.4049/jimmunol.168.6.3088. [DOI] [PubMed] [Google Scholar]
- 76.Zhang Y, McCormick LL, Gilliam AC. Latency-associated peptide prevents skin fibrosis in murine sclerodermatous graft-versus-host disease, a model for human scleroderma. J Invest Dermatol. 2003;121:713–719. doi: 10.1046/j.1523-1747.2003.12517.x. [DOI] [PubMed] [Google Scholar]
- 77.Banovic T, MacDonald KP, Morris ES, et al. TGF-beta in allogeneic stem cell transplantation: friend or foe? Blood. 2005;106:2206–2214. doi: 10.1182/blood-2005-01-0062. [DOI] [PubMed] [Google Scholar]
- 78.MacDonald KP, Rowe V, Filippich C, et al. Chronic graft-versus-host disease after granulocyte colony-stimulating factor-mobilized allogeneic stem cell transplantation: the role of donor T-cell dose and differentiation. Biol Blood Marrow Transplant. 2004;10:373–385. doi: 10.1016/j.bbmt.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 79.Levi-Schaffer F, Weg VB. Mast cells, eosinophils and fibrosis. Clin Exp Allergy. 1997;27 1:64–70. doi: 10.1111/j.1365-2222.1997.tb01829.x. [DOI] [PubMed] [Google Scholar]
- 80.Choi KL, Giorno R, Claman HN. Cutaneous mast cell depletion and recovery in murine graft-vs-host disease. J Immunol. 1987;138:4093–4101. [PubMed] [Google Scholar]
- 81.Nonomura A, Kono N, Mizukami Y, Nakanuma Y. Histological changes of the liver in experimental graft-versus-host disease across minor histocompatibility barriers. VIII. Role of eosinophil infiltration. Liver. 1996;16:42–47. doi: 10.1111/j.1600-0676.1996.tb00702.x. [DOI] [PubMed] [Google Scholar]
- 82.Levi-Schaffer F, Goldenhersh MA, Segal V, Nagler A. Nedocromil sodium ameliorates skin manifestations in a murine model of chronic graft-versus-host disease. Bone Marrow Transplant. 1997;19:823–828. doi: 10.1038/sj.bmt.1700734. [DOI] [PubMed] [Google Scholar]
- 83.Hossain MS, Roback JD, Pollack BP, Jaye DL, Langston A, Waller EK. Chronic GvHD decreases antiviral immune responses in allogeneic BMT. Blood. 2007;109:4548–4556. doi: 10.1182/blood-2006-04-017442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Anderson BE, McNiff JM, Matte C, Athanasiadis I, Shlomchik WD, Shlomchik MJ. Recipient CD4+ T cells that survive irradiation regulate chronic graft-versus-host disease. Blood. 2004;104:1565–1573. doi: 10.1182/blood-2004-01-0328. [DOI] [PubMed] [Google Scholar]
- 85.Shulman HM, Sullivan KM, Weiden PL, et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med. 1980;69:204–217. doi: 10.1016/0002-9343(80)90380-0. [DOI] [PubMed] [Google Scholar]
- 86.Wingard JR, Piantadosi S, Vogelsang GB, et al. Predictors of death from chronic graft-versus-host disease after bone marrow transplantation. Blood. 1989;74:1428–1435. [PubMed] [Google Scholar]
- 87.Fimiani M, De Aloe G, Cuccia A. Chronic graft versus host disease and skin. J Eur Acad Dermatol Venereol. 2003;17:512–517. doi: 10.1046/j.1468-3083.2003.00780.x. [DOI] [PubMed] [Google Scholar]
- 88.Skert C, Patriarca F, Sperotto A, et al. Sclerodermatous chronic graft-versus-host disease after allogeneic hematopoietic stem cell transplantation: incidence, predictors and outcome. Haematologica. 2006;91:258–261. [PubMed] [Google Scholar]
- 89.Flowers ME, Parker PM, Johnston LJ, et al. Comparison of chronic graft-versus-host disease after transplantation of peripheral blood stem cells versus bone marrow in allogeneic recipients: long-term follow-up of a randomized trial. Blood. 2002;100:415–419. doi: 10.1182/blood-2002-01-0011. [DOI] [PubMed] [Google Scholar]
- 90.Mielcarek M, Martin PJ, Heimfeld S, Storb R, Torok-Storb B. CD34 cell dose and chronic graft-versus-host disease after human leukocyte antigen-matched sibling hematopoietic stem cell transplantation. Leuk Lymphoma. 2004;45:27–34. doi: 10.1080/1042819031000151103. [DOI] [PubMed] [Google Scholar]
- 91.Jones-Caballero M, Fernandez-Herrera J, Cordoba-Guijarro S, Dauden-Tello E, Garcia-Diez A. Sclerodermatous graft-versus-host disease after donor leucocyte infusion. Br J Dermatol. 1998;139:889–892. doi: 10.1046/j.1365-2133.1998.02520.x. [DOI] [PubMed] [Google Scholar]
- 92.Ogawa Y, Kodama H, Kameyama K, et al. Donor fibroblast chimerism in the pathogenic fibrotic lesion of human chronic graft-versus-host disease. Invest Ophthalmol Vis Sci. 2005;46:4519–4527. doi: 10.1167/iovs.05-0227. [DOI] [PubMed] [Google Scholar]
- 93.Wolff D, Reichenberger F, Steiner B, et al. Progressive interstitial fibrosis of the lung in sclerodermoid chronic graft-versus-host disease. Bone Marrow Transplant. 2002;29:357–360. doi: 10.1038/sj.bmt.1703386. [DOI] [PubMed] [Google Scholar]
- 94.Banning U, Krutmann J, Korholz D. The role of IL-4 and IL-12 in the regulation of collagen synthesis by fibroblasts. Immunol Invest. 2006;35:199–207. doi: 10.1080/08820130600616714. [DOI] [PubMed] [Google Scholar]
- 95.Liem LM, Fibbe WE, van Houwelingen HC, Goulmy E. Serum transforming growth factor-beta1 levels in bone marrow transplant recipients correlate with blood cell counts and chronic graft-versus-host disease. Transplantation. 1999;67:59–65. doi: 10.1097/00007890-199901150-00009. [DOI] [PubMed] [Google Scholar]
- 96.Pavletic SZ, Smith LM, Bishop MR, et al. Prognostic factors of chronic graft-versus-host disease after allogeneic blood stem-cell transplantation. Am J Hematol. 2005;78:265–274. doi: 10.1002/ajh.20275. [DOI] [PubMed] [Google Scholar]
- 97.Menendez P, Perez-Simon JA, Mateos MV, et al. Influence of the different CD34+ and CD34- cell subsets infused on clinical outcome after non-myeloablative allogeneic peripheral blood transplantation from human leucocyte antigen-identical sibling donors. Br J Haematol. 2002;119:135–143. doi: 10.1046/j.1365-2141.2002.03794.x. [DOI] [PubMed] [Google Scholar]
- 98.Keane MP, Gomperts BN, Weigt S, et al. IL-13 is pivotal in the fibro-obliterative process of bronchiolitis obliterans syndrome. J Immunol. 2007;178:511–519. doi: 10.4049/jimmunol.178.1.511. [DOI] [PubMed] [Google Scholar]
- 99.Simms RW, Korn JH. Cytokine directed therapy in scleroderma: rationale, current status, and the future. Curr Opin Rheumatol. 2002;14:717–722. doi: 10.1097/00002281-200211000-00015. [DOI] [PubMed] [Google Scholar]
- 100.Gabrielli A, Svegliati S, Moroncini G, Luchetti M, Tonnini C, Avvedimento EV. Stimulatory autoantibodies to the PDGF receptor: A link to fibrosis in scleroderma and a pathway for novel therapeutic targets. Autoimmun Rev. 2007;7:121–126. doi: 10.1016/j.autrev.2007.02.020. [DOI] [PubMed] [Google Scholar]
- 101.Svegliati S, Olivieri A, Campelli N, et al. Stimulatory autoantibodies to PDGF receptor in patients with extensive chronic graft-versus-host disease. Blood. 2007;110:237–241. doi: 10.1182/blood-2007-01-071043. [DOI] [PubMed] [Google Scholar]
- 102.Tsunemi Y, Ihn H, Nakamura K, Tamaki K. Post-transplantation chronic graft-versus-host disease with overlapping features similar to those of various collagen diseases. Int J Dermatol. 2003;42:292–294. doi: 10.1046/j.1365-4362.2003.01690.x. [DOI] [PubMed] [Google Scholar]
- 103.Couriel DR, Saliba RM, Giralt S, et al. Acute and chronic graft-versus-host disease after ablative and nonmyeloablative conditioning for allogeneic hematopoietic transplantation. Biol Blood Marrow Transplant. 2004;10:178–185. doi: 10.1016/j.bbmt.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 104.Petersen SL, Ryder LP, Bjork P, et al. A comparison of T-, B- and NK-cell reconstitution following conventional or nonmyeloablative conditioning and transplantation with bone marrow or peripheral blood stem cells from human leucocyte antigen identical sibling donors. Bone Marrow Transplant. 2003;32:65–72. doi: 10.1038/sj.bmt.1704084. [DOI] [PubMed] [Google Scholar]
- 105.Kyewski B, Klein L. A central role for central tolerance. Annu Rev Immunol. 2006;24:571–606. doi: 10.1146/annurev.immunol.23.021704.115601. [DOI] [PubMed] [Google Scholar]
- 106.Bonomo A, Kehn PJ, Payer E, Rizzo L, Cheever AW, Shevach EM. Pathogenesis of post-thymectomy autoimmunity. Role of syngeneic MLR-reactive T cells. J Immunol. 1995;154:6602–6611. [PubMed] [Google Scholar]
- 107.Fukushi N, Arase H, Wang B, et al. Thymus: a direct target tissue in graft-versus-host reaction after allogeneic bone marrow transplantation that results in abrogation of induction of self-tolerance. Proc Natl Acad Sci U S A. 1990;87:6301–6305. doi: 10.1073/pnas.87.16.6301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Krenger W, Rossi S, Piali L, Hollander GA. Thymic atrophy in murine acute graft-versus-host disease is effected by impaired cell cycle progression of host pro-T and pre-T cells. Blood. 2000;96:347–354. [PubMed] [Google Scholar]
- 109.Rossi S, Blazar BR, Farrell CL, et al. Keratinocyte growth factor preserves normal thymopoiesis and thymic microenvironment during experimental graft-versus-host disease. Blood. 2002;100:682–691. doi: 10.1182/blood.v100.2.682. [DOI] [PubMed] [Google Scholar]
- 110.Hauri-Hohl MM, Keller MP, Gill J, et al. Donor T-cell alloreactivity against host thymic epithelium limits T-cell development after bone marrow transplantation. Blood. 2007;109:4080–4088. doi: 10.1182/blood-2006-07-034157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Atkinson K, Incefy GS, Storb R, et al. Low serum thymic hormone levels in patients with chronic graft-versus-host disease. Blood. 1982;59:1073–1077. [PubMed] [Google Scholar]
- 112.Fallen PR, McGreavey L, Madrigal JA, et al. Factors affecting reconstitution of the T cell compartment in allogeneic haematopoietic cell transplant recipients. Bone Marrow Transplant. 2003;32:1001–1014. doi: 10.1038/sj.bmt.1704235. [DOI] [PubMed] [Google Scholar]
- 113.Weinberg K, Blazar BR, Wagner JE, et al. Factors affecting thymic function after allogeneic hematopoietic stem cell transplantation. Blood. 2001;97:1458–1466. doi: 10.1182/blood.v97.5.1458. [DOI] [PubMed] [Google Scholar]
- 114.Poulin JF, Sylvestre M, Champagne P, et al. Evidence for adequate thymic function but impaired naive T-cell survival following allogeneic hematopoietic stem cell transplantation in the absence of chronic graft-versus-host disease. Blood. 2003;102:4600–4607. doi: 10.1182/blood-2003-05-1428. [DOI] [PubMed] [Google Scholar]
- 115.Sakoda Y, Hashimoto D, Asakura S, et al. Donor-derived thymic-dependent T cells cause chronic graft-versus-host disease. Blood. 2007;109:1756–1764. doi: 10.1182/blood-2006-08-042853. [DOI] [PubMed] [Google Scholar]
- 116.Ghayur T, Seemayer TA, Xenocostas A, Lapp WS. Complete sequential regeneration of graft-vs.-host-induced severely dysplastic thymuses. Implications for the pathogenesis of chronic graft-vs.-host disease. Am J Pathol. 1988;133:39–46. [PMC free article] [PubMed] [Google Scholar]