Abstract
We previously demonstrated that monocytes produce IL-23 during Francisella infection, and that IL-23 induces IFNγ from NK cells. Here, we demonstrate that IFNγ-priming of monocytes enhances IL-23 production during Francisella infection. This effect was seen on the IL12/23 p40 subunit. Induction of IL-12/23 p40 is reported to be enhanced by IRF-1 and IRF-8. Consistently, microarray analysis of IFNγ-treated monocytes revealed a significant induction of the IRFs. Interestingly, IFNγ-primed monocytes produced IL-12p70, a more potent inducer of IFNγ than IL-23. We propose that there exists an amplification loop between monocyte IL-23 and NK/T cell IFNγ that leads to IL-12p70 production.
Keywords: Host response, Francisella, IL-23, monocytes
Introduction
Francisella tularensis, a gram-negative facultative bacterium, is the causative agent of tularemia. The Type A subspecies F. tularensis tularensis is classified as a Category A agent because of the low dosage required for lethality [1].
Francisella infects primarily monocytes and macrophages [2], and has the ability to escape from the host cell phagosome and replicate in the cytoplasm. The subspecies F. novicida is commonly used as an infection model because it is nonvirulent in normal humans yet leads to fatal tularemia in mouse models [3]. Further, F. novicida follows the same intracellular lifestyle in human monocytes/macrophages as the virulent F. tularensis tularensis subspecies [4].
Host cell response to Francisella infection involves the release of multiple inflammatory cytokines, which offer protection against the pathogen [5–10]. We have shown that F. novicida elicits IL-23 production in human monocytes and that the IL-23 could induce an IFNγ response from NK cells [10]. IFNγ has been shown to prime monocytes/macrophages to produce enhanced levels of IL-12 in response to a subsequent stimulus such as lipopolysaccharide [11]. Priming with IFNγ has also been recently shown to confer protection against Francisella by promoting phagolysosome fusion and impeding phagosomal escape [4].
Here, we show that IL-23 production is strengthened by IFNγ, and that Francisella elicits IL-12p70 from monocytes only in the presence of sufficient IFNγ.
Materials and Methods
Cells, antibodies and reagents
THP-1 cells were obtained from American Type Culture Collection (Manassas, VA) and maintained in RPMI 1640 supplemented with 10% fetal bovine serum. CD14-positive peripheral blood monocytes (PBM) were isolated as previously described [12].
Cell stimulation, lysis and Western blotting
PBM/THP-1 cells were infected with 100 MOI F. novicida or F. tularensis LVS. The multiplicity of infection (MOI) was approximated by measuring the optical density at 600 nm and confirmed by plating the inocula and counting CFU [6]. Uninfected and infected cells were lysed in TN1 buffer (50mM Tris pH 8.0, 10mM EDTA, 10mM Na4P2O7, 10mM NaF, 1% Triton-X 100, 125mM NaCl, 10mM Na3VO4, 10μg/ml each aprotinin and leupeptin). Post-nuclear lysates were boiled in Laemmli Sample Buffer and were separated by SDS/PAGE, transferred to nitrocellulose filters, probed with the antibody of interest and developed by enhanced chemiluminescence (ECL, Amersham).
Measurement of cytokines by ELISA
Cell supernatants were analyzed by ELISA using cytokine specific kits from eBioscience (San Diego, CA) and R & D Systems (Minneapolis, MN).
Flow cytometry analysis of TLR2 expression
THP-1 cells were tested for the expression of TLR2 by incubating with human TLR2-FITC antibody (or an isotype control antibody) at a concentration of 10μg/ml, in the presence of goat serum for 30 minutes at 4°C. The cells were subsequently washed, fixed in 1% paraformaldehyde and analyzed by flow cytometry on an Elite EPICS fluorescence-activated cell sorter (Coulter, Hialeah, FL). Data from 10,000 cells per condition were recorded to yield the percentage of cells expressing receptors.
Microarray Analysis
Microarray analysis was performed as previously described [10]. Briefly, PBM from four donors were either left untreated, treated with 25 ng/ml human IFNγ, or infected with F. novicida at an MOI of 100 for 24 hours. IFNγ was used at 25ng/ml since pilot experiments indicated that higher concentrations of IFNγ are toxic to primary human monocytes. RNA from each of these separate treatments was extracted using TRIzol Reagent (Invitrogen Life Technologies), column-purified using RNeasy columns (Qiagen), and then hybridized to Human Genome U133 Plus 2.0 Array chips (Affymetrix). Expression values were calculated using the gcrma package in BioConductor (www.bioconductor.org) and the resulting data were analyzed for differential expression using the limma package [13]. Genes with adjusted p values <=0.05 were counted as significantly different. These were ranked by fold change.
Results
IFNγ pretreatment enhances IL-23 production during Francisella infection
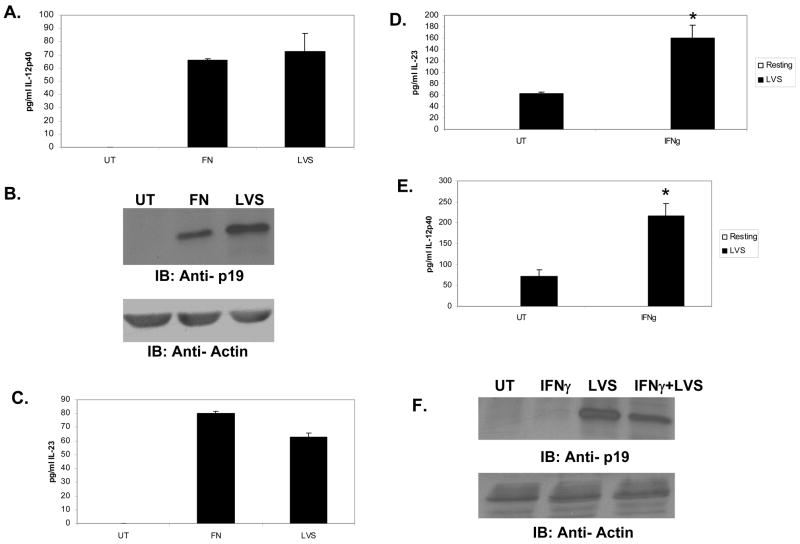
To test whether IFNγ could enhance production of IL-23, we incubated PBM in the presence or absence of recombinant human IFNγ and subsequently infected with F. novicida for 24 hours. Cell supernatants were analyzed by ELISA for the presence of IL-23. The results indicate that IFNγ priming leads to enhanced production of IL-23 in response to F. novicida infection (Figure 1A). To examine the influence of IFNγ on the two subunits of IL-23, supernatants were analyzed by ELISA for IL-12/23p40 (Figure 1B) and lysates were analyzed by Western blotting for the p19 subunit (Figure 1C). IFNγ enhanced the production of IL-12/23 p40 subunit but not the p19 subunit in infected PBM. Of note, IFNγ alone did not induce the production of either IL-12/23 p40 nor IL-23. Similar results were obtained with the THP-1 cells (Figures 1D–F)
Figure 1. IFNγ pretreatment results in enhanced IL-23 production during infection.
PBM from 4 donors were incubated overnight with 25ng/ml hIFNγ, and subsequently infected for 24 hours with F. novicida. Cell supernatants were assayed by ELISA for IL-23 (A); for IL-12/23 p40 (B) The graphs show data from four donors. Error bars indicate standard deviation. * p value ≤0.05. C. Lysates were analyzed by Western blotting for IL-23 p19 (upper panel), followed by a reprobe with actin antibody (lower panel). D–F. THP-1 cells were incubated overnight with 25ng/ml hIFNγ and subsequently infected with F. novicida (MOI of 100). Supernatants and lysates were assayed for IL-23, IL-12/23p40 and the p19 subunit. These results are representative of three independent experiments.
F. tularensis LVS elicits a similar IL-23 response
F. tularensis LVS is an attenuated vaccine strain derived from the Type B holarctica subspecies [14] and is commonly used to study host response. To test whether these IL-23 findings were applicable to this strain, THP-1 cells were infected with either F. novicida or with F. tularensis LVS for 24 hours. Production of IL-12/23 p40 and IL-23 was measured by ELISA, and the p19 subunit was analyzed by Western blotting. Both strains of the pathogen elicited equivalent responses (Figures 2A–C).
Figure 2. F. tularensis LVS elicits a similar IL-23 response.
A–C THP-1 cells were infected for 24 hours with either F. novicida or F. tularensis LVS. Cell supernatants were assayed by ELISA for IL-12/23 p40 and IL-23, and lysates were analyzed by Western blotting for the p19 subunit. D–F. THP-1 cells were incubated overnight with 25ng/ml hIFNγ and subsequently infected for 24 hours with F. tularensis LVS. Supernatants were assayed by ELISA for IL-12/23 p40 and IL-23. Data from three independent experiments were analyzed by the student’s t-test. Error bars indicate standard deviation. * p value ≤0.05. Lysates were analyzed by Western blotting for the p19 subunit.
The results shown in Figures 2A–C demonstrate that IL-23 is produced in response to F. tularensis LVS infection. IFNγ priming resulted in enhanced IL-23 production (Figure 2D). IFNγ specifically upregulated the production of IL-12/23p40 but not the p19 subunit (Figures 2E and F). Indeed, the p19 subunit appeared to be somewhat reduced in the IFNγ-treated cells (Figures 1F and 2F), perhaps reflecting the increased secretion of IL-23 into the supernatant.
Role of IFNγ-priming during infection
We recently demonstrated that the production of IL-23 is dependent on the PI3K/Akt pathway and NFκB [10]. Thus, THP-1 cells were incubated overnight with or without IFNγ and subsequently infected with F. novicida and analyzed for phosphorylation of Akt, NFκB and IKKα. The results indicated that all three molecules are phosphorylated in infected cells (Figure 3). Interestingly, IFNγ priming did not enhance these upstream signaling events required for cytokine production.
Figure 3. IFNγ-priming does not enhance the activation of the PI3K pathway nor NFκB during infection.
THP-1 cells were incubated overnight with 25ng/ml IFNγ and then infected with F. novicida for the time points indicated. Lysates were analyzed by Western blotting with antibodies for the phosphorylated forms of Akt (A), NFκB (B) or IKKα (C). All membranes were reprobed with actin antibody. These results are representative of three independent experiments. D. Cells were labeled with a FITC-conjugated TLR2 antibody or a control antibody and analyzed by Flow Cytometry.
Host response to Francisella has been shown to be critically dependent on the expression of TLR2 [15–18]. Consistent with a lack of effect on the infection-induced signaling events, IFNγ treatment did not alter TLR2 levels (Figure 3D).
The enhancement of IL-12 production in IFNγ primed monocytes has been well documented. Although IFNγ alone does not induce the production of IL-12p40, it primes the IL-12p40 promoter so that transcription can occur in response to a second stimulus such as LPS. IFNγ promotes IL-12 p40 transcription through the induction of IRF-1 and IRF-8/ICSBP [19]. Thus, we next examined whether these regulatory factors were induced in the IFNγ-primed PBM. Microarray analysis of IFNγ-primed PBM demonstrated an induction of both IRF-1 and IRF-8 (8.3 fold change, p=0.01 and 6.13 fold change, p=0.0001 for IRF-1 and IRF-8 respectively), thus accounting for the enhancement in IL-12p40 production.
IFNγ-primed monocytes produce IL-12 p70
IL-12 p70 has been shown to be a strong inducer of IFNγ leading to protection from bacterial infections [20,21,21]. Our recent studies demonstrated that although IL-12/23 p40 is produced readily upon Francisella infection of PBM, there was no detectable IL-12 p70 [10]. Since IFNγ has been shown to prime cells for the production of the p35 subunit [22], we examined whether IFNγ-primed PBM could produce IL-12 p70 in response to Francisella infection. For this, IFNγ-primed PBM and mixed peripheral blood mononuclear cells (PBMC) were infected with F. novicida for 24 hours and the cell supernatants were assayed by ELISA for IL-12 p70. The results demonstrate that IFNγ-primed cells are indeed capable of producing detectable amounts of IL-12 p70 upon infection (Figure 4).
Figure 4. IFNγ-primed monocytes produce IL-12 p70.
(A) PBM and (B) PBMC from 3 donors were incubated overnight with hIFNγ, infected for 24 hours with F. novicida and cell supernatants were assayed for IL-12 p70. C. Proposed model of an amplification loop between IL-23 and IFNγ.
Discussion
The microbicidal activity of monocytes/macrophages is mediated by various effectors such as cytokines, and this anti-microbial action is greatly enhanced by IFNγ exposure. Thus the cytokine-enhancing activity of IFNγ is an important component of IFNγ-mediated protection [5,6,8,9,23].
In our previous study we reported that Francisella-infected PBM secrete IL-23, which can induce secretion of IFNγ by NK cells [10]. Here we show that IFNγ priming of monocytes results in enhanced production of IL-23. Collectively then, our previous and present studies suggest that an IL-23 – IFNγ loop may arise upon Francisella infection.
One important point is that IL-12 p70 was only detectable after IFNγ-priming. This suggests that IL-23 may take part in the early stages of an amplification loop, where the resulting NK cell-derived IFNγ feeds back to the monocytes/macrophages to elicit IL-12 p70 production. Hence, it is conceivable that IL-23 begins the monocyte – NK cell loop and these initial iterations of the loop would then result in IL-12 p70 production. It is known that IL-12 p70 is far more effective than IL-23 at eliciting IFNγ from NK cells [21], so the secreted IL-12 p70 would then bring the amplification loop to its fullest level.
One major question is that, given the previous studies showing that IFNγ is required for IL-12 p40 production and that p40 is a subunit of IL-23, how do the monocytes produce IL-23 to begin with? Our earlier study showed that monocytes produce small amounts of IFNγ during Francisella infection [10]. This suggests that the IFNγ signaling could be at levels sufficient to act in an autocrine/paracrine fashion to enable p40 production but not yet high enough to elicit p35 production. In this sense, the concentration of IFNγ could act as a rheostat, where low levels elicit IL-23 production and high levels elicit more IL-23 but also permit IL-12 p70 production.
In summary, our proposed model (Figure 4C) shows an initial relatively weak IFNγ response by monocytes to Francisella infection. This IFNγ leads to IL-23 secretion, which then acts upon NK cells and causes moderate IFNγ secretion. The IFNγ then feeds back to the monocytes, prompting them to 1) secrete more IL-23 and to 2) begin secreting IL-12 p70. In this sense, IL-23 is an early yet critical player in the pro-inflammatory cytokine loop between monocytes and NK cells, serving to initiate the stronger IL-12 p70/IFNγ amplification loop.
Acknowledgments
We thank Dr. John Gunn for the Francisella isolates and Ms. Donna Cain for assistance with ELISAs. This work was sponsored by the NIH/NIAID Region V ‘Great Lakes’ RCE (NIH award 1-U54-AI-057153). JPB is supported by T32CA090223.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Fortier AH, Green SJ, Polsinelli T, Jones TR, Crawford RM, Leiby DA, Elkins KL, Meltzer MS, Nacy CA. Life and death of an intracellular pathogen: Francisella tularensis and the macrophage. Immunol Ser. 1994;60:349–61. [PubMed] [Google Scholar]
- 2.Hall JD, Craven RR, Fuller JR, Pickles RJ, Kawula TH. Francisella tularensis replicates within alveolar type II epithelial cells in vitro and in vivo following inhalation. Infect Immun. 2007;75:1034–1039. doi: 10.1128/IAI.01254-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kieffer TL, Cowley S, Nano FE, Elkins KL. Francisella novicida LPS has greater immunobiological activity in mice than F. tularensis LPS, and contributes to F. novicida murine pathogenesis. Microbes Infect. 2003;5:397–403. doi: 10.1016/s1286-4579(03)00052-2. [DOI] [PubMed] [Google Scholar]
- 4.Santic M, Molmeret M, Abu KY. Modulation of biogenesis of the Francisella tularensis subsp. novicida-containing phagosome in quiescent human macrophages and its maturation into a phagolysosome upon activation by IFN-gamma. Cell Microbiol. 2005;7:957–967. doi: 10.1111/j.1462-5822.2005.00529.x. [DOI] [PubMed] [Google Scholar]
- 5.Telepnev M, Golovliov I, Sjostedt A. Francisella tularensis LVS initially activates but subsequently down-regulates intracellular signaling and cytokine secretion in mouse monocytic and human peripheral blood mononuclear cells. Microb Pathog. 2005;38:239–247. doi: 10.1016/j.micpath.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 6.Gavrilin MA, I, Bouakl J, Knatz NL, Duncan MD, Hall MW, Gunn JS, Wewers MD. Internalization and phagosome escape required for Francisella to induce human monocyte IL-1{beta} processing and release. Proc Natl Acad Sci USA. 2006;103(1):141–6. doi: 10.1073/pnas.0504271103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parsa KV, Ganesan LP, Rajaram MV, Gavrilin MA, Balagopal A, Mohapatra NP, Wewers MD, Schlesinger LS, Gunn JS, Tridandapani S. Macrophage Pro-Inflammatory Response to Francisella novicida Infection Is Regulated by SHIP. PLoS Pathog. 2006;2:e71. doi: 10.1371/journal.ppat.0020071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mariathasan S, Weiss DS, Dixit VM, Monack DM. Innate immunity against Francisella tularensis is dependent on the ASC/caspase-1 axis. J Exp Med. 2005;202:1043–1049. doi: 10.1084/jem.20050977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bolger CE, Forestal CA, Italo JK, Benach JL, Furie MB. The live vaccine strain of Francisella tularensis replicates in human and murine macrophages but induces only the human cells to secrete proinflammatory cytokines. J Leukoc Biol. 2005;77:893–897. doi: 10.1189/jlb.1104637. [DOI] [PubMed] [Google Scholar]
- 10.Butchar JP, Rajaram MVS, Ganesan LP, Parsa KVL, Clay CD, Schlesinger LS, Tridandapani S. Francisella tularensis induces IL-23 production in human monocytes. Journal of Immunology. 2007;178:4445–4454. doi: 10.4049/jimmunol.178.7.4445. [DOI] [PubMed] [Google Scholar]
- 11.Hayes MP, Wang J, Norcross MA. Regulation of interleukin-12 expression in human monocytes: selective priming by interferon-gamma of lipopolysaccharide-inducible p35 and p40 genes. Blood. 1995;86:646–650. [PubMed] [Google Scholar]
- 12.Tridandapani S, Wardrop R, Baran CP, Wang Y, Opalek JM, Caligiuri MA, Marsh CB. TGF-beta1 Supresses Myeloid Fcgamma Receptor Function by Regulating the Expression and Function of the Common gamma-Subunit. J Immunol. 2003;170:4572–4577. doi: 10.4049/jimmunol.170.9.4572. [DOI] [PubMed] [Google Scholar]
- 13.Smyth GK. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol. 2004;3(Article3) doi: 10.2202/1544-6115.1027. [DOI] [PubMed] [Google Scholar]
- 14.SASLAW S, EIGELSBACH HT, PRIOR JA, WILSON HE, CARHART S. Tularemia vaccine study. II. Respiratory challenge. Arch Intern Med. 1961;107:702–14. 702–714. doi: 10.1001/archinte.1961.03620050068007. [DOI] [PubMed] [Google Scholar]
- 15.Cole LE, Shirey KA, Barry E, Santiago A, Rallabhandi P, Elkins KL, Puche A, Michalek SM, Vogel SN. TLR2-Mediated Signaling Requirements for Francisella tularensis LVS Infection of Murine Macrophages. Infect Immun. 2007;75(8):4127–37. doi: 10.1128/IAI.01868-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Katz J, Zhang P, Martin M, Vogel SN, Michalek SM. Toll-like receptor 2 is required for inflammatory responses to Francisella tularensis LVS. Infect Immun. 2006;74:2809–2816. doi: 10.1128/IAI.74.5.2809-2816.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li H, Nookala S, Bina XR, Bina JE, Re F. Innate immune response to Francisella tularensis is mediated by TLR2 and caspase-1 activation. J Leukoc Biol. 2006;80:766–773. doi: 10.1189/jlb.0406294. [DOI] [PubMed] [Google Scholar]
- 18.Malik M, Bakshi CS, Sahay B, Shah A, Lotz SA, Sellati TJ. Toll-like receptor 2 is required for control of pulmonary infection with Francisella tularensis. Infect Immun. 2006;74:3657–3662. doi: 10.1128/IAI.02030-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang IM, Contursi C, Masumi A, Ma X, Trinchieri G, Ozato K. An IFN-gamma-inducible transcription factor, IFN consensus sequence binding protein (ICSBP), stimulates IL-12 p40 expression in macrophages. J Immunol. 2000;165:271–279. doi: 10.4049/jimmunol.165.1.271. [DOI] [PubMed] [Google Scholar]
- 20.Trinchieri G, Wysocka M, D’Andrea A, Rengaraju M, Aste-Amezaga M, Kubin M, Valiante NM, Chehimi J. Natural killer cell stimulatory factor (NKSF) or interleukin-12 is a key regulator of immune response and inflammation. Prog Growth Factor Res. 1992;4:355–368. doi: 10.1016/0955-2235(92)90016-b. [DOI] [PubMed] [Google Scholar]
- 21.Oppmann B, Lesley R, Blom B, Timans JC, Xu Y, Hunte B, Vega F, Yu N, Wang J, Singh K, Zonin F, Vaisberg E, Churakova T, Liu M, Gorman D, Wagner J, Zurawski S, Liu Y, Abrams JS, Moore KW, Rennick D, Waal-Malefyt R, Hannum C, Bazan JF, Kastelein RA. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000;13:715–725. doi: 10.1016/s1074-7613(00)00070-4. [DOI] [PubMed] [Google Scholar]
- 22.Goriely S, Demonte D, Nizet S, De Wit D, Willems F, Goldman M, Van Lint C. Human IL-12(p35) gene activation involves selective remodeling of a single nucleosome within a region of the promoter containing critical Sp1-binding sites. Blood. 2003;101:4894–4902. doi: 10.1182/blood-2002-09-2851. [DOI] [PubMed] [Google Scholar]
- 23.Stenmark S, Sunnemark D, Bucht A, Sjostedt A. Rapid local expression of interleukin-12, tumor necrosis factor alpha, and gamma interferon after cutaneous Francisella tularensis infection in tularemia-immune mice. Infect Immun. 1999;67:1789–1797. doi: 10.1128/iai.67.4.1789-1797.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]