Abstract
The Cystic Fibrosis Transmembrane conductance Regulator (CFTR) is present on the apical membrane of corneal endothelial cells. Increasing intracellular [cAMP] with forskolin stimulates an NPPB and glibenclamide-inhibitable apical Cl- and HCO3- permeability. To definitively determine that the increased permeability is dependent on CFTR, we used an siRNA knockdown approach. Apical Cl- and HCO3- permeability and steady-state HCO3- flux were measured in the presence or absence of forskolin using cultured bovine corneal endothelial cells that were transfected with CFTR siRNA or a scrambled sequence control. CFTR protein expression was reduced by ∼80% in CFTR siRNA treated cultures. Forskolin (10 μM) increased apical chloride permeability by 7 fold, which was reduced to control level in siRNA treated cells. CFTR siRNA treatment had no effect on baseline apical chloride permeability. Apical HCO3- permeability was increased 2 fold by 10 μM forskolin, which was reduced to control level in siRNA treated cultures. Similarly, there was no effect on baseline apical HCO3- permeability by knocking down CFTR expression. The steady-state apical-basolateral pH gradient (ΔpH) at four hours in control cultures was increased ∼2.5 fold by forskolin. In CFTR siRNA treated cells, the baseline ΔpH was similar to control, however forskolin did not have a significant effect. We conclude that forskolin induced increases in apical HCO3- permeability in bovine corneal endothelium requires CFTR. However, CFTR does not have a major role in determining baseline apical chloride or HCO3- permeability.
The corneal endothelium is responsible for maintaining the hydration and transparency of the cornea by counteracting the fluid imbibition properties of the glycosaminoglycan rich stroma. Numerous studies have shown that this process is dependent on the presence of HCO3- and slowed by inhibition of carbonic anhydrase activity (Hodson 1971; Barfort and Maurice 1974; Fischbarg and Lim 1974; Hodson 1974; Kuang et al. 1990; Riley et al. 1995). The endothelium develops a small apical side negative transendothelial potential (0.5 mV) that is significantly reduced by HCO3- withdrawal or by carbonic anhydrase inhibitors (Fischbarg 1972; Barfort and Maurice 1974; Fischbarg and Lim 1974; Hodson et al. 1977). Corneal swelling is also induced by the Na+/K+ ATPase inhibitor ouabain, indicating that the endothelial pump involves active transport.
HCO3- uptake at the basolateral (stromal) membrane has been identified as being predominantly via the 1Na+:2HCO3- cotransporter (NBCe1) (Sun et al. 2000; Sun and Bonanno 2003). A recent study showed that siRNA knockdown of NBC significantly reduced basolateral HCO3- permeability and eliminated net basolateral to apical fluxes (Li et al. 2005). Interestingly, studies using cultured cells indicate that basolateral HCO3- permeability is 3-4 times greater than apical permeability (Bonanno et al. 1999). The low HCO3- permeability of the apical membrane suggests that it is rate limiting for HCO3- fluxes and that agents that could enhance HCO3- flux would act on the apical membrane.
The contributors to the smaller apical HCO3- permeability are unclear. Immunofluorescence micrographs suggest that a Sodium Bicarbonate cotransporter (NBCe1) is expressed on both the basolateral and apical membranes (Diecke et al. 2004). In this case, the apical NBC would operate in the 1Na+/3HCO3- stoichiometry needed for efflux. On the other hand, studies with cultured endothelial cells show that apical HCO3- permeability is not dependent on the presence of Na+ (Bonanno et al. 1999; Sun and Bonanno 2003) or Cl- (Bonanno et al. 1998; Bonanno et al. 1999), indicating that it is not contributed by an anion exchanger or Na+ -coupled transporter, suggesting that anion channels may be responsible. Two apical anion channels that are permeable to HCO3-, CFTR (Cystic Fibrosis Transmembrane Conductance Regulator) (Sun et al. 2001; Sun and Bonanno 2002) and bCLCA1 (Ca2+ activated chloride channels) (Zhang et al. 2002; Zhang et al. 2006) have been conclusively identified in bovine corneal endothelial cells. The calcium induced increase in apical HCO3- permeability was eliminated in bCLCA1 siRNA treated cells, however baseline permeability was unaffected (Zhang et al. 2006), indicating that bCLCA1 did not contribute to baseline apical anion permeability.
Adenosine has long been recognized as an agonist for corneal endothelial fluid transport (Dikstein and Maurice 1972; Fischbarg et al. 1977; Riley et al. 1996). Enhanced fluid transport by adenosine is via activation of A2 purinergic receptors, specifically A2b, which increase intracellular [cAMP] (Riley et al. 1998; Tan-Allen et al. 2005). Consistent with this notion, forskolin (Riley et al. 1998), which directly activates transmembrane adenylate cyclases, and rolipram (Wigham et al. 2000), a specific PDE4 inhibitor, each enhance fluid transport. Previous studies have shown that adenosine and forskolin increase endothelial cell apical chloride and HCO3- permeability (Bonanno and Srinivas 1997; Sun and Bonanno 2002; Tan-Allen et al. 2005). Whereas the presence of Cl- is important for maintenance of corneal deturgescence (Winkler et al. 1992), a net flux of Cl- has not been established (Maurice 1985) and it does not appear to have a role in baseline fluid transport (Riley et al. 1997). A role for Cl- in stimulated transport however, has not been tested. Interestingly, increasing [cAMP] and PKA activity also increases paracellular resistance (Srinivas et al. 2004), through a myosin light chain kinase mediated process. This leads to a small increase in endothelial electrical resistance, rather than a decrease that would be predicted based solely on apical anion permeability. Since CFTR is activated by cAMP/PKA, its presence on the apical membrane of corneal endothelium suggests that it has a role in cAMP mediated increases in apical anion permeability. Previous work has shown that two inhibitors of CFTR conductance, NPPB and glibenclamide, inhibit cAMP stimulated apical Cl- and HCO3- permeability, but not unstimulated anion permeability (Sun and Bonanno 2002) suggesting that CFTR does not contribute directly to baseline anion permeability.
To definitively determine that cAMP stimulated apical anion permeability is dependent on CFTR, we used an siRNA knockdown procedure. We examined both baseline and forskolin mediated increases in apical Cl- and HCO3- permeability. We found that CFTR has a very significant role in forskolin stimulated apical anion permeability, but no apparent role in contributing to baseline permeability.
METHODS
Cell culture
Bovine corneal endothelial cells (BCEC) were cultured to confluence onto 25-mm round coverslips, 13-mm Anodisc filters, or T-25 flasks as previously described (Bonanno and Giasson 1992). Briefly, primary cultures from fresh cow eyes were established in T-25 flasks with 3 ml of Dulbecco’s modified Eagle’s medium (DMEM), 10% bovine calf serum, and antibiotic (penicillin 100U/ml, streptomycin 100 U/ml, and Fungizone 0.25 μg/ml), gassed with 5 % CO2-95% air at 37 °C and fed every 2 to 3 days. Primary cultures were subcultured to three T-25 flasks and grown to confluence in 5 to 7 days. The resulting second passage cultures were then further subcultured onto coverslips or Anodiscs and allowed to reach confluence within 5 to 7 days.
siRNA Transfection
Construction and testing of siRNAs was performed as previously described (Li et al. 2005; Yang et al. 2005; Zhang et al. 2006). Briefly, five Sense and anti-sense oligonucleotides corresponding to the following CFTR cDNAs were designed and blasted by using the Ambion siRNA targeting design tool and were purchased from Invitrogen: #1 AAG AAT CAT AGC TTC CTA TGA, #2 AAT GAT GAT GAA GTA CAG AGA, #3 AAG AAT ATA AGA CAT TGG AAT, #4 AAT TAA GCA CAG TGG AAG AAT, #5 AAA GCT CTG AAT TTA CAT ACT. Using these oligonucleotides, five siRNAs for CFTR were synthesized using the Silencer siRNA construction kit from Ambion. An siCONTROL non-targeting siRNA (no known mammalian homology) was purchased from Dharmacom. Cells were transfected when 70-80% confluent using Oligofectamine (Invitrogen) according to the manufacturer’s protocol in the presence of siRNA. Cell coated coverslips or Anodiscs in six-well plates were incubated with 1 ml OPTI-MEM I (Gibco) containing siRNA for four hours followed by addition of 2 ml of standard DMEM with serum. T-25 flasks were treated with 2 ml OPTI-MEM I containing siRNA followed by addition of 4 ml of culture media. Media was then changed every 2 days.
Immunoprecipitation
Cultured cells in T-25 flasks were washed with PBS and dissolved directly in immunoprecipitation (IP) buffer [1.0% Nonidet P-40, 150 mM NaCl, 0.1% SDS, 0.5% sodium deoxycholate, 50 mM Tris · HCl pH 8.0 containing a protease inhibitor cocktail, (Complete™, Boehringer Mannheim)]. Preparations were sonicated on ice. Sonicated samples were centrifuged at 10,000 g for 10 min at 4°C. The supernatant was transferred and then incubated for 16-18 h with a monoclonal antibody (MAB25031; 2 μg antibody · mg protein-1 · ml IP buffer-1) directed against the COOH terminus of CFTR (R&D systems, Minneapolis, MN). Immobilized protein A agarose was added to the solution during the final 2 h of incubation. The immune complexes were collected by centrifugation at 10,000g for 15 seconds at 4°C and washed three times with ice-cold IP buffer (1 ml). The immune complexes were resuspended with 50 μl of Laemmli sample buffer (2% SDS, 10% glycerol, 100 mM DTT, 60 mM Tris, pH 6.8, and 0.01% bromophenol blue) and heated to 80°C for 10 min before gel loading. After being separated by 8% SDS-PAGE, samples were transferred to a polyvinylidene fluoride (PVDF) membrane. The membrane was blocked with 5% nonfat dry milk for 1 h at room temperature and then probed with the anti-CFTR antibody (1:1000) in PBS containing 5% nonfat dry milk for one hour at room temperature with shaking. Next, the blots were washed five times for 5 min. each with PBS/Tween-20, incubated with goat anti-mouse secondary antibody coupled to horseradish peroxidase (Sigma) for one hour at room temperature, then washed with PBS/Tween-20 five times for 5 min. each and developed by enhanced chemiluminescence (ECL). Films were scanned to produce digital images that were then assembled and labeled using Adobe Photoshop software.
Microscope perfusion
For measurement of apical Cl- or HCO3- permability, cells were cultured to confluence on 13 mm diameter, 0.2 μm pore AnoDisc membranes. AnoDiscs were placed in a double-sided perfusion chamber designed for independent perfusion of the apical and basolateral sides. The assembled chamber was placed on a water-jacketed (37°C) brass collar held on the stage of an inverted microscope (Nikon Diaphot 200) and viewed with a long working distance (2 mm) water-immersion objective (Nikon, X 40). Apical and basolateral compartments were connected to hanging syringes, which contained Ringer solution in a Plexiglas warming box (37°C), using Phar-Med tubing. The flow of the perfusate (∼0.5 ml/min) was achieved by gravity. Two independent eight-way valves were employed to select the desired perfusate for the apical and basolateral chambers. The composition of the standard HCO3--rich Ringer solution used throughout the study was (in mM) 150 Na+, 4 K+, 0.6 Mg2+, 1.4 Ca2+, 118 Cl-, 1 HPO4-, 10 HEPES-, 28.5 HCO3-, 2 gluconate-, and 5 glucose, equilibrated with 5% CO2 and pH adjusted to 7.50 at 37°C. Low-HCO3- (LB) Ringer solution (2.85 mM HCO3-, pH6.5) was prepared by replacing 25.65 mM NaHCO3 with sodium gluconate. For HCO3- free Ringer, NaHCO3 was substituted with equimolar NaGluconate. Cl--free Ringer was prepared by equimolar replacement of NaCl and KCl with sodium nitrate and potassium nitrate. Osmolarity was adjusted to 295 ± 5 mosM with sucrose.
Measurement of Apical Cl- Permeability
Apical Cl- permeability was assessed with the halide-sensitive fluorescent dye MEQ, as previously described (Sun and Bonanno 2002). Briefly, corneal endothelial cells on Anodiscs were exposed to the nonfluorescent cell-permeant reduced quinoline derivative of MEQ (diH-MEQ), which is oxidized to MEQ within the cytoplasm. Cells were exposed to 10 μM diH-MEQ for 10 minutes at room temperature in Cl--free Ringer’s solution, and washed for 30 min with Cl--free Ringer’s solution. Cellular fluorescence was measured (1s-1) with a microscope spot fluorimeter (DeltaRam, Photon Technology International, Monmouth Junction, NJ). Fluorescence was excited at 365±10nm and emission collected at 420-450nm. Relative differences in Cl- permeability between control and experimental conditions in the same cells were determined by comparing the maximum slope of the percentage change in MEQ fluorescence (F/F0) after addition of Cl- to the apical bath, where F0 is the fluorescence in the absence of Cl-.
Measurement of HCO3- Permeability
BCEC cultured onto permeable Anodisc filters were loaded with the pH-sensitive fluorescent dye 2′, 7′-bis(2-carboxyethyl)-5(6)-carboxyfluorescein (BCECF) by incubation in HCO3--free Ringer solution that contained 1 μM BCECF-AM at room temperature for 30-60 min. Dye- loaded cells were then kept in Ringer solution for at least 30 min before use. Fluorescence ratios (Ex 495 and 440 nM; Em 520) were obtained at 1 s-1 and were calibrated against pHi by the high potassium-nigericin technique. A calibration curve, which follows a pH titration equation, has been constructed for BCEC (Bonanno and Giasson 1992). Apical HCO3- permeability was determined using the constant CO2 protocol as described previously (Bonanno et al. 1999). Briefly, the apical HCO3- rich Ringer (28.5 mM, 5%CO2, pH 7.5) is replaced with a low HCO3- (LB) solution (2.85 mM, 5% CO2, pH 6.5). Under this protocol the initial pHi change is predominantly due to HCO3- efflux since there is no CO2 gradient. However there is a pH gradient that contributes (∼15%) to the pHi decrease(Bonanno et al. 1999).
Measurement of Steady-State HCO3-- Flux
Measurement of steady-state HCO3- flux was performed as previously described (Li et al. 2005). Briefly, BCEC cultured to confluence on 0.2 μm Anopore membrane tissue culture inserts were washed with DMEM containing 2% bovine calf serum. 200 μl of this culture medium containing 1 μM BCECF free acid was placed on the apical side and 300 μl on the basolateral side. After 4 h in a standard 5% CO2 incubator, 37°C, cultures were placed in a large glove box equilibrated with 5% CO2, 37°C. 50 μl samples were taken from apical and basolateral sides with separate glass capillary tubes and both ends sealed with wax. The tubes were then taken to the microscope fluorimeter and the fluorescence ratio of BCECF was measured. The pH of each sample was then determined using a standard curve constructed using solutions of known pH that had been placed within capillary tubes. The difference in pH was calculated as, ΔpH = Apical pH - Basolateral pH. A positive ΔpH indicates relative apical alkalinization.
RESULTS
Five siRNA constructs at 4 concentrations (10, 20, 50 and 100 nM) were tested for knockdown of CFTR. Construct #1, 4, and 5 had no effect on CFTR expression, while #3 showed modest reduction at 100 nM (data not shown). Figure 1 shows a representative western blot using construct #2. At 5 nM, construct #2 knocked down ∼50% and at 20 nM knocked down CFTR expression to ∼15% of control four days following transfection. The mean knockdown was 82 ± 6% (n=3). Higher concentrations had no further effect. Therefore, 20 nM of construct #2 was used in subsequent experiments.
Figure 1.
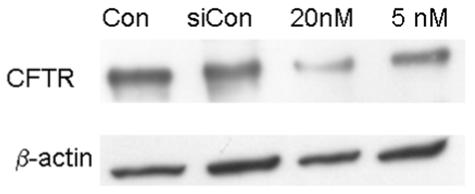
Western blot for CFTR and βactin using protein from control, siControl, and CFTR siRNA treated (20 and 5 nM) cultured bovine corneal endothelial cells. Representative of three experiments.
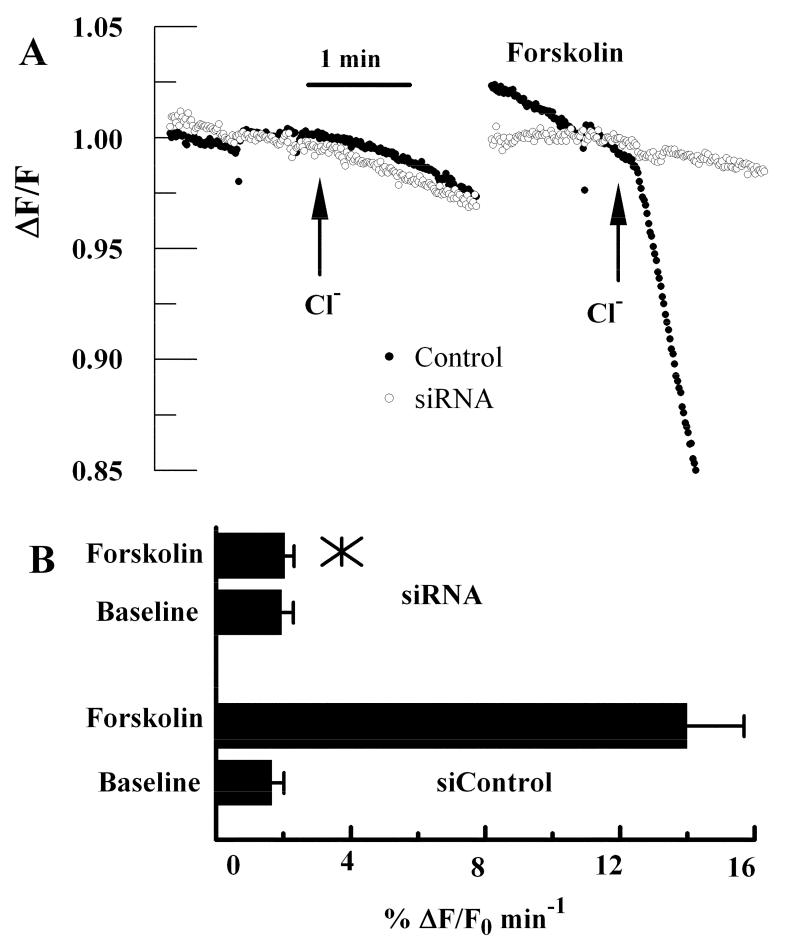
Apical Cl- permeability is increased several fold by forskolin treatment and is partially inhibited by the anion channel blockers, NPPB and glibenclamide (Sun and Bonanno 2002), suggesting that CFTR is responsible for the increase in apical permeability. To further confirm this notion we used BCEC treated with CFTR siRNA. Figure 2A shows representative traces of MEQ fluorescence changes in response to 10 μM forskolin in siControl and CFTR siRNA treated cells. In this assay, cells are perfused in chloride free ringer on both apical and basolateral sides. Cl- is added to the apical side only and the initial quenching rate (ΔF/F0, where F0 is the baseline fluorescence) of MEQ fluorescence is taken as the baseline apical Cl- permeability. Chloride free ringer is returned, forskolin is introduced, and apical Cl- is then added in the presence of forskolin, which significantly increases the rate of MEQ quenching. Figure 2A shows that baseline MEQ quenching by apical Cl- is slow (<2%/min) and that there was no significant difference between siControl and CFTR siRNA treated anodisc cultures. In contrast, whereas forskolin increased apical Cl- permeability by 7 fold in siControl treated cells, CFTR siRNA treated cells showed little response. Figure 2B summarizes the data from eight experiments.
Figure 2.
Apical Cl- Permeability in control and CFTR siRNA treated cells. Cells were depleted of Cl-, loaded with the halide sensitive fluorescent dye MEQ and perfused on basolateral and apical sides with Cl- free ringer’s solution. Relative apical Cl- permeability is measured as the initial rate of MEQ fluorescence quenching upon addition of Cl- to the apical perfusing solution. A. Representative experiments showing the change in MEQ fluorescence relative to the starting fluorescence value in response to addition of chloride on the apical side in the absence and presence of 10 μM forskolin for siControl and CFTR siRNA treated cultures. B. Bar graph summarizes the Cl- permeability data (n=8). *, mean value significantly different compared to control (p<0.05); error bars show Standard Deviation.
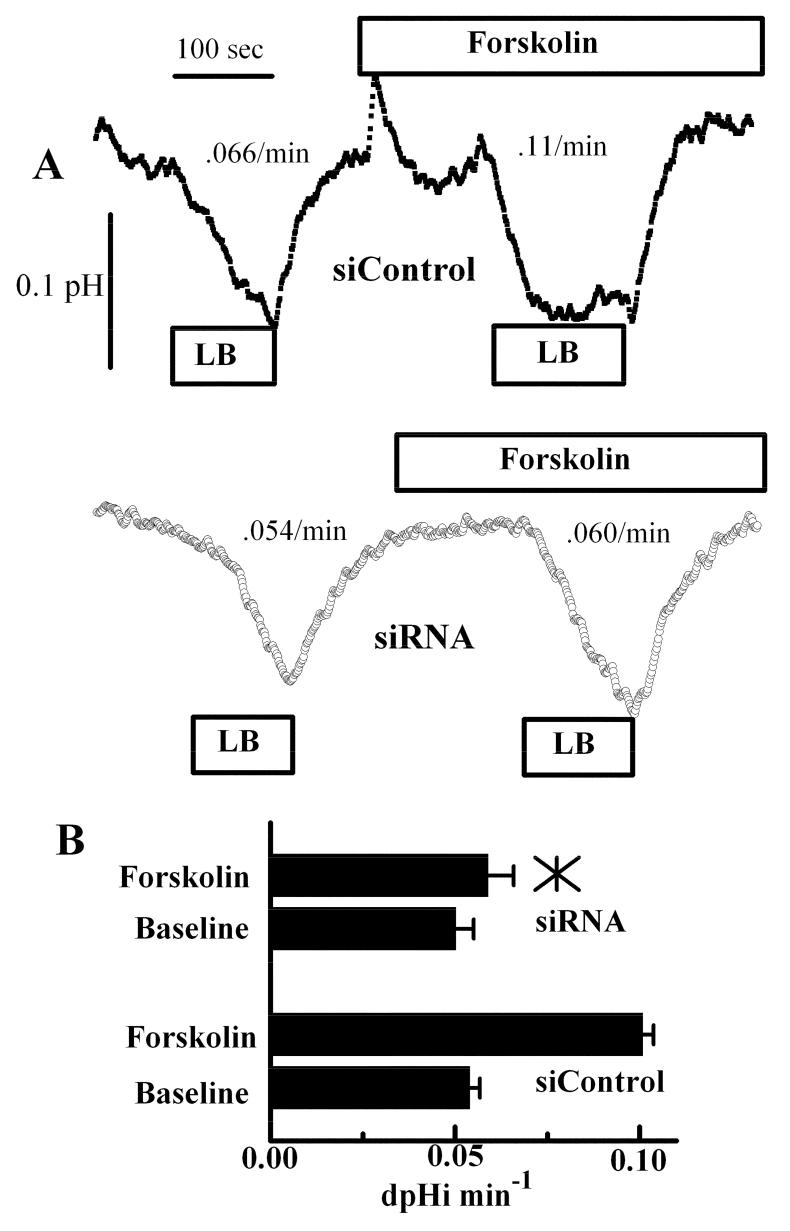
Apical HCO3- permeability is also increased by forskolin treatment, but much less than Cl- permeability, and is also partially inhibited by NPPB and glibenclamide, suggesting that CFTR is responsible (Sun and Bonanno 2002). In this assay, apical HCO3- permeability is estimated by pulsing the apical surface with a low [HCO3-], at constant CO2, and measuring the initial rate of pHi decrease secondary to HCO3- efflux. Figure 3A shows representative traces of pHi changes in response to apical low bicarbonate in the presence and absence of 10 μM forskolin for siControl and CFTR siRNA treated cells, respectively. Forskolin increased apical HCO3- permeability by 2-fold in siControl cells, but had no significant effect in CFTR siRNA treated cells. Interestingly, CFTR knockdown did not have a significant effect on baseline apical HCO3- permeability. This data is summarized in figure 3B.
Figure 3.
Apical HCO3- Permeability in control and CFTR siRNA treated cells. A. Cells were perfused in bicarbonate-rich ringer’s on both sides. Where indicated the apical ringer’s was changed to low bicarbonate (LB) in the absence and then presence of 10 μM forskolin. Upper trace: siControl; Lower trace: CFTR siRNA. Rates (dpHi/min) are indicated adjacent to the trace for these experiments. B. Bar graph summarizes the HCO3- permeability data (n=8). *, mean value significantly different compared to control (p<0.05); error bars show Standard Deviation.
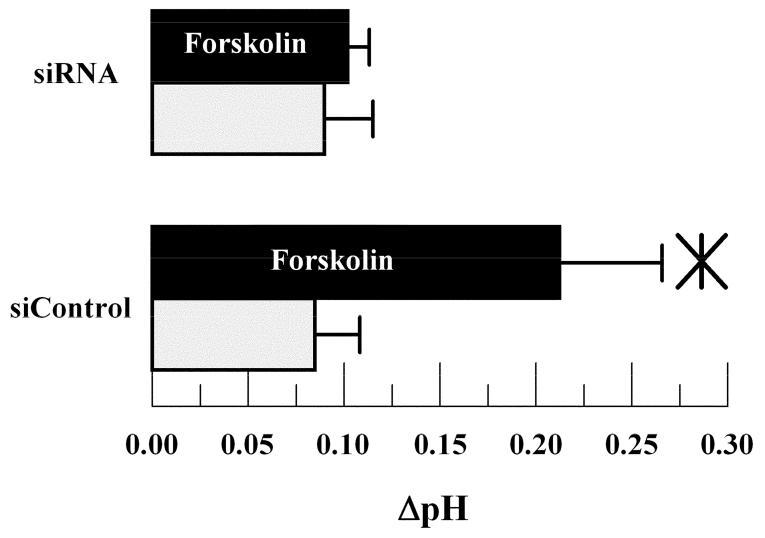
Lastly, we examined the effects of CFTR knockdown on steady-state ΔpH. Previously we have shown that the apical bath is relatively alkaline in reference to the basolateral bath after several hours incubation in a standard CO2 incubator (Li et al. 2005). This alkalinity is reversed in cultures where the basolateral 1Na+/2HCO3- cotransporter expression is significantly reduced by siRNA treatment (Li et al. 2005). We expect that CFTR could contribute to apical alkalinity because of the permeability to HCO3-. Figure 4 shows that in control cultures, ΔpH was ∼ +0.08 (apical side alkaline relative to basolateral) after four hours, which is similar to what was previously reported (Li et al. 2005). Forskolin increased ΔpH significantly to +0.215. In CFTR siRNA treated cells baseline ΔpH was not significantly different from siControl treated cells. However, the forskolin dependent increase in ΔpH was almost totally eliminated. These results are consistent with significant HCO3- flux through activated CFTR and indicate that CFTR does not significantly contribute to baseline apical HCO3- flux.
Figure 4.
Effect of CFTR siRNA knockdown on steady-state ΔpH (apical-basolateral pH) after 4 hours. Light bars unstimulated cells; Dark bars 10 μM forskolin stimulation. *significantly different from unstimulated (p<0.05, n=6); error bars show Standard Deviation.
DISCUSSION
This study demonstrates, using an siRNA approach, that CFTR has a significant role in cAMP-activated anion transport in corneal endothelium, but not baseline anion permeability or HCO3- flux. This is consistent with previous studies that showed that CFTR channel inhibitors had no effect on baseline Cl- and HCO3- permeability (Sun and Bonanno 2002) or on maintenance of corneal thickness of in vitro mounted rabbit corneas (Diecke et al. 2004). These authors also conclude that CFTR is unlikely to contribute to baseline endothelial function. Since apical HCO3- permeability is not altered in the absence of Na+ or Cl- (Bonanno et al. 1998; Bonanno et al. 1999; Sun et al. 2000), an anion channel seems the best candidate to support the relatively small apical permeability. While this putative channel is not CFTR, the small amount of corneal swelling induced by the non-specific anion channel blocker niflumic acid (Diecke et al. 2004) is consistent with this notion. Whether knockdown of CFTR expression in rabbit cornea endothelium blocks cAMP mediated activation of endothelial fluid transport however, remains to be tested.
There are no established ocular abnormalities in cystic fibrosis. CFTR is highly expressed on the ocular surface (Zaidi et al. 1999; Al-Nakkash and Reinach 2001; Levin et al. 2006) and there are some reports that CF patients are prone to dry eye (Botelho et al. 1973; Kalayci et al. 1996; Mrugacz et al. 2007), but this is likely related to malabsorption of vitamin A (Lindenmuth et al. 1989; Mrugacz et al. 2005). CFTR is also expressed in the retinal pigment epithelium (Blaug et al. 2003; Wu et al. 2006). Although the electroretinogram is altered in cystic fibrosis consistent with a defective anion channel, there are no clinical retinal abnormalities (Schupp et al. 2004). Clinical studies have also shown that Cystic Fibrosis patients have normal corneal thickness and corneal endothelial morphology, consistent with little or no role in baseline corneal endothelial function. Interestingly, the endothelial cell density of CF patients may be slightly higher than in controls (Lass et al. 1985). Possibly this is to compensate for the defective channel.
The in vivo conditions under which cAMP activation of endothelial function (i.e. increased anion flux and increased transepithelial resistance (Srinivas et al. 2004)) occurs are not known. We have hypothesized that endothelial stress, which leads to ATP release and subsequent conversion to adenosine, would activate A2b receptors and increase [cAMP] in an attempt to counteract the negative effects of the stress. This hypothesis would predict that CF patients may have an altered corneal response, for example to anterior chamber inflammation.
Recent studies have shown that CFTR is involved in some other interesting functions. For example, CFTR is permeable to glutathione (Kariya et al. 2007) and is activated by vitamin C (Fischer et al. 2004). Glutathione is an antioxidant that is in relatively high concentrations in aqueous humor and has important anti-oxidant functions in the lens and cornea. Furthermore, vitamin C is also at high concentrations in aqueous humor and in addition to its own anti-oxidant function, vitamin C may act to maintain high levels of corneal glutathione. SAGE analysis of mRNA from Fuchs’ Dystrophy endothelium, which is characterized by low endothelial cell densities, indicates that expression of Glutathione-S-Transferase-π is significantly reduced relative to control endothelium (Gottsch et al. 2003), suggesting that transfer of reducing equivalents is important for endothelial protection from oxidative stress and that a ready supply of glutathione is needed. If true, CF patients may have increased corneal oxidative stress. However, since they rarely survive past the third decade, this may not have time to become clinically apparent.
In summary, our data show that CFTR is responsible for the cAMP-induced increase in apical anion permeability of corneal endothelium. CFTR does not however, contribute to baseline apical anion permeability. These findings together with the lack of clinically significant corneal problems in CF patients suggest that the role of CFTR in baseline corneal endothelial function may be unrelated to its anion transport properties.
Acknowledgements
Supported by NIH grant EY08834.
REFERENCES
- Al-Nakkash L, Reinach PS. Activation of a CFTR-mediated chloride current in a rabbit corneal epithelial cell line. Invest Ophthalmol Vis Sci. 2001;42:2364–2370. [PubMed] [Google Scholar]
- Barfort P, Maurice D. Electrical potential and fluid transport across the corneal endothelium. Exp Eye Res. 1974;19:11–19. doi: 10.1016/0014-4835(74)90067-0. [DOI] [PubMed] [Google Scholar]
- Blaug S, Quinn R, Quong J, Jalickee S, Miller SS. Retinal pigment epithelial function: a role for CFTR? Doc Ophthalmol. 2003;106:43–50. doi: 10.1023/a:1022514031645. [DOI] [PubMed] [Google Scholar]
- Bonanno J, Guan Y, Jelamskii S, Kang X. Apical and basolateral CO2-HCO3- permeability in cultured bovine corneal endothelial cells. Am. J. Physiol. 1999;277:C545–C553. doi: 10.1152/ajpcell.1999.277.3.C545. [DOI] [PubMed] [Google Scholar]
- Bonanno J, Srinivas S. Cyclic AMP activates anion channels in cultured bovine corneal endothelial cells. Exp Eye Res. 1997;64:953–962. doi: 10.1006/exer.1997.0290. [DOI] [PubMed] [Google Scholar]
- Bonanno JA, Giasson C. Intracellular pH regulation in fresh and cultured bovine corneal endothelium. I. Na/H exchange in the absence and presence of HCO3- Invest. Ophthalmol. Vis. Sci. 1992;33:3058–3067. [PubMed] [Google Scholar]
- Bonanno JA, Yi G, Kang XJ, Srinivas SP. Reevaluation of Cl-/HCO3- exchange in cultured bovine corneal endothelial cells. Invest. Ophthalmol. Vis. Sci. 1998;39:2713–2722. [PubMed] [Google Scholar]
- Botelho SY, Goldstein AM, Rosenlund ML. Tear sodium, potassium, chloride, and calcium at various flow rates: children with cystic fibrosis and unaffected siblings with and without corneal staining. J Pediatr. 1973;83:601–606. doi: 10.1016/s0022-3476(73)80221-5. [DOI] [PubMed] [Google Scholar]
- Diecke FP, Wen Q, Sanchez JM, Kuang K, Fischbarg J. Immunocytochemical localization of Na+-HCO3- cotransporters and carbonic anhydrase dependence of fluid transport in corneal endothelial cells. Am J Physiol Cell Physiol. 2004;286:C1434–1442. doi: 10.1152/ajpcell.00539.2003. [DOI] [PubMed] [Google Scholar]
- Dikstein S, Maurice DM. The active control of corneal hydration. Isr J Med Sci. 1972;8:1523–1528. [PubMed] [Google Scholar]
- Fischbarg J. Potential difference and fluid transport across rabbit corneal endothelium. Biochim Biophys Acta. 1972;288:362–366. doi: 10.1016/0005-2736(72)90257-x. [DOI] [PubMed] [Google Scholar]
- Fischbarg J, Lim J. Role of cations, anions, and carbonic anhydrase in fluid transport across rabbit corneal endothelium. J. Physiol. 1974;241:647–675. doi: 10.1113/jphysiol.1974.sp010676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischbarg J, Lim J, Bourguet J. Adenosine stimulation of fluid transport across rabbit corneal endothelium. J Memb Biol. 1977;35:95–112. doi: 10.1007/BF01869942. [DOI] [PubMed] [Google Scholar]
- Fischer H, Schwarzer C, Illek B. Vitamin C controls the cystic fibrosis transmembrane conductance regulator chloride channel. Proc Natl Acad Sci U S A. 2004;101:3691–3696. doi: 10.1073/pnas.0308393100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottsch JD, Bowers AL, Margulies EH, Seitzman GD, Kim SW, Saha S, Jun AS, Stark WJ, Liu SH. Serial analysis of gene expression in the corneal endothelium of Fuchs’ dystrophy. Invest Ophthalmol Vis Sci. 2003;44:594–599. doi: 10.1167/iovs.02-0300. [DOI] [PubMed] [Google Scholar]
- Hodson S. Evidence for a bicarbonate-dependent sodium pump in corneal endothelium. Exp Eye Res. 1971;11:20–29. doi: 10.1016/s0014-4835(71)80060-x. [DOI] [PubMed] [Google Scholar]
- Hodson S. The regulation of corneal hydration by a salt pump requiring the presence of sodium and bicarbonate ions. J. Physiol. 1974;236:271–302. doi: 10.1113/jphysiol.1974.sp010435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodson S, Miller F, Riley M. The electrogenic pump of rabbit corneal endothelium. Exp Eye Res. 1977;24:249–253. doi: 10.1016/0014-4835(77)90162-2. [DOI] [PubMed] [Google Scholar]
- Kalayci D, Kiper N, Ozcelik U, Gocmen A, Hasiripi H. Clinical status, ocular surface changes and tear ferning in patients with cystic fibrosis. Acta Ophthalmol Scand. 1996;74:563–565. doi: 10.1111/j.1600-0420.1996.tb00735.x. [DOI] [PubMed] [Google Scholar]
- Kariya C, Leitner H, Min E, van Heeckeren C, van Heeckeren A, Day BJ. A role for CFTR in the elevation of glutathione levels in the lung by oral glutathione administration. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1590–1597. doi: 10.1152/ajplung.00365.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang K, Xu M, Koniarek J, Fischbarg J. Effects of ambient bicarbonate, phosphate and carbonic anhydrase inhibitors on fluid transport across rabbit endothelium. Exp. Eye Res. 1990;50:487–493. doi: 10.1016/0014-4835(90)90037-u. [DOI] [PubMed] [Google Scholar]
- Lass JH, Spurney RV, Dutt RM, Andersson H, Kochar H, Rodman HM, Stern RC, Doershuk CF. A morphologic and fluorophotometric analysis of the corneal endothelium in type I diabetes mellitus and cystic fibrosis. Am J Ophthalmol. 1985;100:783–788. doi: 10.1016/s0002-9394(14)73367-7. [DOI] [PubMed] [Google Scholar]
- Levin MH, Kim JK, Hu J, Verkman AS. Potential difference measurements of ocular surface Na+ absorption analyzed using an electrokinetic model. Invest Ophthalmol Vis Sci. 2006;47:306–316. doi: 10.1167/iovs.05-1082. [DOI] [PubMed] [Google Scholar]
- Li J, Sun XC, Bonanno JA. Role of NBC1 in apical and basolateral HCO3- permeabilities and transendothelial HCO3- fluxes in bovine corneal endothelium. Am J Physiol Cell Physiol. 2005;288:C739–746. doi: 10.1152/ajpcell.00405.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenmuth KA, Del Monte M, Marino LR. Advanced xerophthalmia as a presenting sign in cystic fibrosis. Ann Ophthalmol. 1989;21:189–191. [PubMed] [Google Scholar]
- Maurice DM. Passive ion fluxes cross the corneal endothelium. Cur Eye Res. 1985;4:339–349. doi: 10.3109/02713688509025147. [DOI] [PubMed] [Google Scholar]
- Mrugacz M, Kasacka I, Bakunowicz-Lazarczyk A, Kaczmarski M, Kulak W. Impression cytology of the conjunctival epithelial cells in patients with cystic fibrosis. Eye. 2007 doi: 10.1038/sj.eye.6702867. [DOI] [PubMed] [Google Scholar]
- Mrugacz M, Tobolczyk J, Minarowska A. Retinol binding protein status in relation to ocular surface changes in patients with cystic fibrosis treated with daily vitamin A supplements. Eur J Pediatr. 2005;164:202–206. doi: 10.1007/s00431-004-1587-6. [DOI] [PubMed] [Google Scholar]
- Riley M, Winkler B, Czajkowski C, Peters M. The roles of bicarbonate and CO2 in transendothelial fluid movement and control of corneal thickness. Invest. Ophthalmol. Vis. Sci. 1995;36:103–112. [PubMed] [Google Scholar]
- Riley M, Winkler B, Starnes C, Peters M. Adenosine promotes regulation of corneal hydration through cyclic adenosine monophosphate. Invest. Ophthalmol. Vis. Sci. 1996;37:1–10. [PubMed] [Google Scholar]
- Riley M, Winkler B, Starnes C, Peters M. Fluid and ion transport in corneal endothelium: insensitivity to modulators of Na-K-2Cl cotransport. Am. J. Physiol. 1997;273:C1480–C1486. doi: 10.1152/ajpcell.1997.273.5.C1480. [DOI] [PubMed] [Google Scholar]
- Riley MV, Winkler BS, Starnes CA, Peters MI, Dang L. Regulation of corneal endothelial barrier function by adenosine, cyclic AMP, and protein kinases. Invest Ophthalmol Vis Sci. 1998;39:2076–2084. [PubMed] [Google Scholar]
- Schupp C, Olano-Martin E, Gerth C, Morrissey BM, Cross CE, Werner JS. Lutein, zeaxanthin, macular pigment, and visual function in adult cystic fibrosis patients. Am J Clin Nutr. 2004;79:1045–1052. doi: 10.1093/ajcn/79.6.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivas SP, Satpathy M, Gallagher P, Lariviere E, Van Driessche W. Adenosine induces dephosphorylation of myosin II regulatory light chain in cultured bovine corneal endothelial cells. Exp Eye Res. 2004;79:543–551. doi: 10.1016/j.exer.2004.06.027. [DOI] [PubMed] [Google Scholar]
- Sun XC, Bonanno JA. Expression, localization, and functional evaluation of CFTR in bovine corneal endothelial cells. Am J Physiol Cell Physiol. 2002;282:C673–683. doi: 10.1152/ajpcell.00384.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun XC, Bonanno JA. Identification and cloning of the Na/HCO(3-) cotransporter (NBC) in human corneal endothelium. Exp Eye Res. 2003;77:287–295. doi: 10.1016/s0014-4835(03)00150-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun XC, Bonanno JA, Jelamskii S, Xie Q. Expression and localization of NaHCO3 cotransporter in bovine corneal endothelium. Am J Physiol Cell Physiol. 2000;279:C1648–C1655. doi: 10.1152/ajpcell.2000.279.5.C1648. [DOI] [PubMed] [Google Scholar]
- Sun XC, McCutheon C, Bertram P, Xie Q, Bonanno JA. Studies on the expression of mRNA for anion transport related proteins in corneal endothelial cells. Curr Eye Res. 2001;22:1–7. doi: 10.1076/ceyr.22.1.1.6981. [DOI] [PubMed] [Google Scholar]
- Tan-Allen KY, Sun XC, Bonanno JA. Characterization of adenosine receptors in bovine corneal endothelium. Exp Eye Res. 2005;80:687–696. doi: 10.1016/j.exer.2004.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigham CG, Turner HC, Swan J, Hodson SA. Modulation of corneal endothelial hydration control mechanisms by Rolipram. Pflugers Arch. 2000;440:866–870. doi: 10.1007/s004240000357. [DOI] [PubMed] [Google Scholar]
- Winkler B, Riley M, Peters M, Williams F. Chloride is required for fluid transport by the rabbit corneal endothelium. Am J Physiol. 1992;262:C1167–C1174. doi: 10.1152/ajpcell.1992.262.5.C1167. [DOI] [PubMed] [Google Scholar]
- Wu J, Marmorstein AD, Peachey NS. Functional abnormalities in the retinal pigment epithelium of CFTR mutant mice. Exp Eye Res. 2006;83:424–428. doi: 10.1016/j.exer.2006.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Mergler S, Sun X, Wang Z, Lu L, Bonanno JA, Pleyer U, Reinach PS. TRPC4 knockdown suppresses epidermal growth factor-induced store-operated channel activation and growth in human corneal epithelial cells. J Biol Chem. 2005;280:32230–32237. doi: 10.1074/jbc.M504553200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidi TS, Lyczak J, Preston M, Pier GB. Cystic fibrosis transmembrane conductance regulator-mediated corneal epithelial cell ingestion of Pseudomonas aeruginosa is a key component in the pathogenesis of experimental murine keratitis. Infect Immun. 1999;67:1481–1492. doi: 10.1128/iai.67.3.1481-1492.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Li J, Xie Q, Bonanno JA. Molecular expression and functional involvement of the bovine calcium-activated chloride channel 1 (bCLCA1) in apical HCO3- permeability of bovine corneal endothelium. Exp Eye Res. 2006;83:1215–1224. doi: 10.1016/j.exer.2006.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Xie Q, Sun XC, Bonanno JA. Enhancement of HCO3- Permeability across the Apical Membrane of Bovine Corneal Endothelium by Multiple Signaling Pathways. Invest Ophthalmol Vis Sci. 2002;43:1146–1153. [PubMed] [Google Scholar]