Abstract
The cysteinyl leukotrienes, LTC4, LTD4, and LTE4, play an integral role in the pathophysiology of asthma. Acting via the type 1 leukotriene (CysLT1) receptor, these proinflammatory mediators have numerous effects in the lungs, including decreased activity of respiratory cilia, increased mucus secretion, increased venopermeability, and promotion of eosinophil migration into airway mucosa. Blocking studies show that Cys-LTs are pivotal mediators in the pathophysiology of asthma. Cys-LTs are key components in the early and late allergic airway response and also contribute to bronchial obstruction after exercise and hyperventilation of cold, dry air in asthmatics. Effects of the cysteinyl leukotrienes are blocked by leukotriene receptor antagonists; these agents inhibit bronchoconstriction in normal subjects provoked with inhaled cysteinyl leukotrienes, as well as in patients with asthma undergoing allergen, exercise, cold air, or aspirin challenge. Montelukast is a potent and selective blocker of the CysLT1 receptor. For treatment of chronic asthma, montelukast is administered once daily to adults as a 10-mg film-coated tablet, to children aged 6–14 years as a 5-mg chewable tablet, and to children aged 2–5 years as a 4-mg chewable tablet form. Given their efficacy, antiinflammatory activity, oral administration, and safety, leukotriene modifiers will play an important role in the treatment of asthmatic children.
Keywords: montelukast, asthma, children, efficacy
Introduction
Asthma is the most common chronic disease of childhood and its prevalence has substantially increased worldwide, particularly in pre-school children. It is associated with significant morbidity and economic burden (Global Strategy for Asthma Management and Prevention 1995, updated 2006). Chronic inflammation and smooth muscle dysfunction are consistent features of asthma pathophysiology, responsible for disease progression and airway remodeling (National Heart Lung and Blood Institute 2002).
For more than two decades, no new drug has been introduced for asthma, and we have used the same old drugs in various dosage forms and combinations to give relief to the millions who suffer from this widespread illness (Mehta 2000). The two classes of drugs most commonly used for treating childhood asthma, namely the β2-agonist bronchodilators and inhaled corticosteroids, have both come under increasing scrutiny in the last few years. The development of tolerance resulting from continuous use of β2-agonists is of concern, as is the risk of adverse systemic effects with inhaled corticosteroids, particularly in children requiring high dosages. In addition, ensuring adequate compliance with inhaled therapy continues to be a major difficulty. Against this background, the development of an orally active, once-daily, disease-modifying drug with additional bronchodilator properties would represent a major advance for managing young patients with asthma (Wenzel 1998; Warner 2001).
Leukotriene modifiers are an entirely new class of drugs for the treatment of asthma. We now know that asthma is basically a disorder of airway inflammation. The last few years have seen extensive research on mediators of inflammation, including leukotrienes, prostaglandins, neuropeptides, lymphokines, and interleukins. The knowledge gained about these mediators is being put to use to develop new drugs for this old affliction of mankind. Montelukast is one of the results of this scientific search (Salvi et al 2001).
Leukotriene modifiers (LTRs)
Leukotrienes are chemical mediators of asthmatic airway inflammation (Figure 1). They are formed from arachidonic acid, and are secreted by eosinophils, mast cells, neutrophils, lymphocytes, macrophages, and basophils (Turner et al 1996) (Figure 2). After the discovery in the late 1970s that the cysteinyl leukotrienes LTC4 and LTE4 (formerly known collectively as the slow-reacting substance of anaphylaxis [SRS-A]) play a key role in the pathophysiology of asthma a number of specific antagonists of their actions have been developed. The leukotriene receptor antagonists (LTRAs) selectively block the binding of cysteinyl leukotrienes to the CysLT1 receptor, which has been identified as the receptor through which most of their actions are mediated (Drazen et al 1999). These actions include bronchoconstriction, mucus hypersecretion, and increased vascular permeability and eosinophil migration. Consequently, the LTRs inhibit bronchconstriction. Moreover, LTRAs prevent many types of provoked asthmatic responses, including allergen-induced, exercise- and cold-air-hyperventilation-induced, and aspirin-induced asthma (Wright et al 1998). Three drugs of this class are in use at present – zafirlukast, pranlukast, and montelukast. All three are specifically active against the cysteinyl leukotrienes by blocking their receptor, CysLT1. Only montelukast has been extensively studied in children (Jones et al 1995).
Figure 1.
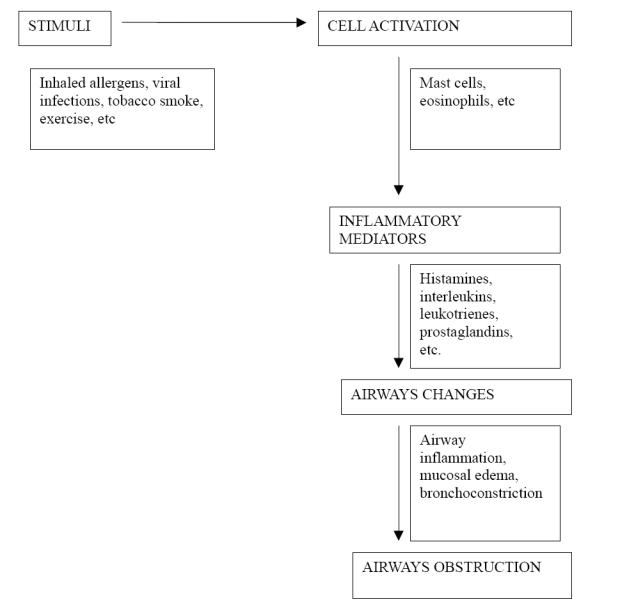
Pathogenesis of airway obstruction in asthma.
Figure 2.
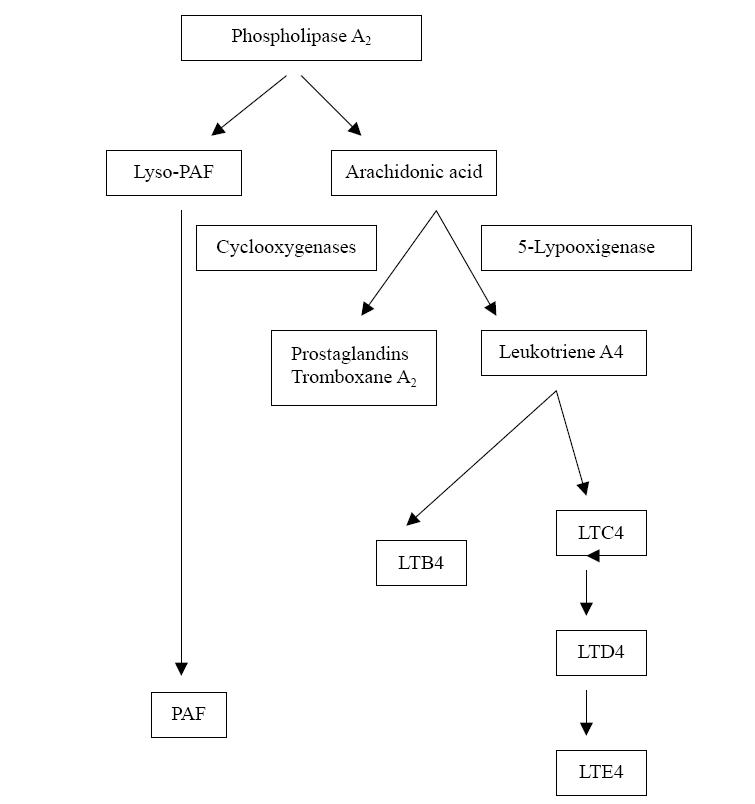
Schematic representation of the arachidonic acid cascade. LTC4 is generated by the action of 5-LO on cell membrane-derived arachidonic acid. It is rapidly converted to the equipotent LTD4 and then to the stable excretory product LTE4.
Montelukast
Montelukast (Merck and Co, Inc, Whitehouse Station, NJ) is an orally bioavailable Cys-LTRA administered once daily (Noonan et al 1998). The drug has been approved for the treatment of asthma in children 6 months and older (Skoner 2001). There is no difference in bioavailability in young and elderly patients, and food does not have a clinically important influence with chronic administration (Altman et al 1998). Therapeutic concentrations of montelukast do not inhibit the cytochrome P450 isoenzymes. Dose-ranging studies evaluating multiple doses and dosage schedules of montelukast have been reported in adults with chronic asthma (Bisgaard 2001). These studies have evaluated measures of asthma control, including lung function, use of rescue treatment, and symptom scores. Doses of 10 to 200 mg had similar efficacy, while 2 mg produced suboptimal response. BID dosing provided no additional benefit over once-daily dosing (Altman et al 1998). The bronchoprotective effect against exercise-induced bronchoconstriction (EIB) was also dose-related up to 10 mg in adult asthmatics, and there was no additional improvement with higher doses (Bronsky et al 1997). Dose-ranging studies have not been performed in children. Instead, the pediatric dosage was chosen as the dosage yielding a pharmacokinetic profile (single-dose area under the plasma concentration-time curve) in children comparable to that achieved with the 10-mg tablet in adults (Knorr et al 1999).
Adverse effects of montelukast
Pediatric studies on montelukast found that it was well tolerated. The majority of the reported adverse effects were mild and included headache, ear infection, nausea, abdominal pain, and pharyngitis. In clinical trials the incidence of these adverse effects was not higher than with placebo (Bisgaard 2001). No dose adjustment with montelukast is necessary for patients with renal and mild-moderate hepatic dysfunction (Salvi et al 2001).
Montelukast for managing childhood asthma: compared with placebo
Several randomized double blind comparative studies in pediatric patients have compared therapeutic efficacy of montelukast and placebo (Knorr et al 2001a, b; Strauch et al 2003; Becker et al 2004; Bisgaard 2005) (Table 1). The asthma severity was mild to moderate persistent in these trials. The results from these studies depicted significant improvements in multiple parameters of asthma control with montelukast compared to placebo: day-time asthma symptoms (cough, wheeze, breathing difficulty, and activity limitation), overnight asthma symptoms (cough); percentage of days with asthma symptoms, percentage of days without asthma, need for beta-agonist or oral corticosteroids; physician global evaluations and peripheral blood eosinophils. There was a significantly greater improvement in forced expiratory volume in 1 second (FEV1) from baseline for the montelukast group compared to placebo group. Studies comparing the effect on fractional exhaled nitric oxide (FeNO), a marker of airway inflammation in asthma, found a significant reduction in FeNO in the montelukast arm (Bisgaard et al 1999). A recent study conducted among preschool children revealed that 4 weeks treatment with montelukast resulted in a decrease in bronchial hyperreactivity compared with placebo (Hakim et al 2007).
Table 1.
Studies comparing the efficacy of montelukast vs placebo in childhood asthma
| Study | Design | Study population | Intervention | Main outcome measures | Conclusions |
|---|---|---|---|---|---|
| Bisgaard et al 2005 | RCT, | 2–5 y (n = 549) | MT (4 or 5 mg) | Asthma | Reduced |
| DB | intermittent asthma | vs placebo, 12 mo | exacerbation episodes | exacerbation | |
| Becker et al 2004 | RCT, | 6–14 y (n = 138) | MT (5 mg) vs | % change | Significant |
| DB | mild persistent asthma | placebo 8 wk | FEV1 | improvement (p = 0.005) | |
| Strauch et al 2003 | RCT | 6–14 y (n = 25) | MT (5 mg) vs | Sputum ECP, | MT suppressed |
| steroid-dependent asthma | placebo 4 wk | sputum Eo count FeNo, Qol | sputum ECP, improved QOL no change in rest of parameters | ||
| Knorr et al 2001b | RCT, | 2-5 y (n = 689) | MT vs | Clinical | Clinically |
| DB | persistent asthma | placebo, 12 wk | parameters of asthma control, Adverse effects. QOL scores | significant, efficacy well tolerated | |
| Knorr et al 1998 | RCT, | 6-14 y (n = 336) | MT (5 mg) vs | Morning FEV1 | Significant |
| DB | persistent asthma | placebo 8 wk | change | improvement (p < 0.001) |
Abbrevations: DB, double-blind; ECP, eosinophilic cationic protein; FeNO, fractional exhaled nitric oxide; RCT, randomized control trial; MT, montelukast; QOL, quality of life.
Epidemiologic studies have detected viral upper respiratory tract infections (URTIs) in 85% of childhood asthma exacerbations. Cycteinyl leukotrienes are released during infection with respiratory syncytial virus infection in infants and in virus-associated wheeze in preschool children. Cysteinyl leukotrienes appear to mediate abnormalities of lung function, including mucus production, decreased mucociliary clearence, changes in vascular permeability, and smooth muscle contraction. Montelukast is an oral specific cysteinyl LTRA with bronchoprotective effects for 20–24 hours after dosing (Robertson et al 2007). In this regard it was reported that 12 months of treatment with montelukast decreased viral-induced attacks of preschool children with intermittent asthma (Bisgaard et al 2005).
Montelukast for managing childhood asthma: compared with inhaled corticosteroids
Many studies have compared leukotriene inhibitors with other asthma treatments. Two Cochrane Reviews evaluated research comparing leukotriene inhibitors with inhaled corticosteroids in the management of recurrent and persistent asthma in children (Ducharme and Di Salvio 2002; Ng et al 2004). Ducharme and Di Salvio conducted a bibliographic search of randomized controlled clinical trials comparing the efficacy of antileukotrienes with inhaled corticosteroids (ICSs) in asthmatic patients and identified 27 trials of which 13 were of high methodological quality. Mild-to-moderate chronic asthmatic patients treated with LTRAs were 60% more likely to experience an asthma exacerbation requiring oral steroids than those treated with ICSs (in most trials the daily dose of ICSs was 400 mg of beclomethasone or equivalent). After 6 weeks of treatment, those patients who received ICS showed a significantly greater improvement in baseline FEV1, morning peak expiratory flow rate, fewer nocturnal awakenings and respiratory symptoms, and less use of rescue medication. Ng et al (2004) confirmed the earlier findings by Ducharme and Di Salvio that patients on antileukotrienes are more likely to suffer an exacerbation requiring systemic steroids, to exhibit a lesser improvement in lung function, and to report more nocturnal awakenings and respiratory symptoms and greater use of rescue medication. The available evidence convincingly persuades against the use of LTRAs as first-line monotherapy in patients with mild-to-moderate asthma (Table 2). It must be noted that only 3 of the 13 studies taken in the meta-analysis were conducted among children. With only 3 published trials for a total of 216 patients, there is insufficient evidence to make any firm conclusions about the use of LTRAs as monotherapy in children with asthma (Table 2). LTRAs appear to be safe. At present, the scientific evidence does not support the substitution of LTRA for low doses of ICSs, which remain the first-line therapy for asthma (Ducharme 2004).
Table 2.
Studies comparing efficacy of montelukast and inhaled corticosteroids in childhood asthma
| Study | Study population | Intervention | Main outcome measures | Conclusions |
|---|---|---|---|---|
| Stelmach et al 2004 | Children (n = 51) | MT vs Inh BD 6 mo | IgE | Inh BD and MT decreased S IgE levels |
| Garcia et al 2005 | 6–14 years Children (n = 914) | MT vs Inh FP 12 mo | FeV1 | Those with low pulmonary function, high markers, better response to FP |
| Maspero et al 2001 | 10 years children (n = 124) | MT vs BDP, 6 mo | FeV1 | MT = BDP |
Abbreviations: BD, budesonide; BDP, beclomethasone dipropionate; FEV1, forced expiratory volume in 1 s; FP, fluticasone propionate; ICS, inhaled corticosteroid; MT, montelukast; PEFR, peak expiratory flow rate.
Montelukast for managing childhood asthma: compared with long-acting beta-2 agonists (LABA) as add-on therapy to ICSs
Pediatric studies comparing montelukast with LABA as add-on therapy to ICSs in persistent asthma are limited. One study by (Buchvald et al 2003) found that FeNO levels were significantly higher after salmeterol add on treatment compared with both placebo and montelukast. FEV1 levels were comparable between the two groups. Another study by Bjermer et al (2000) revealed that adddition of montelukast in patients whose symptoms remain uncontrolled by ICSs could provide equivalent clinical control to salmeterol. Ram et al (2005) recently summarized the addition of a LABA compared with a LTRA in patients receiving inhaled steroids.This study concluded that in adults with asthma inadequately controlled by low-dose inhaled steroids, the addition of a LABA was superior in preventing exacerbations requiring systemic steroids.
The only study conducted among children is a very recent report which has revealed that add on therapy with montelukast plus low-dose budesonide was more effective than the addition of LABA or doubling the dose budesonide for controlling FeNO in asthmatic children (Miraglia et al 2007).
Exercise-induced asthma
During exercise, evaporation of water from the airway surface is the stimulus for release of inflammatory mediators such as histamine and cysteinyl leukotrienes (Stelmach et al 2004). Current options for attenuating exercise-induced asthma are inhaled beta agonists, cromolyn, or oral theophylline. All of these, with the exception of LABA, must be taken shortly before starting the exercise activity, and they provide protection for 1–2 hours only (Pearlman et al 1999). Exhaled breath condensate Cys-LT values are shown to be higher in asthmatic children with EIB and correlate with the dicrease in FEV1 after exercise (Carraro et al 2005). LTRAs are reported to decrease exhaled LTE4 in atopic children with asthma (Montuschi et al 2006). Accordingly, montelukast was shown to be effective in controlling asthma symptoms after exercise in children. Specifically, it is approved for the prevention of EIB from 2 years of age (Pajaron-Fernandez et al 2006). Notably, over an 8-week study period, the tolerance that some patients developed to LABA did not occur in patients receiving leukotriene inhibitors (Edelman et al 2000). One review suggests that using leukotriene inhibitors may be preferable to increasing the dose of beta-2 agonists (Anderson 2004). Leukotriene inhibitors can provide a useful alternative in preventing exercise-induced asthma, especially in young children for whom the use of an inhaler may be difficult, or for persons who receive incomplete protection from short-acting beta-2 agonists (Thomas et al 2007).
Aspirin-induced asthma
The cysteinyl leukotrienes are the leading mediators of the airway reaction that occurs in persons with aspirin-sensitive asthma after exposure to aspirin (O’Byrne et al 1997). LTRs resulted in almost complete inhibition of aspirin-induced bronchoconstriction as well as symptoms of the skin and gastrointestinal tract (Israel et al 1993). For this reason LTRs are able to prevent this reaction (Drazen et al 1999) and are the treatment of choice for these patients (Wenzel 1998; Mehta 2000).
Predicting the response to montelukast
Heterogeneous response has been documented for asthma treatments, including LTRAs (Meyer et al 2003). This issue is further complicated in young children who present with asthma-like symptoms that might represent other disease pathologies. Therefore it is a critical clinical question whether a particular therapy will be effective in an individual child with symptoms of asthma, and examination of this question is pertinent.
Studies have demonstrated that genetic variation in some genes encoding key proteins in the leukotriene pathway (ALOX5, LTA4H, LTC4S, and ABCC1) influences response to LTRAs. Also plasma concentrations of LTRAs vary considerably among patients. Physiochemical characteristics make it likely that membrane efflux and update transporters mediate the absorbtion of LTRAs into the systemic circulation following oral administration. Genes that encode efflux and uptake transport proteins harbor many varients that could influence the pharmacocinetics, and particularly the bioavailability of LTRAs, and could contribute to heterogeneity in response.
Efforts to determine clinical indicators of response to LTRAs have not been very successful. Results from a primary trial analysis of 2- to 5-year-old patients indicated no difference in response to montelukast according to study center, age, sex, or race. In another study among 6- to 14-year-old patients, no treatment differences were observed according to categories of age, ethnicity, Tanner stage, or history of exercise-induced asthma. Additionally, in subjects aged 15 years or older, baseline peak expiratory flow variability was not associated with montelukast-associated decreases in peak expiratory flow variability over follow-up. The findings of a recent study demonstrated that characteristics predictive of asthma prognosis (such as family history of asthma, eosinophilia, and personal history of allergy) were not, in general, predictive of response to montelukast.
Studies have demonstrated that some clinical indicators can predict a better clinical response to inhaled corticosteroids compared with LTRAs. Intra-individual analyses revealed lower levels of pulmonary function, greater bronchodilator use, and higher levels of biomarkers of inflammation, including FeNO, at baseline related to a better differential pulmonary response to ICSs compared with montelukast (Szefler 2005). The report of Zeiger et al (2006) extends the findings of this study to several other measures of asthma control, including asthma control days. Strong evidence of greater mean improvements after 8 weeks of therapy with an ICS compared with an LTRA across many other outcomes (asthma control days, asthma control questionnaire score, albuterol use, FeNO, peak expiratory flow rate variability, morning peak expiratory flow rate, and measures of impedance). FeNO, as a predictor of clinical and pulmonary responses, might be a useful marker to identify individual children solely receiving as-needed bronchodilators who achieve a greater improvement in asthma control days (present study) and FEV1 with an ICS compared with a LTRA (Zeiger et al 2006).
LTRAs decrease exhaled LTE4 in atopic children with asthma. This reduction is dependent on baseline exhaled LTE4 values. Measurement of exhaled LTE4 might help identify children with asthma most likely to benefit from LTRAs (Montuschi et al 2006).
Position of montelukast in current pediatric asthma management guidelines
The international guideline for asthma mangement, GINA (Global INiative for Asthma) was updated at 2006. In this reviewed addition, asthma treatment is based on “asthma control” and has 5 steps (Global Strategy for Asthma Management and Prevention, 1995 updated 2006). For children whose asthma is partially controlled with only short-acting bronchodilators, LTRAs are recommended as an alternative to low-dose ICSs (in step 2). In step 3, adding LTRA to low-dose ICSs is given as an alternative to using other add-on therapies. In this guideline, it is stated that the available literature on treatment of asthma in children 5 years and younger precludes detailed treatment recommendations. The best-documented treatment to control asthma in these age groups is ICSs and at Step 2, a low-dose inhaled glucocorticosteroid is recommended as the initial controller treatment.
British Thoracic Society (BTS) Guidelines, updated in 2006, give special recommendations for children under 5 years. According to this guideline, LTRAs can be used as a regular preventive therapy when ICSs cannot be used or as an add on therapy when disease is not under control with 200–400 μ g ICSs. For children under 2 years, it is recommended to refer the patient to a respiratory pediatrician before adding LTRAs. For children over 5 years, LTRAs are recommended when ICSs cannot be used and as an add-on treatment when only a good response to LABA cannot be achieved, LABAs are recommended as the first choice of add on therapy for this group of children.
Conclusion
The current evidence indicates that first-line monotherapy with antileukotrienes is not generally recommended in asthma sufferers, with perhaps the exclusion of those who have aspirin-intolerant asthma and exercise-induced asthma. Its efficacy and cost-effectiveness in comparison to ICSs for the management of mild persistent asthma is inferior, but there are studies supporting that adddition of montelukast in patients whose symptoms remain uncontrolled by ICSs could provide equivalent clinical control to salmeterol (Bjermer et al 2000; Miraglia et al 2007). Nonetheless, by virtue of their high systemic bioavailability, antileukotrienes may be valuable in those asthmatic patients who find it difficult to use inhaled medications. In summary, Cys-LTRAs have proved moderately effective in asthmatic children, an effect which appears to be complementary to current corticosteroid treatment.
References
- Anderson SD. Single-dose agents in the prevention of exercise-induced asthma: a descriptive review. Treat Respir Med. 2004;3:365–79. doi: 10.2165/00151829-200403060-00004. [DOI] [PubMed] [Google Scholar]
- Altman LC, Munk Z, Seltzer J. A placebo-controlled, dose-ranging study of montelukast, a cysteinyl leukotriene-receptor antagonist. J Allergy Clin Immunol. 1998;102:50–6. doi: 10.1016/s0091-6749(98)70054-5. [DOI] [PubMed] [Google Scholar]
- Becker A, Swern A, Tozzi CA, et al. Montelukast in asthmatic patients 6 years-14 years old with an FEV1 > 75% Curr Med Res Opin. 2004;20:1651–9. doi: 10.1185/030079904X4644. [DOI] [PubMed] [Google Scholar]
- Bisgaard H. Leukotriene modifiers in pediatric asthma management. Pediatrics. 2001;107:381–90. doi: 10.1542/peds.107.2.381. [DOI] [PubMed] [Google Scholar]
- Bisgaard H, Loland L, Oj JA. No in exhaled air asthmatic children is reduced by the leukotriene receptor antagonist montelukast. Am J Respir Crit Care Med. 1999;160:1227–31. doi: 10.1164/ajrccm.160.4.9903004. [DOI] [PubMed] [Google Scholar]
- Bisgaard H, Zielen S, Garcia-Garcia ML, et al. Montelukast reduces asthma exacerbations in 2- to 5-year-old children with intermittent asthma. Am J Respir Crit Care Med. 2005;171:315–22. doi: 10.1164/rccm.200407-894OC. [DOI] [PubMed] [Google Scholar]
- British Thoracic Society. The British guidelines on asthma management revised edition. 2005 Nov; National Asthma Campaign, Royal College of Physicians of London in association with the General Practitioner in Asthma Group, et al. [Google Scholar]
- Bjermer L, Bisgaard H, Bousquet J, et al. Montelukast or salmeterol combined with an inhaled steroid in adult asthma: design and rationale of a randomized, double-blind comparative study (the IMPACT Investigation of Montelukast as a Partner Agent for Complementary Therapy-trial) Resp Med. 2000;94:612–21. doi: 10.1053/rmed.2000.0806. [DOI] [PubMed] [Google Scholar]
- Bronsky EA, Kemp JP, Zhang J, et al. Dose-related protection of exercise bronchoconstriction by montelukast, a cysteinyl leukotriene-receptor antagonist, at the end of a once-daily dosing interval. Clin Pharmacol Ther. 1997;62:556–61. doi: 10.1016/S0009-9236(97)90051-5. [DOI] [PubMed] [Google Scholar]
- Buchvald F, Bisgaard H. Comparisons of the complementary effect on exhaled nitric oxide of salmeterol vs montelukast in asthmatic children taking regular inhaled budesonide. Ann Allergy Asthma Immunol. 2003;91:309–13. doi: 10.1016/S1081-1206(10)63536-3. [DOI] [PubMed] [Google Scholar]
- Carraro S, Corradi M, Zanconato S, et al. Exhaled breath condensate cysteinyl leukotrienes are increased in children with exercise-induced bronchoconstriction. J Allergy Clin Immunol. 2005;115:764–70. doi: 10.1016/j.jaci.2004.10.043. [DOI] [PubMed] [Google Scholar]
- Drazen JM, Israel E, O’Byrrne PM. Treatment of asthma with drugs modifying the leukotriene pathway. N Engl J Med. 1999;340:197–206. doi: 10.1056/NEJM199901213400306. [DOI] [PubMed] [Google Scholar]
- Ducharme FM. Inhaled corticosteroids versus leukotriene antagonists as first-line therapy for asthma: a systematic review of current evidence. Treat Respir Med. 2004;3:399–405. doi: 10.2165/00151829-200403060-00006. [DOI] [PubMed] [Google Scholar]
- Ducharme FM, Di Salvio F. Antileukotriene agents compared to inhaled corticosteroids in the management of recurrent and/or chronic asthma in adults and children. Cochrane Database Syst Rev. 2002;(1):CD002314. doi: 10.1002/14651858.CD002314. [DOI] [PubMed] [Google Scholar]
- Edelman JM, Turpin JA, Bronsky EA, et al. Oral montelukast compared with inhaled salmeterol to prevent exercise-induced bronchoconstriction. A randomized, double-blind trial. Exercise Study Group. Ann Intern Med. 2000;132:97–104. doi: 10.7326/0003-4819-132-2-200001180-00002. [DOI] [PubMed] [Google Scholar]
- Fernandez MP, Rubai SG, Solis MS, et al. Montelukast administered in the morning or evening to prevent exercise-induced bronchoconstriction in children. Pediatr Pulmonol. 2006;41:222–7. doi: 10.1002/ppul.20377. [DOI] [PubMed] [Google Scholar]
- Global Strategy for Asthma Management and Prevention. 2006 NIH Publication No 02-3659, issued January, 1995 (updated 2006), Management Segment (Chapter 7) [Google Scholar]
- Hakim F, Vizlozni D, Adler A, et al. The effect of montelukast on bronchial hyperreactivity in preschool children. Chest. 2007;131:180–6. doi: 10.1378/chest.06-1402. [DOI] [PubMed] [Google Scholar]
- Israel E, Fischer AR, Rosenberg MA. The pivotal role of 5-lipoxygenase products in the reaction of aspirin-sensitive asthmatics to aspirin. Am Rev Respir Dis. 1993;148:1447–51. doi: 10.1164/ajrccm/148.6_Pt_1.1447. [DOI] [PubMed] [Google Scholar]
- Jones TR, Labelle M, Belley M. Pharmacology of montelukast sodium (Singulair), a potent and selective leukotriene D4 receptor antagonist. Can J Physiol Pharmacol. 1995;73:191–201. doi: 10.1139/y95-028. [DOI] [PubMed] [Google Scholar]
- Knorr B, Franchi LM, Bisgaard H, et al. Montelukast, a leukotriene receptor antagonist, for the treatment of persistent asthma in children aged 2 to 5 years. Pediatrics. 2001a;108:E48. doi: 10.1542/peds.108.3.e48. [DOI] [PubMed] [Google Scholar]
- Knorr B, Holland S, Schwartz J, et al. Clinical pharmacology of montelukast. Clin Exp Allergy Rev. 2001b;1:254–61. [Google Scholar]
- Knorr B, Larson P, Nguyen HH. Montelukast dose selection in 6- to 14-year-olds: comparison of single-dose pharmacokinetics in children and adults. J Clin Pharmacol. 1999;39:786–93. doi: 10.1177/00912709922008434. [DOI] [PubMed] [Google Scholar]
- Knorr B, Matz J, Bernstein JA, et al. Montelukast for chronic asthma in 6- to 14-year-old children: a randomized, double-blind trial. Pediatric Montelukast Study Group. JAMA. 1998;279:1181–6. doi: 10.1001/jama.279.15.1181. [DOI] [PubMed] [Google Scholar]
- Mehta NP. Montelukast in childhood asthma. Indian Pediatr. 2000;37:12019. [PubMed] [Google Scholar]
- Meyer KA, Arduino JM, Santanello NC, et al. Response to montelukast among subgroups of children aged 2 to 14 years with asthma. J Allergy Clin Immunol. 2003;111:757–62. doi: 10.1067/mai.2003.1391. [DOI] [PubMed] [Google Scholar]
- Miraglia DGM, Piacentini GL, Caposso M, et al. Formoterol, montelukast, and budesonide in asthmatic children: Effect on lung function and exhaled nitric oxide. Respir Med. 2007 doi: 10.1016/j.rmed.2007.02.010. Apr 4 (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- Montuschi P, Mondino C, Koch P, et al. Effects of a leukotriene receptor antagonist on exhaled leukotriene E4 and prostanoids in children with asthma. J Allergy Clin Immunol. 2006;118:347–53. doi: 10.1016/j.jaci.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Ng D, Salvio F, Hicks G. Antileukotriene agents compared to inhaled corticosteroids in the management of recurrent and/or chronic asthma in adults and children. Cochrane Database Syst Rev. 2004;(2):CD002314. doi: 10.1002/14651858.CD002314.pub2. [DOI] [PubMed] [Google Scholar]
- National Heart Lung and Blood Institute. NAEPP Expert Panel Report. Guidelines for the Diagnosis and Management of Asthma – Update on Selected Topics. 2002 [Google Scholar]
- Noonan MJ, Chervinsky P, Brandont M. Montelukast, a potent leukotriene receptor antagonist, causes dose related improvements in chronic asthma. Eur Respir J. 1998;11:1232–9. doi: 10.1183/09031936.98.11061232. [DOI] [PubMed] [Google Scholar]
- O’Byrne PM, Israel E, Drazen JM. Antileukotrienes in the treatment of asthma. Ann Intern Med. 1997;127:472–80. doi: 10.7326/0003-4819-127-6-199709150-00009. [DOI] [PubMed] [Google Scholar]
- Pajaron-Fernandez M, Garcia-Rubia S, Sanchez-Solis M, et al. Montelukast administered in the morning or evening to prevent exercise-induced bronchoconstriction in children. Pediatr Pulmonol. 2006;41:222–7. doi: 10.1002/ppul.20377. [DOI] [PubMed] [Google Scholar]
- Pearlman DS, Ostrom NK, Bronsky EA, et al. The leukotriene D4 receptor antagonist zafirlukast attenuates exercise – induced broncho-constriction in children. J Pediatr. 1999;134:273–9. doi: 10.1016/s0022-3476(99)70449-x. [DOI] [PubMed] [Google Scholar]
- Ram FS, Cates CJ, Ducharme FM. Long-acting beta2-agonists versus anti-leukotrienes as add-on therapy to inhaled corticosteroids for chronic asthma. Cochrane Database Syst Rev. 2005;25:CD003137. doi: 10.1002/14651858.CD003137.pub2. [DOI] [PubMed] [Google Scholar]
- Robertson CF, Price D, Henry R, et al. Short-course montelukast for intermittent asthma in children. Am J Respir Crit Care Med. 2007;175:323–9. doi: 10.1164/rccm.200510-1546OC. [DOI] [PubMed] [Google Scholar]
- Salvi SS, Krishna MT, Sampson AP, et al. The anti-inflammatory effects of leukotriene-modifying drugs and their use in asthma. Chest. 2001;119:1533–46. doi: 10.1378/chest.119.5.1533. [DOI] [PubMed] [Google Scholar]
- Skoner D. Montelukast in 2- to 5-year-old children with asthma. Pediatr Pulmonol. 2001;21(Suppl):46–8. doi: 10.1002/ppul.2006. [DOI] [PubMed] [Google Scholar]
- Stelmach I, Grzelewski T, Stelmach W, et al. Effect of triamcinolone acetonide, montelukast, nedocromil sodium, formoterol on eosinophil blood conts, ECP serum levels and clinical parameters in children with asthma. Pol Merkuriusz Lek. 2002;12:208–13. [PubMed] [Google Scholar]
- Stelmach I, Korzeniewska A, Smejda K, et al. Effect of montelukast on lung function and clinical symptoms in patients with cystic fibrosis. Pneumonol Alergol Pol. 2004;72:85–9. [PubMed] [Google Scholar]
- Strauch E, Moske O, Thoma S, et al. A randomized controlled trial on the effect of montelukast on sputum eosinophils cationic protein in children with corticosteroid-dependent asthma. Pediatr Res. 2003;54:198–203. doi: 10.1203/01.PDR.0000072328.28105.06. [DOI] [PubMed] [Google Scholar]
- Szefler SJ. Facing the challenges of childhood asthma: What changes are necessary? J Allergy Clin Immunol. 2005;115:685–8. doi: 10.1016/j.jaci.2005.01.031. [DOI] [PubMed] [Google Scholar]
- Thomas SD, Luttermoser GK, Dickerson KS. Leukotriene inhibitors in the treatment of allergy and asthma. Am Fam Physician. 2007;75:65–70. [PubMed] [Google Scholar]
- Turner CR, Breslow R, Conklyn MJ, et al. In vitro and in vivo effects of leukotriene B4 antagonism in a primate model of asthma. J Clin Inves. 1996;97:381–7. doi: 10.1172/JCI118426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner OJ. The role of leukotriene receptor antagonists in the treatment of chronic asthma in childhood. Allergy. 2001;56(Suppl 66):22–9. doi: 10.1034/j.1398-9995.2001.00005.x. [DOI] [PubMed] [Google Scholar]
- Wenzel SE. Antileukotriene drugs in the management of asthma. JAMA. 1998;280:2068–9. doi: 10.1001/jama.280.24.2068. [DOI] [PubMed] [Google Scholar]
- Wright AL, Taussig LM. Lessons from long term cohort studies: childhood asthma. Eur Respir J. 1998;27(Suppl):17S–22S. [PubMed] [Google Scholar]
- Zeiger RS, Szefler SJ, Phillips BR, et al. Response profiles to fluticasone and montelukast in mild-to-moderate persistent childhood asthma. J Allergy Clin Immunol. 2006;117:45–52. doi: 10.1016/j.jaci.2005.10.012. [DOI] [PubMed] [Google Scholar]


