Abstract
Deferiprone is an orally active iron chelator which has emerged from an extensive search for new treatment of iron overload. Comparative studies have shown that at comparable doses deferiprone may be as effective as deferoxamine in removing body iron. Retrospective and prospective studies have shown that deferiprone monotherapy is significantly more effective than deferoxamine in improving myocardial siderosis in thalassemia major. Agranulocytosis is the most serious side effect associated with the use of deferiprone, occurring in about 1% of the patients. More common but less serious side effects are gastrointestinal symptoms, arthralgia, zinc deficiency, and fluctuating transaminases levels. Deferiprone can be used in combination with desferrioxamine. This regimen of chelation is tolerable and attractive for patients unable to comply with standard deferoxamine infusions or with inadequate response to deferiprone alone. Combination therapy has been effectively used in the management of severe cardiac siderosis.
Keywords: deferiprone, iron overload, thalassemia, deferoxamine
Introduction
Deferiprone (DFP, Ferriprox™, Kelfer™, L1, CP20) is one of a series of hydroxypyridinone iron chelators synthesized by Dr. Kontoghiorghes in the early to mid 1980s in the laboratory of Professor R. Hider at the University of Essex in London (Kontoghiorghes 1985). The medicinal chemists in this laboratory were researching a molecule that could be taken orally, bind iron in conditions of iron overload, such as thalassemia, and excrete it from the body. When screening techniques revealed efficacy in 59Fe-labeled liver macrophages and leukemic cell lines, they tested this chelator in iron-loaded mice, rats, and rabbits and found that it was absorbed into the body and did excrete excess iron (Hoffbrand 2005).
The excitement over the discovery of a potentially effective oral iron chelator led the investigators to initiate a development program of animal studies that would lead them to the most rapid route to a trial in humans. The first publication of the use of DFP in man was published in 1987 (Kontoghiorghes et al 1987).
Iron is essential to survival of virtually all species and there is no physiologic excretory pathway for this essential element (Andrews 1999). In conditions of primary iron overload (eg, hemochromatosis) or secondary (eg, transfusion-dependant thalassemia), accumulation of this potentially toxic element, due to the lack of a formal mechanism for iron excretion, results in massive iron accumulation, followed by iron-induced morbidity and early death, much of which is attributed to the generation of iron-induced free radical damage (Rund and Rachmilewitz 2005).
Prior to the discovery of DFP, the only option for treatment of iron overload was deferoxamine (DFO), an iron chelator that is not orally absorbed and thus needed to be administered parenterally, typically as an 8- to 12-hour nightly infusion, 5–7 nights a week (Thalassemia International Federation Guidelines 2000). While the use of DFO for about 2 decades prior to the introduction of DFP decreased morbidity and mortality among those who were able to comply with night-long infusions, a consistent proportion of patients refused therapy or at least were non-compliant, limiting the usefulness of this chelator, and a key factor in spurring on scientists to find an effective alternative chelator. Thus the introduction of DFP was accompanied by much hope among hematologists and thalassemia patients alike.
The regulatory approval of Ferriprox™ in Europe (August 1999) was a key advance in the treatment of iron overload. Recently, another oral iron chelator, ICL670, has been approved for clinical use and additional compounds are in various stages of development (Donovan et al 2005; Cappellini et al 2006; Galanello et al 2006a).
Pharmacologic properties
DFP (3-hydroxy-1,2-dimethylpyridin-4-one) is a synthetic analogue of mimosine, an iron chelator isolated from the legume Mimosa paduca (Clarke and Martell 1992). It has 2 pKas, one of 3.6 and the other of 9.9 (Hider and Liu 2003). DFP has strong iron binding properties, with a pFe3+ of 19.6 and a pFe2+ of only 5.6, indicating a high degree of relative specificity for the trivalent form of iron, binding it in a 3:1 complex (Figure 1). Key pharmacologic properties of the compound are shown in Table 1 (Clarke and Martell 1992; Tam et al 2003). As a water-soluble compound having a partition coefficient of 0.11 and with a molecular weight of only 139 Da, it would be expected to move freely through cell membranes throughout the body.
Figure 1.
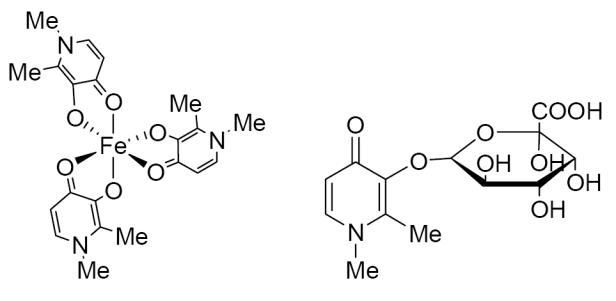
Deferiprone 3:1 complex with iron and DFP-O-glucuronide
Table 1.
Deferiprone main pharmacologic properties
| Denticity | Bidentate |
|---|---|
| Molecular weight | 139 Da |
| pM for Fe+++ | 19.6 |
| Cmax (fasting state) | 100 μmol/L |
| Elimination, t ½ | 2–3 h |
| Cell penetration | Lipophilic |
| Charge of chelator-iron complex | neutral |
DFP appears to be rapidly and completely absorbed after oral administration, with peak plasma levels typically occurring about 1 hour after administration. Food slows the rate of absorption and thus reduces the peak concentration observed, with a Cmax of about 100 μmol/L in the fasting state and about 85 μmol/L when fed (Matsui et al 1991; Al-Refaie et al 1995a), but does not have much effect on the total amount absorbed.
The drug is rapidly eliminated from the body with a half-life of about 2 hours due to hepatic biotransformation, with glucuronidation accounting for almost all of the metabolism. About 90% of the drug is excreted in the urine as the glucuronide. The t½ in healthy subjects (1.3 hours) may be shorter than that in thalassemia subjects (2.3 hours) (Stobie et al 1993). Since the clearance (CL/F) did not differ between the two subject populations, this indicates a different volume of distribution in transfused patients, most likely related to large differences in iron stores and the ability of DFP to access intracellular iron pools.
Comparative efficacy
The purpose of an iron chelator is to minimize the risk of iron-induced toxicity. Ideally, this is accomplished by the combined mechanisms of inactivating current iron deposits and removing excess iron from the body through the urine and/or feces. By reducing tissue iron stores to levels that can be tolerated by the respective organs, the benefits of a targeted transfusion program can be achieved without the sequelae of iron overload.
Whereas 1–2 mg of iron is absorbed from the diet in healthy subjects, patients with transfusion-dependent thalassemia typically have an intake many times that amount. Since there is no iron elimination pathway, the iron load accumulates in the body, leading to toxicity in key organs such as the liver, endocrine organs, and heart. In transfusion-dependent thalassemia, the greatest cause of increased body iron is the hemoglobin in transfused blood. Transfusion requirements are normally determined on the basis of a patient’s hemoglobin level, but a rough approximation of the increased iron load can be estimated if one assumes that splenectomized patients might receive about 120 mL red blood cells (RBC)/kg each year, and non-splenectomized patients, who tend to have greater requirements, of about 160 mL RBC/kg each year because of enhanced degradation by overactive spleens (Thalassemia International Federation Guidelines 2000). Since 1 mL of pure RBC contains approximately 1 mg iron, the total iron received from transfusions, when averaged for a year in a “typical” patient, would be about 120–160 mg iron/kg divided by 365 days or a total of about 0.3–0.4 mg/kg iron/day. Thus to maintain a negative iron balance, one should normally seek to eliminate 0.3–0.4 mg iron/kg body weight per day.
Assessment of efficacy of an iron chelator can be determined by measuring the amount of iron eliminated during use of the chelator and comparing that with the amount of iron input. However, efficacy assessment may be somewhat more complicated, because one is concerned not only in achieving a negative iron balance, but also in minimizing iron-induced organ toxicity. Since some chelators may be more effective in removing iron from some tissues/organs than others, in part based upon their ability to access these sites, organ-specific iron removal is another key factor in the assessment of efficacy.
Consequently, there are several methods used to track changes in body iron concentrations resulting from the response to chelation therapy, each with its own unique advantages. Iron balance provides explicit information on the actual removal of iron from the body, but gives no precise information on the concentration of iron within any particular tissue, or its change subsequent to chelation. The measurement of iron within a given tissue, such as the liver, provides information specifically about that tissue, but cannot be relied upon for levels of iron loading in other tissues. The same applies to circulating levels of ferritin, a storage protein for iron. The newest development in assessing chelation efficacy is measuring cardiac iron loading and heart function (left ventricular ejection fraction, LVEF) with modern MRI techniques (Anderson et al 2001). The response to DFP for each of the measures of iron chelator effectiveness is addressed below.
Iron balance
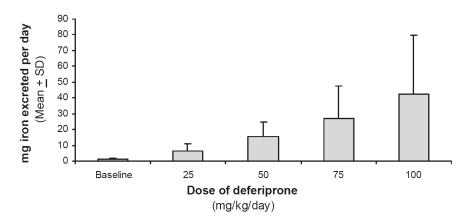
Measurement of the amount of iron eliminated in urine and feces provides an unequivocal determination of the effectiveness of an iron chelator in removing iron from the body, because these excreta contain negligible amounts of iron (about 1 mg/day), except for desquamation of cells (gastrointestinal and menses) (Andrews 1999). Since DFP may remove iron either from the liver, via the bile, or the rest of the body, via the urine, both excreta need to be measured for iron content in the case of this chelator. Iron excretion was found to be about 25 mg per day in transfusion-dependent patients, in patients receiving a standard dose of 75 mg/kg/day (Kontoghiorghes et al 1990; Agarwal et al 1992; Collins et al 1994; Al-Refaie et al 1995a). On average, this amount of excreted iron is sufficient to keep them in net negative iron balance, in spite of continued transfusions. Agarwal et al measured urinary iron excretion in thalassemia patients receiving various doses of DFP, ranging from 25 to 100 mg/kg/day, and demonstrated an increasing amount of total iron excretion as the dose increased (Figure 2) (Agarwal et al 1992).
Figure 2.
Urinary iron excretion in thalassemia patients receiving different doses of deferiprone.
Although Agarwal’s data predicted a dose-response effect for iron elimination with DFP, he used different patients having different iron input and measured only urinary iron excretion, leading to tentative conclusions that could be confirmed only by systematic studies. The techniques used in each of the centers that have carried out such studies varied, as did the number of days of urine and/or fecal collections (Addis et al 1999). Combined with the high level of inherent day-to-day variability in iron excretion, it became apparent that definitive data on total body iron excretion could be achieved only by detailed study of patients in a systematic fashion obtained with cumulative daily collection of excreta averaged over several days, under highly controlled conditions.
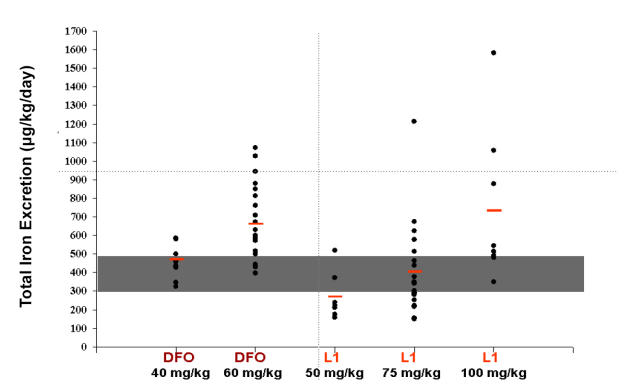
The most thorough approach to iron excretion studies has been carried out by Grady et al, who used full iron balance studies to measure iron excreted in both urine and feces and compared the output to the total iron intake with food and blood transfusion. Grady’s DFP and DFO-induced iron excretion results are summarized in Figure 3, and demonstrate a dose-response relationship for DFP between 50 and 100 mg/kg/day and for DFO for 40 and 60 mg/kg/day. Grady’s total iron balance studies in iron overloaded thalassemia patients revealed that 75 mg/kg/day of DFP was comparable to DFO 40 mg/kg/day, and increasing the dose to 60 mg/kg/day of DFO or 100 mg/kg/day of DFP would increase the proportion of patients achieving negative iron balance with these chelators (Grady et al 2001, 2002).
Figure 3.
Total iron excretion with different doses of deferoxamine or deferiprone. Red bars indicate mean values. Grey area represents the total iron excretion needed to maintain negative iron balance. Reproduced with permission of RW Grady, Weill Cornell Medical Center.
Serum ferritin concentrations
The ferritins are a family of iron storage and detoxification proteins which play a critical role in cellular iron homeostasis in humans, other animals, plants and microbes. Shortly after chelation therapy for iron overload became available, an immunoassay technique for measuring serum ferritin concentrations was introduced as a means estimating body iron load (Siimes et al 1974) and following serum ferritin concentration has been used ever since then to monitor response to chelation therapy in conditions such as thalassemia (McLaren et al 1983; Modell and Berdoukas 1984). While widely used as an index of total body iron load, it has the disadvantage that it is also an acute phase reactant and thus spurious values can occur in the presence of an inflammatory process, particularly hepatitis, and neoplasia (Siimes et al 1974). Although single values can be misleading, serum ferritin concentrations can be used long term to compare monthly or quarterly changes, providing evidence of the success of chelation therapy based on the trends in response over time.
Understanding the utility and limitations of serum ferritin concentrations, enables one to assess patient response to DFP in thalassemia and other conditions. Table 2 summarizes the results of several publications which examined the effectiveness of various doses of DFP in lowering iron overload in thalassemia patients throughout the world. The data reveal that DFP is effective at decreasing or stabilizing serum ferritin concentrations during continued blood transfusions. In general, those patients who had high serum ferritin concentrations before starting DFP, experienced the greatest decline, whereas those who were well-treated prior to starting to DFP, generally experienced a stabilization of values, indicative of control of iron load (Cohen et al 2000). When using serum ferritin concentrations as a measure of iron overload, response to DFP therapy can be monitored and doses can be tailored to the needs of the patient to maintain concentrations below those associated with iron-induced heart disease.
Table 2.
Changes in serum ferritin levels in thalassemia major patients treated with deferiprone
| Study | Number of enrolled | Dose | Length of study | Mean serum ferritin (μg/L) | |
|---|---|---|---|---|---|
| patients | (mg/kg/d) | (months) | First | Last | |
| Al-Refaie et al 1992 | 11 | 185–119 | 6–12 | 5549 | 4126 |
| Kersten et al 1996 | 36 | 50–100 | 1 to 36 | 3563 | 2560 |
| Mazza et al 1998 | 29 | 70 | >12 | 3748 | 2550 |
| Olivieri et al 1998 | 18 | 75 | 55.2 | 4455 | 2831 |
| Hoffbrand et al 1998 | 26 | 75 | 4 to 49 | 2937 | 2323 |
| Maggio et al 2002 | 71 | 75 | 12 | 2283 | 2061 |
| Rombos et al 2000 | 11 | 75–100 | 12 | 4732 | 3849 |
| Peng et al 2003 | 11 | 75 | 36 | 4652 | 2792 |
| Lucas et al 2002 | 54 | 75 | 9 | 5743 | 3558 |
| Taher et al 2004 | 12 | 75 | 60 | 4302 | 2229 |
| Cohen et al 2000 | 187 | 75 | 12 | 2696 | 2633 |
| Cohen et al 2003 | 84 | 75 | 48 | 2696 | 2889 |
Liver iron concentrations (LIC)
The liver is the organ most suited to accommodate excess stores of iron because of its histological composition, size and anatomical location. Even though the liver is relatively well-designed to tolerate high concentrations of iron due to lysosomal storage mechanisms and other factors, high levels of iron over prolonged periods do induce hepatic fibrosis and even cirrhosis (Prati et al 2004). Early studies revealed that LIC increased linearly with the number of units of transfused blood (Cazzola et al 1983). Upon initiating chelation therapy, the transfusion regimen as well as the dose of chelating agent used become the major determinants of LIC and the key is to adjust the dose to the needs of the patient, depending largely on the patient’s transfusion regimen and efficacy of the chelator in that individual.
Until recently, most hepatic iron concentrations were measured by hepatic biopsy, an invasive procedure that patients are not eager to have repeated. Consequently, the data evaluating sequential assessments of liver iron concentration over time is sparse. The introduction of a superconducting quantum interference device (SQUID) was promoted for a period of time, but the scarcity of these expensive instruments, together with a lack of validation, minimized their use (Fischer et al 2005). More recently, MRI has been used to assess hepatic iron concentrations (Anderson et al 2001; St Pierre and Clark 2005). It has been clearly established that liver iron correlates linearly with the total iron body while there is little evidence for the value of liver iron concentrations as a predictor of cardiac iron load or of iron induced cardiac disease in patients undergoing chelation treatment (Angelucci et al 2000; Anderson et al 2001; Wood et al 2004). Although LIC may not have good predictive value for cardiac disease-free survival, hepatocellular damage is related to the level of hepatic iron loading (Jensen et al 2003). To minimize the risk of iron-induced liver damage as well as to reduce total body iron stores, a chelator needs to be capable of reducing LIC, or maintaining acceptable levels if they have already been reduced (Jensen et al 2003).
As is the case for DFO, little information is available to assess the effect of DFP on LIC following serial hepatic concentrations over time (Hoffbrand et al 2003).
Maggio et al compared the efficacy of DFP versus DFO in a large multicenter randomized clinical trial (Maggio et al 2002). They found no difference in the reduction of liver iron content measured by MRI or liver biopsy between the two groups. Another randomized controlled study designed to compare the abilities of DFP and DFO to remove excess iron from the heart, used MRI to assess hepatic iron concentrations. In 61 randomized patients (32 on DFO), a mean dose of 92 mg/kg/day of DFP reduced hepatic iron concentrations by 0.93 mg/g dry weight vs 1.54 mg/g dry weight in patients receiving a mean of 43 mg/kg/day DFO 5.7 days/week (p = 0.40) (Pennell et al 2006).
Cardiac magnetic resonance imaging
Magnetic resonance imaging, particularly employing the recent MRI T2* methodology, is the most recent parameter to be employed in the assessment of iron overload to evaluate cardiac iron load and predict the risk of iron-induced cardiac damage. MRI was first used more than a decade ago to estimate, semi-quantitatively, cardiac iron levels in patients with iron overload (Olivieri et al 1992). Newer instrumentation and sophisticated software have greatly improved its capability, and it has now been validated against physical measurements of iron in liver biopsy samples, as well as showing reproducibility in multiple sites with different scanners (Westwood et al 2003; Anderson et al 2001). Data generated over the last 5 years have shown that MRI T2* values have good reproducibility for the measurement of iron concentration, with a CV of approximately 5% (Tanner et al 2006a). A quantitative assessment of the magnitude of cardiac iron loading in transfusion-dependent thalassemia major patients has only recently been published, revealing that two thirds of 167 patients in Sardinia maintained on DFO therapy exhibited cardiac siderosis (T2* <20 ms) (Tanner et al 2006b). Simultaneous assessment of the iron content in heart and liver has shown that patients may have high concentrations of iron in the liver but low concentrations of iron in the heart, or vice versa (Anderson et al 2001; Wood et al 2004). Together, these findings point to the critical need to monitor cardiac iron load during chelation therapy to avoid false security regarding the apparent efficacy of chelation.
DFP and heart
Iron-induced heart failure and arrhythmias are the most common causes of death in patients with thalassemia major, accounting for about 70% of deaths (Borgna-Pignatti et al 2004). Several studies have shown that DFP is more effective than DFO in removing cardiac iron. Anderson et al (2002) retrospectively compared myocardial iron content, using magnetic resonance T2* and cardiac function in 15 patients receiving long-term DFP with 30 matched thalassemia major controls on long-term DFO. The DFP groups had significantly reduced myocardial iron (median 34 msec vs 11.4 msec; p = 0.02) and higher ejection fraction (mean 70.0% ± 6.5% vs 63% ± 6.9%; p = 0.004) than the DFO group. The cardiac benefit of chelation therapy with DFP has been observed in another retrospective study with more than 4 years of follow-up (Piga et al 2003). Cardiac dysfunction was newly diagnosed in 4% of the DFP treated patients and in 20% of the DFO-treated patients (p = 0.007). Several prospective trials have compared the myocardial effects of DFP and DFO (Maggio et al 2002; Peng et al 2003; Galia et al 2003; Pennell et al 2006). In two studies DFP at 75 mg/kg/day was as effective as DFO, at 50 mg/kg/day 5–6 days per week, at reducing cardiac iron by magnetic resonance signal intensity ratio (SIR) (Maggio et al 2002; Galia et al 2003).
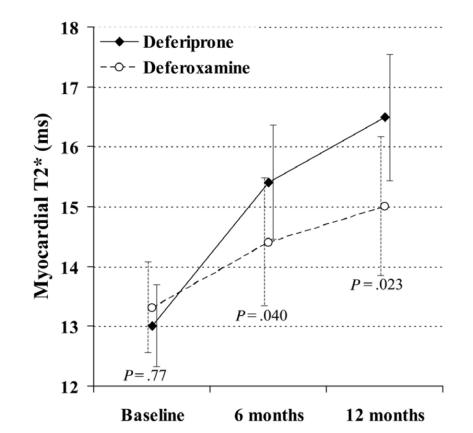
Peng et al (2003) prospectively compared cardiac iron, estimated by magnetic resonance signal intensity, and LVEF over 3 years in 13 patients allocated to DFO (50 mg/kg/day at least 5 days per week) with 11 patients taking DFP (75 mg/kg/day). Cardiac iron was markedly improved in 5 patients on DFP and only in 2 of the patients on DFO treatment. Mean LVEF improved in the patients taking DFP (from 58.6 ± 6.8 to 65.2% ± 7.1%), whereas there was no significant change in the DFO group (from 63.3 ± 6.3 to 64.6% ± 7.0%). The above-reported results have been recently confirmed by a larger, prospective controlled trial, where 61 patients previously treated with DFO were randomized to be maintained on DFO (43 mg/kg for 5–7 days per week) or switched to DFP (92 mg/kg/d 7 days per week) (Pennell et al 2006). After 1 year of treatment there was significantly greater improvement in myocardial T2* for patients taking DFP than those on DFO (27% vs 13%; p = 0.023) (Figure 4). Ventricular ejection fraction also increased significantly more in the DFP group (3.1% vs 0.3%; p = 0.0034).
Figure 4.
Change in myocardial T2* at 6 and 12 months in patients taking deferiprone or deferoxamine. Reproduced with permission from Pennell DJ, Berdoukas V, Karagiorga M, et al 2006. Randomized controlled trial of deferiprone or deferoxamine in beta-thalassemia major patients with asymptomatic myocardial siderosis.
The available data show that DFP may have greater cardiac benefit than DFO. The apparent greater efficacy of DFP in removing excess cardiac iron may be related to the higher ability to enter myocardial cells due to its lower molecular weight, neutral charge, and lipophilicity.
DFP safety profile
Safety is an issue of major concern for a drug that patients take long term. Several factors are responsible for side effects of an iron chelator, including route of administration, iron removal from iron-dependent enzymes, removal of other divalent cations such as zinc and calcium, body iron redistribution, and direct toxicity to tissue and organs. The safety of DFP has been extensively evaluated over the last 15 years and many studies have provided a detailed safety profile of its long-term use.
Transient gastrointestinal symptoms (GI) such as nausea, vomiting, and abdominal pain, are the most frequently reported DFP-related adverse drug reactions (ADR). In a long-term prospective study, which monitored 187 patients with thalassemia major, most of them followed for 4 years for a total drug exposure of 531 patient-years, GI symptoms occurred overall in 33% of patients in the first year (Cohen et al 2003). Those events were mild to moderate in intensity and resolved without discontinuation of therapy in most patients. The 33% decreased to 3% in subsequent years. Despite the high frequency, only a restricted number of patients (3) withdrew because of GI symptoms. Similar results have been reported in other studies (Al-Refaie 1995b; Ceci et al 2002; Maggio et al 2002).
Joint symptoms (pain and/or swelling) are the second most frequent ADR reported in 3.9%–20% of patients taking DFP (Al-Refaie 1995b; Olivieri et al 1995; Ceci et al 2002; Cohen et al 2003). There seems to be a particular high incidence of arthropathy in Indian patients treated with DFP (up to 41%), which has been attributed to their severe iron overload or undefined ethnic genetic background (Choudry et al 2004). Joint symptoms, sometimes associated with myalgia, are mild/moderate in most patients, but occasionally may be severe enough to warrant interruption of the drug, reduction of the dose, or discontinuation. Overall about 2% of the patients discontinued DFP because of joint symptoms. Unlike GI problems, which usually manifest in the first weeks of treatment, joint problems may occur even after years of treatment. Sequential assessments of antinuclear antibody, rheumatoid factor, anti-double stranded DNA, and anti-histone antibodies indicate that the episodes of arthropathy are not associated with an autoimmune disease (Cohen et al 2000).
Overall fluctuating serum alanine aminotransferase (ALT) have been reported in about 7% of the patients particularly in the first months of treatment. The transient nature of the changes in ALT levels and the absence of a progressive increase over time has been reported in several studies (Al-Refaie et al 1995b; Olivieri et al 1995; Maggio et al 2002; Cohen et al 2003). However, careful monitoring of ALT levels at regular intervals is recommended and DFP interruption or dose reduction should be considered for patients with substantial and persistent increase of transaminases.
In 1998 one report suggested that DFP use was associated with an increase in liver fibrosis in 5 out 14 patients (Olivieri et al 1998). An accompanying editorial outlined that the design of the study, the small liver biopsy samples, and the lack of an appropriate control group precluded any definitive conclusion (Kowdley and Kaplan 1998). A separate review of the same 14 biopsies did not confirm the original results (Callea 1998). Since then, several studies conducted to evaluate liver histology changes during therapy with DFP showed no evidence of DFP-induced liver fibrosis (Hoffbrand et al 1998; Piga et al 1998; Galanello 1999). The analysis by 3 independent pathologists of the largest collection of liver biopsies from patients treated with DFP for a mean of 3.1 years demonstrated the lack of progressive hepatic fibrosis during long term therapy with DFP (Wanless et al 2002).
Low plasma zinc levels have been reported in patients treated with DFP, particularly in those with diabetes mellitus. In a long-term controlled study a slight decrease in plasma zinc was observed over time, but the mean value remained within the normal reference range (Taher et al 2002; Cohen et al 2003). Overall low plasma zinc levels are not a common problem in patients treated with DFP, but periodic monitoring can be suggested especially in patients with diabetes.
Reddish discoloration of urine due to excretion of the iron-deferiprone complex has been reported in DFP-treated patients.
Studies in non-iron loaded animals have shown that DFP is embryotoxic and teratogenic (Berdoukas et al 1993). Although few uneventful pregnancies with healthy newborns have been reported, women of child bearing age should be counseled to avoid pregnancy while on therapy with DFP (Goudsmit and Jaeger et al 1992; Kersten 1992; Gogtay and Agarwal 2002). DFP should be also avoided in breast feeding mothers.
Agranulocytosis, defined as a confirmed absolute neutrophil count (ANC) less than 0.5 ×109/L, is considered the most serious adverse effect of DFP. In a prospective, long-term study including 187 patients followed for 4 years, specifically designed to determine the incidence of severe adverse events, agranulocytosis occurred in 0.5% of the patients (0.2 episodes per 100 patient-years) (Cohen et al 2003). Similar results have been reported in a large Italian study including 532 patients (1% of the patients, 0.3 episodes per 100 patient-years) (Ceci et al 2002). Agranulocytosis usually resolves with interruption of DFP, but sometimes for faster recovery treatment with G-CSF is needed. Fatal outcome due to severe infections associated with DFP-related agranulocytosis has been reported in some patients (Apotex Research Inc. 2003; Muller et al 2000).
Milder episodes of neutropenia, defined as an ANC between 0.5 and 1.5 ×109/L, have been reported in 3.6%–8.5% of the patients (Al-Refaie et al 1995b; Ceci et al 2002; Cohen et al 2003). Milder neutropenia is usually reversible on discontinuation of the drug. The reason for the reduced neutrophil count during DFP treatment is uncertain. Agranulocytosis is not dose-dependent. It seems to be an idiosyncratic response and although it is more frequent in the first year of treatment, it has been reported up to 4 years from starting DFP therapy. It appears that the mechanism for agranulocytosis and milder neutropenia may be different. Neutropenia occurs significantly more often in non-splenectomized patients and in association with viral infections (Cohen et al 2000). Because of risk of recurrence, reintroduction of DFP after an initial episode of agranulocytosis is not recommended. It should be emphasized that careful monitoring of blood counts remains a critical component of therapy with DFP.
Association of deferiprone with deferoxamine
The availability of two chelators (DFO and DFP), besides monotherapy with one of them, makes possible two chelation strategies: alternate chelation and combined chelation. Alternate therapy means that in each single day only one of the two chelators is taken by the patient, while combination therapy indicates that the two chelators are taken in the same day.
Alternate therapy
Alternate therapy is very common in clinical practice probably because by introducing oral DFP and reducing the weekly number of DFO infusions, it may be a practical approach to improve compliance with iron chelation for patients poorly compliant with DFO monotherapy. The effectiveness of alternating treatment was initially reported by Aydinok et al (1999) in a small non-controlled study. A prospective, randomized, controlled trial on the safety and efficacy of alternating DFO and DFP has been recently reported (Galanello et al 2006b). In this study 60 patients with thalassemia major regularly transfused, were randomized either to continue the standard therapy with DFO at 30–40 mg/kg/day for 5–7 days per week, or to receive an alternating regimen of DFP 75 mg/kg body weight, divided into 3 doses 5 days a week and DFO (30–40 mg/kg/day) the other 2 days of the week. After 1 year of treatment both arms resulted in equivalent decreases of serum ferritin (−248 ± 791 μg/L for the alternating regimen vs −349 ± 573 μg/L for the DFO monotherapy group; p = 0.58) and liver iron concentration determined by SQUID (−65 ± 615 vs –239 ± 474 μgFe/g liver; p = 0.22). Overall the alternating use of both chelators was not associated with increased toxicity and no significant difference was observed in the proportion of patients with adverse events in the two therapy groups, although the nature of the adverse events differed according to the chelation regimen: DFO was associated with local reactions at the site of infusion, DFP with GI symptoms.
Combined therapy
Combined use of DFO and DFP was introduced into the clinical practice by Wonke et al (1998) in a small group of patients. Combined chelation offers several potential advantages. Drugs with distinct physicochemical properties have different iron-carrying capacities and may access different iron pools. Combined therapy may achieve levels of iron excretion that cannot be achieved by either drug alone without loss of compliance and without increased toxicity (Hoffbrand et al 2003). This approach is attractive for patients unable to comply with standard DFO infusions (5–7 per week) or with inadequate response to DFP alone. Several non-controlled studies using different doses of chelators, different regimens (number of days per week on DFO and DFP), for variable periods have been reported (Wonke et al 1998; Balveer et al 2001; Alymara et al 2002; Farmaki et al 2002; Kattamis et al 2002; Mourad et al 2003; Origa et al 2005). In most of these studies serum ferritin significantly decreased compared with initial levels and a higher urinary iron excretion was observed. Some of these studies evaluated the clinical efficacy of the combination therapy. Origa et al (2005) reported a significant improvement in LVEF from 48.6% ± 9% at baseline to 57.0% ± 6% (p < 0.0001) without modifying cardiac therapy. The effect of combination therapy on glucose metabolism has been described by Farmaki et al (2006). Glucose responses were improved at all times during an oral glucose tolerance test, particularly in patients in early stages of glucose intolerance.
The effect of combined therapy versus DFO monotherapy on myocardial iron overload was evaluated in a prospective randomized placebo controlled trial using cardiovascular magnetic resonance (Tanner et al 2007). Sixty-five patients with mild to moderate cardiac iron loading (heart T2* 8–20 msec) were randomized to receive either DFO and placebo or DFO and DFP. There were significant improvements in the combined treatment group compared with the placebo group in myocardial T2* (ratio of change in geometric means 1.50 vs 1.24; p = 0.02) and LVEF (2.6% vs 0.6%; p = 0.05).
The role of combination therapy in the management of severe cardiac siderosis has been specifically addressed in some case report and in prospective studies (Wu et al 2004; Tsironi et al 2005; Tavecchia et al 2006; Porcu et al 2007). Patients with severe end stage heart failure had a dramatic improvement in symptoms and myocardial function after initiation of treatment with combination of DFP and DFO. A prospective study, including 15 patients with severe myocardial siderosis (heart T2*< 8 msec) and reduced LVEF, has shown that combined chelation provides an effective means of reducing myocardial iron and improving cardiac function. In this study myocardial T2* increased from 5.7 ± 0.98 at baseline to 7.1 ± 1.96 after 6 months of combined chelation and LVEF improved significantly (Tanner M, personal communication).
Based on the above studies and on data showing that DFP may have a greater effect in removing cardiac iron than DFO (see below) combination therapy should be considered as an alternative to continuous intravenous DFO monotherapy.
Careful metabolic iron balance studies assessing the total (urinary and fecal) iron excretion have shown an additive effect when DFO and DFP were given sequentially and a synergistic effect, in some patients, when the drugs were given simultaneously (Grady et al 2002). The hypothesis to explain the synergy is that the small DFP molecule acts as a shuttle mobilizing iron from intracellular compartments to the bloodstream, where DFP may exchange iron with the larger DFO molecule which has a higher affinity for iron.
Over the past 6 years numerous clinical studies have provided data on the safety of the association of DFP with DFO (Alymara et al 2002; Farmaki et al 2002; Kattamis et al 2002; Origa et al 2005). Combination regimens have shown no unanticipated adverse drug reactions.
Deferiprone and survival
The published data showing superior efficacy of DFP in the management of cardiac siderosis and cardiac disease have received support from outcome studies looking at patient’s survival. A retrospective 5-year observational study comparing a group of 54 DFP-treated patients with 75 DFO-treated patients indicated that cardiac-disease-free survival was significantly better in the DFP group (p = 0.0003) (Piga et al 2003). A recent multicenter study involving 359 patients receiving DFO and 157 patients switched to DFP showed that during the 9-year observation period there was a total of 52 cardiac events, including 15 cardiac deaths, all occurring in the patients treated with DFO (Borgna-Pignatti et al 2006). Overall 14.5% of the DFO patients developed a cardiac event vs 0% of the DFP patients. Telfer et al (2006) analyzed the survival trends in Cypriot patients with thalassemia major born after 1974 and found significant decline of cardiac deaths and a marked improvement in survival since 2000, probably due to the introduction of combination therapy.
Conclusion
The available data appear to demonstrate that DFP is an effective oral iron chelator able to reduce iron overload and to maintain a safe body iron level. Moreover, deferiprone alone or in combination with DFO seems to be superior to DFO monotherapy in improving myocardial siderosis and cardiac function. The safety of DFP has been extensively evaluated over the last 10 years. The relatively large number of patients and the extended period of intensive follow-up provide a detailed long-term safety profile. In general, adverse drug reactions with DFP are predictable and the risk manageable provided a continuous and careful monitoring of the patients.
Acknowledgments
This study has been supported by Regione Sardegna (L.R. 11, 1990) and by Ithanet project (Electronic Infrastructure for Thalassemia Research Network). We thank Franca Rosa Demartis for editorial assistance.
Disclosures
Prof. Renzo Galanello has participated in chelation drug trials sponsored by Apotex and has received research support and speaker’s honoria from Apotex.
Reference
- Addis A, Loebstein R, Koren G, et al. Meta–analytic review of the clinical effectiveness of oral deferiprone (L1) Eur J Clin Pharmacol. 1999;55:1–6. doi: 10.1007/s002280050584. [DOI] [PubMed] [Google Scholar]
- Agarwal MB, Gupte SS, Viswanathan C, et al. Long–term assessment of efficacy and safety of L1, an oral iron chelator, in transfusion dependent thalassaemia: indian trial. Br J Haematol. 1992;82:460–6. doi: 10.1111/j.1365-2141.1992.tb06445.x. [DOI] [PubMed] [Google Scholar]
- Al–Refaie FN, Hershko C, Hoffbrand AV, et al. Results of long–term deferiprone (L1) therapy: a report by the International Study Group on Oral Iron Chelators. Brit J Haematol. 1995b;91:224–9. doi: 10.1111/j.1365-2141.1995.tb05274.x. [DOI] [PubMed] [Google Scholar]
- Al–Refaie FN, Sheppard L, Nortey P, et al. Pharmacokinetics of the Oral Iron Chelator Deferiprone (L1) in Patients with Iron Overload. Br J Haematol. 1995a;89:403–8. doi: 10.1111/j.1365-2141.1995.tb03318.x. [DOI] [PubMed] [Google Scholar]
- Al–Refaie FN, Wonke B, Hoffbrand AV, et al. Efficacy and possible adverse effects of the oral iron chelator 1, 2–dimethyl–3–hydroxypyrid–4–one (L1) in thalassemia major. Blood. 1992;80:593–9. [PubMed] [Google Scholar]
- Alymara V, Bourantas DK, Chaidos A, et al. Combined iron chelation therapy with desferrioxamine and deferiprone in thalassemic patients [abstract] Hematol J. 2002;3(Suppl 1):81. [Google Scholar]
- Anderson LJ, Holden S, Davis B, et al. Cardiovascular T2–star (T2*) magnetic resonance for the early diagnosis of myocardial iron overload. Eur Heart J. 2001;22:2171–9. doi: 10.1053/euhj.2001.2822. [DOI] [PubMed] [Google Scholar]
- Anderson LJ, Wonke B, Prescott E, et al. Comparison of effects of oral deferiprone and subcutaneous desferrioxamine on myocardial iron concentrations and ventricular function in beta–thalassaemia. Lancet. 2002;360:516–20. doi: 10.1016/s0140-6736(02)09740-4. [DOI] [PubMed] [Google Scholar]
- Andrews NC. Disorders of iron metabolism. N Engl J Med. 1999;341:1986–95. doi: 10.1056/NEJM199912233412607. [DOI] [PubMed] [Google Scholar]
- Angelucci E, Brittenham GM, McLaren CE, et al. Hepatic iron concentration and total body iron stores in thalassemia major. N Engl J Med. 2000;343:327–31. doi: 10.1056/NEJM200008033430503. [DOI] [PubMed] [Google Scholar]
- Apotex Research Inc. Ferriprox: Periodic Safety Update Report, PSUR. Toronto, Canada: 2003. Feb, [Google Scholar]
- Aydinok Y, Nisli G, Kavakli K, et al. Sequential use of deferiprone and desferrioxamine in primary school children with thalassaemia major in Turkey. Acta Haematol. 1999;102:17–21. doi: 10.1159/000040962. [DOI] [PubMed] [Google Scholar]
- Balveer K, Pryor K, Wonke B. Combined oral and parenteral iron chelation in beta thalassaemia major. Med J Malaysia. 2001;55:493–7. [PubMed] [Google Scholar]
- Berdoukas V, Bentley P, Frost H, et al. Toxicity of oral iron chelator L1 [letter] Lancet. 1993;341:1088. doi: 10.1016/0140-6736(93)92443-w. [DOI] [PubMed] [Google Scholar]
- Borgna–Pignatti C, Rugolotto S, De Stefano P, et al. Survival and complications in patients with thalassemia major treated with transfusion and deferoxamine. Haematologica. 2004;89:1187–93. [PubMed] [Google Scholar]
- Borgna–Pignatti C, Cappellini MD, De Stefano P, et al. Cardiac morbidity and mortality in deferoxamine– or deferiprone–treated patients with thalassemia major. Blood. 2006;107:3733–7. doi: 10.1182/blood-2005-07-2933. [DOI] [PubMed] [Google Scholar]
- Callea F. Iron chelation with oral deferiprone in patients with thalassemia. N Engl J Med. 1998;339:1710–1. [PubMed] [Google Scholar]
- Cappellini MD, Cohen A, Piga A, et al. A phase 3 study of deferasirox (ICL670), a once–daily oral iron chelator, in patients with beta–thalassemia. Blood. 2006;107:3455–62. doi: 10.1182/blood-2005-08-3430. [DOI] [PubMed] [Google Scholar]
- Cazzola M, Borgna–Pignatti C, De Stefano P, et al. Internal distribution of excess iron and sources of serum ferritin in patients with thalassaemia. Scand J Haematol. 1983;30:289–96. doi: 10.1111/j.1600-0609.1983.tb01494.x. [DOI] [PubMed] [Google Scholar]
- Ceci A, Baiardi P, Felisi M, et al. The safety and effectiveness of deferiprone in a large–scale, 3–year study in Italian patients. Br J Haematol. 2002;118:330–6. doi: 10.1046/j.1365-2141.2002.03554.x. [DOI] [PubMed] [Google Scholar]
- Clarke ET, Martell AE. Stabilities of 1, 2–dimethyl–3–hydroxy–4–pyridinone chelates of divalent and trivalent metal ions. Inorg Chim Acta. 1992;191:57–63. [Google Scholar]
- Cohen AR, Galanello R, Piga A, et al. Safety profile of the oral iron chelator deferiprone: a multicentre study. Br J Haemat. 2000;108:305–12. doi: 10.1046/j.1365-2141.2000.01866.x. [DOI] [PubMed] [Google Scholar]
- Cohen AR, Galanello R, Piga A, et al. Safety and effectiveness of long–term therapy with the oral iron chelator deferiprone. Blood. 2003;102:1583–7. doi: 10.1182/blood-2002-10-3280. [DOI] [PubMed] [Google Scholar]
- Collins AF, Fassos FF, Stobie S, et al. Iron–balance and dose–response studies of the oral iron chelator 1, 2–dimethyl–3–hydroxypyrid–4–one (L1) in iron–loaded patients with sickle cell disease. Blood. 1994;83:2329–33. [PubMed] [Google Scholar]
- Choudhry VP, Pati HP, Saxena A, et al. Deferiprone, efficacy and safety. Indian J Pediatr. 2004;71:213–6. doi: 10.1007/BF02724272. [DOI] [PubMed] [Google Scholar]
- Donovan JM, Plone M, Dagher R, et al. Preclinical and clinical development of deferitrin, a novel, orally available iron chelator. Ann N Y Acad Sci. 2005;1054:492–4. doi: 10.1196/annals.1345.071. Review. [DOI] [PubMed] [Google Scholar]
- Farmaki K, Anagnostopoulos G, Platis O, et al. Combined chelation therapy in patients with thalassemia major: a fast and effective method of reducing ferritin levels and cardiological complications [abstract] Hematol J. 2002;3(Suppl 1):79. [Google Scholar]
- Farmaki K, Angelopoulos N, Anagnostopoulos G, et al. Effect of enhanced iron chelation therapy on glucose metabolism in patients with beta–thalassaemia major. Br J Haematol. 2006;134:438–44. doi: 10.1111/j.1365-2141.2006.06203.x. [DOI] [PubMed] [Google Scholar]
- Fischer R, Piga A, Harmatz P, et al. Monitoring long–term efficacy of iron chelation treatment with biomagnetic liver susceptometry. Ann N Y Acad Sci. 2005;1054:350–7. doi: 10.1196/annals.1345.043. [DOI] [PubMed] [Google Scholar]
- Galanello R, De Virgiliis S, Agus A, et al. Sequential liver fibrosis grading during deferiprone treatment in patients with thalassemia major [abstract]. 9th International Conference on Oral Chelation in the Treatment of Thalassaemia and Other Diseases; Mar 25–28; Hamburg Germany. 1999. p. 50. [Google Scholar]
- Galanello R, Kattamis A, Piga A, et al. A prospective randomized controlled trial on the safety and efficacy of alternating deferoxamine and deferiprone in the treatment of iron overload in patients with thalassemia. Haematologica. 2006b;91:1241–3. [PubMed] [Google Scholar]
- Galanello R, Piga A, Forni GL, et al. Phase II clinical evaluation of deferasirox, a once–daily oral chelating agent, in pediatric patients with beta–thalassemia major. Haematologica. 2006a;91:1343–51. [PubMed] [Google Scholar]
- Galia M, Midiri M, Bartolotta V, et al. Multicenter Trial Group of the Society for the Study of Thalassemia and Haemoglobinopathies. Potential myocardial iron content evaluation by magnetic resonance imaging in thalassemia major patients treated with deferoxamine or deferiprone during a randomized multicenter prospective clinical study. Hemoglobin. 2003;27:63–76. doi: 10.1081/hem-120021538. [DOI] [PubMed] [Google Scholar]
- Gogtay JA, Agarwal MB. Clinical experience with kelfer (deferiprone) in India over the last 12 years [abstract]. 12th International Conference on Oral Chelation in the Treatment of Thalassemia & Other Diseases; Jul 4–7; Santorini, Greece. 2002. p. 89. [Google Scholar]
- Goudsmit R, Kersten MJ. Long–term treatment of transfusion hemosiderosis with the oral iron chelator L1. Drugs of Today. 1992;28:133–5. [Google Scholar]
- Grady RW, Berdoukas VA, Rachmilewitz EA, et al. Iron chelation therapy: Metabolic aspects of combining deferiprone and desferrioxamine [abstract]. Proceedings of the 11th International Conference on Oral Chelation in the Treatment of Thalassemia and Other Diseases; 2001. pp. 74–78. [Google Scholar]
- Grady RW, Berdoukas VA, Rachmilewitz EA, et al. Combination of desferrioxamine and deferiprone markedly enhance iron excretion [abstract] Proceedings of the American Society of Hematology 44th Annual Meeting and Exposition. 2002;100:241a. [Google Scholar]
- Hider RC, Liu ZD. Emerging understanding of the advantage of small molecules such as hydroxypiridones in the treatment of iron overload. Curr Med Chem. 2003;10:1051–64. doi: 10.2174/0929867033457629. [DOI] [PubMed] [Google Scholar]
- Hoffbrand AV. Deferiprone therapy for transfusional iron overload. Best Pract Res Clin Haematol. 2005;18:299–317. doi: 10.1016/j.beha.2004.08.026. [DOI] [PubMed] [Google Scholar]
- Hoffbrand AV, Al–Refaie FN, Davis B, et al. Long–term trial of deferiprone in 51 transfusion–dependent iron overloaded patients. Blood. 1998;91:295–300. [PubMed] [Google Scholar]
- Hoffbrand AV, Cohen A, Hershko C. Role of deferiprone in chelation therapy for transfusional iron overload. Blood. 2003;102:17–24. doi: 10.1182/blood-2002-06-1867. [DOI] [PubMed] [Google Scholar]
- Jaeger M, Aul C, Sohngen D, et al. Iron overload in polytransfused patients with MDS: use of L1 for oral iron chelation. Drugs Today. 1992;28:143–7. [Google Scholar]
- Jensen PD, Jensen FT, Christensen T, et al. Relationship between hepatocellular injury and transfusional iron overload prior to and during iron chelation with desferrioxamine: a study in adult patients with acquired anemias. Blood. 2003;101:91–6. doi: 10.1182/blood-2002-06-1704. [DOI] [PubMed] [Google Scholar]
- Kattamis A, Kassou C, Ladis V, et al. Safety and efficacy of combining deferiprone and deferoxamine in iron chelation therapy in patients with thalassemia [abstract] Blood. 2002;100:120a. [Google Scholar]
- Kersten MJ, Lange R, Smeets MEP, et al. Longterm treatment of transfusional iron overload with the oral iron chelator deferiprone (L1): a Dutch multicenter trial. Ann Hematol. 1996;73:247–52. doi: 10.1007/s002770050236. [DOI] [PubMed] [Google Scholar]
- Kontoghiorghes GJ. New orally active iron chelators. Lancet. 1985;1:817. doi: 10.1016/s0140-6736(85)91472-2. [DOI] [PubMed] [Google Scholar]
- Kontoghiorghes GJ, Aldouri MA, Sheppard L, et al. 1, 2–dimethyl–3–hydroxypyrid–4–one, an orally active chelator for treatment of iron overload. Lancet. 1987;1:1294–5. doi: 10.1016/s0140-6736(87)90545-9. [DOI] [PubMed] [Google Scholar]
- Kontoghiorghes GJ, Bartlett AN, Hoffbrand AV, et al. Long–term trial with the oral iron chelator 1, 2–dimethyl–3– hydroxypyrid–4–one (L1). I. Iron chelation and metabolic studies. Br J Haematol. 1990;76:295–300. doi: 10.1111/j.1365-2141.1990.tb07887.x. [DOI] [PubMed] [Google Scholar]
- Kowdley KV, Kaplan MM. Iron–chelation therapy with oral deferiprone–toxicity or lack of efficacy? N Engl J Med. 1998;339:468–9. doi: 10.1056/NEJM199808133390709. [DOI] [PubMed] [Google Scholar]
- Lucas GN, Perera BJ, Fonseka EA, et al. Experience with the oral iron chelator deferiprone in transfusion–dependent children. Ceylon Med J. 2002;47:119–21. doi: 10.4038/cmj.v47i4.3413. [DOI] [PubMed] [Google Scholar]
- McLaren GD, Muir WA, Kellermeyer RW. Iron overload disorders: natural history, pathogenesis, diagnosis, and therapy. Crit Rev Clin Lab Sci. 1983;19:205–66. doi: 10.3109/10408368309165764. [DOI] [PubMed] [Google Scholar]
- Maggio A, D’Amico G, Morabito A, et al. Deferiprone versus deferoxamine in patients with thalassemia major: a randomized clinical trial. Blood Cells Mol Dis. 2002;28:196–208. doi: 10.1006/bcmd.2002.0510. [DOI] [PubMed] [Google Scholar]
- Matsui D, Klein J, Hermann C, et al. Relationship between the pharmacokinetics and iron excretion pharmacodynamics of the new oral iron chelator 1, 2–dimethyl–3–hydroxypyrid–4–one in patients with thalassemia. Clin Pharmacol Ther. 1991;50:294–298. doi: 10.1038/clpt.1991.139. [DOI] [PubMed] [Google Scholar]
- Mazza P, Amurri B, Lazzari G, et al. Oral iron chelating therapy: a single center interim report on deferiprone (L1) in thalassaemia. Haematologica. 1998;83:496–501. [PubMed] [Google Scholar]
- Modell B, Berdoukas V. The Clinical Approach to Thalassaemia. London: Grune & Stratton; 1984. [Google Scholar]
- Mourad FH, Hoffbrand AV, Sheikh–Taha M, et al. Comparison between desferrioxamine and combined therapy with desferrioxamine and deferiprone in iron overloaded thalassaemia patients. Br J Haematol. 2003;121:187–9. doi: 10.1046/j.1365-2141.2003.04240.x. [DOI] [PubMed] [Google Scholar]
- Müller A, Soyano A, Soyano–Müller A, et al. Irreversible aplastic anemia after treatment with deferiprone in a patient with blackfan diamond anemia and hemochromatosis [abstract] Blood. 2000;96(11):13b. [Google Scholar]
- Olivieri NF, Koren G, Matsui D, et al. Reduction of tissue iron stores and normalization of serum ferritin during treatment with the oral iron chelator L1 in thalassemia intermedia. Blood. 1992;79:2741–8. [PubMed] [Google Scholar]
- Olivieri NF, Brittenham GM, Matsui D, et al. Iron–chelation therapy with oral deferiprone in patients with thalassemia major. N Engl J Med. 1995;332:918–22. doi: 10.1056/NEJM199504063321404. [DOI] [PubMed] [Google Scholar]
- Olivieri NF, Brittenham GM, McLaren CE, et al. Long–term safety and effectiveness of iron–chelation therapy with deferiprone for thalassemia major. N Engl J Med. 1998;339:417–23. doi: 10.1056/NEJM199808133390701. [DOI] [PubMed] [Google Scholar]
- Origa R, Bina P, Agus A, et al. Combined therapy with deferiprone and desferrioxamine in thalassemia major. Haematologica. 2005;90:1309–14. [PubMed] [Google Scholar]
- Peng CT, Chow KC, Chen JH, et al. Safety monitoring of cardiac and hepatic systems in beta–thalassemia patients with chelating treatment in Taiwan. Eur J Haematol. 2003;70:392–7. doi: 10.1034/j.1600-0609.2003.00071.x. [DOI] [PubMed] [Google Scholar]
- Pennell DJ, Berdoukas V, Karagiorga M, et al. Randomized controlled trial of deferiprone or deferoxamine in beta–thalassemia major patients with asymptomatic myocardial siderosis. Blood. 2006;107:3738–44. doi: 10.1182/blood-2005-07-2948. [DOI] [PubMed] [Google Scholar]
- Piga A, Facello S, Gaglioti C, et al. No progression of liver fibrosis in thalassemia major during deferiprone or desferrioxomine iron chelation [abstract] Blood. 1998;92(10 Suppl 1):3065. [Google Scholar]
- Piga A, Gaglioti C, Fogliacco E, et al. Comparative effects of deferiprone and deferoxamine on survival and cardiac disease in patients with thalassemia major: a retrospective analysis. Haematologica. 2003;88:489–96. [PubMed] [Google Scholar]
- Porcu M, Landis N, Salis S, et al. Effects of combined deferiprone and desferrioxamine iron chelating therapy in beta–thalassemia major end–stage heart failure A case report. Eur J Heart Fail. 2007;9:320–2. doi: 10.1016/j.ejheart.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Prati D, Maggioni M, Milani S, et al. Cooleycare Cooperative Group. Clinical and histological characterization of liver disease in patients with transfusion–dependent beta–thalassemia. A multicenter study of 117 cases. Haematologica. 2004;89:1179–86. [PubMed] [Google Scholar]
- Rombos Y, Tzanetea R, Konstantopoulos K, et al. Chelation therapy in patients with thalassemia using the orally active iron chelator deferiprone (L1) Haematologica. 2000;85:115–7. [PubMed] [Google Scholar]
- Rund D, Rachmilewitz E. Beta–thalassemia. N Engl J Med. 2005;353:1135–46. doi: 10.1056/NEJMra050436. [DOI] [PubMed] [Google Scholar]
- Siimes MA, Addiego JE, Jr, Dallman JR. Ferritin in Serum: Diagnosis of Iron Deficiency and Iron Overload in Infants and Children. Blood. 1974;43:581–90. [PubMed] [Google Scholar]
- St Pierre TG, Clark PR, Chua–anusorn W, et al. Noninvasive measurement and imaging of liver iron concentrations using proton magnetic resonance. Blood. 2005;105:855–61. doi: 10.1182/blood-2004-01-0177. [DOI] [PubMed] [Google Scholar]
- Stobie S, Tyberg J, Matsui D, et al. Comparison of the pharmacokinetics of 1, 2–dimethyl–3–hydroxypyrid–4–one (L1) in healthy volunteers, with and without co–administration of ferrous sulfate, to thalassemia patients. Int J Clin Pharmacol Ther Toxicol. 1993;31:602–5. [PubMed] [Google Scholar]
- Taher A, Mourad FH, Koussa S, et al. Effect of desferrioxamine, deferiprone or a combination therapy in iron overload thalassemia patients: the Lebanese experience [abstract]. 12th International Conference on Oral Chelation in the Treatment of Thalassemia & Other Diseases; Santorini, Greece. 2002. p. 18. [Google Scholar]
- Taher A, Aoun E, Sharara AI, et al. Five–year trial of deferiprone chelation therapy in thalassaemia major patients. Acta Haematol. 2004;112:179–83. doi: 10.1159/000081268. [DOI] [PubMed] [Google Scholar]
- Tam TF, Leung–Toung R, Li W, et al. Iron chelator research: past, present, and future. Curr Med Chem. 2003;10:983–95. doi: 10.2174/0929867033457593. [DOI] [PubMed] [Google Scholar]
- Tanner MA, Galanello R, Dessi C, et al. A randomized, placebo–controlled, double–blind trial of the effect of combined therapy with deferoxamine and deferiprone on myocardial iron in thalassemia major using cardiovascular magnetic resonance. Circulation. 2007;115:1876–84. doi: 10.1161/CIRCULATIONAHA.106.648790. [DOI] [PubMed] [Google Scholar]
- Tanner MA, He T, Westwood MA, et al. Multi–center validation of the transferability of the magnetic resonance T2* technique for the quantification of tissue iron. Haematologica. 2006a;91:1388–91. [PubMed] [Google Scholar]
- Tanner MA, Galanello R, Dessi C, et al. Myocardial Iron Loading in Patients with Thalassemia Major on Deferoxamine Chelation. J Cardiovasc Mag Res. 2006b;8:543–7. doi: 10.1080/10976640600698155. [DOI] [PubMed] [Google Scholar]
- Tavecchia L, Masera N, Russo P, et al. Successful recovery of acute hemosiderotic heart failure in beta–thalassemia major treated with a combined regimen of desferrioxamine and deferiprone. Haematologica. 2006;91:ECR19. [PubMed] [Google Scholar]
- Telfer P, Coen PG, Christou S, et al. Survival of medically treated thalassemia patients in Cyprus. Trends and risk factors over the period 1980–2004. Haematologica. 2006;91:1187–92. [PubMed] [Google Scholar]
- Tsironi M, Deftereos S, Andriopoulos P, et al. Reversal of heart failure in thalassaemia major by combined chelation therapy: a case report. Eur J Haematol. 2005;74:84–5. doi: 10.1111/j.1600-0609.2004.00335.x. [DOI] [PubMed] [Google Scholar]
- Wanless I, Sweeney G, Dhillon AP, et al. Lack of progressive hepatic fibrosis during long term therapy with deferiprone in subjects with transfusion–dependent beta–thalassemia. Blood. 2002;10:1566–9. doi: 10.1182/blood-2002-01-0306. [DOI] [PubMed] [Google Scholar]
- Westwood MA, Anderson LJ, Firmin, et al. Interscanner reproducibility of cardiovascular magnetic resonance T2* measurements of tissue iron in thalassemia. J Magn Reson Imaging. 2003;18:616–20. doi: 10.1002/jmri.10396. [DOI] [PubMed] [Google Scholar]
- Wonke B, Wright C, Hoffbrand AV. Combined therapy with deferiprone and desferrioxamine. Br J Haematol. 1998;103:361–4. doi: 10.1046/j.1365-2141.1998.01002.x. [DOI] [PubMed] [Google Scholar]
- Wood JC, Tyszka M, Carson S, et al. Myocardial iron loading in transfusion–dependent thalassemia and sickle–cell disease. Blood. 2004;103:1934–5. doi: 10.1182/blood-2003-06-1919. [DOI] [PubMed] [Google Scholar]
- Wu KH, Chang JS, Tsai CH, et al. Combined therapy with deferiprone and desferrioxamine successfully regresses severe heart failure in patients with beta–thalassaemia major. Ann Hematol. 2004;83:471–3. doi: 10.1007/s00277-003-0820-0. [DOI] [PubMed] [Google Scholar]