Abstract
Ulcerative colitis (UC) is a chronic inflammatory condition of unclear etiology affecting the large bowel, most commonly the rectum and extending proximally in a continuous fashion. The overall principle in the pathophysiolgy of ulcerative colitis is the dysregulation of the normal immune system against an antigenic trigger leading to a prolonged mucosal inflammatory response. The diagnosing of UC is made by combining the clinical picture, tissue biopsy with the endoscopic appearance of mucosal ulceration, friable, edematous, erythematous granular appearing mucus. The approach to therapy of UC has been dependent on severity of symptoms with frontline therapy being salicylate based sulfasalazine. Newer formulations of salicylates based drugs with fewer side-effects have been developed. These are free of the sulphur component and are composed of 5-ASA, without the sulfapyridine carrier molecule. Mesalamine is one of these 5-ASA based agents that are currently available and indicated for treatment of UC. In mild/moderate active disease mesalamine has response rates between 40%–70% and remission rates of 15%–20%. Considering that the efficacy of 5-ASA is dose dependent, 4.8 g/day and 2.4 g/day have been shown to be the optimal dosages for mild-moderate distal active disease and for maintenance therapy, respectively. Patients with moderately active ulcerative colitis treated with 4.8 g/d of mesalamine are significantly more likely to achieve overall improvement at week 6 compared to patients treated with 2.4 g/d. In the setting of left-sided distal colitis (proctitis), topical (rectal) formulations have been found to be superior to oral aminosalicylates at inducing remission. Mesalamine has been shown to be safe in short term use with a dose-response efficacy without dose-related toxicity.
Keywords: ulcerative colitis (UC), mesalamine, 5-ASA therapy, inflammatory bowel disease, remission
Introduction
Ulcerative colitis (UC) is a chronic inflammatory condition of unclear etiology affecting the large bowel, most commonly the rectum and extending proximally in a continuous fashion. Patients often present with abdominal pain, diarrhea and may also manifest other systemic features of fever and weight loss. The overall incidence and prevalence rates for UC range from 3 to 15:100,000 and 50–80:100,000, respectively, with higher rates reported in the industrial world (Stevenson and Korzenik 2003). The prevalence in the United States has been reported as high as 229 cases per 100,000, with approximately 25,000 new cases a year (Loftus et al 2000). It can affect any age group; however, it is more common in younger populations with a peak onset between 15 and 25 years old. There is an equal incidence found in both genders with bimodal incidence commonly seen in IBD. In the United States, African–Americans have the lowest incidence rate of 1.4 per 100,000 while Jews have the highest of 13 per 100,000.
Etiology
Ulcerative colitis is characterized as an idiopathic disease, however various possible etiologies have been proposed. The major hypotheses regarding the etiology of UC have included infection, allergy to dietary components, immune responses to bacterial or self-antigens and environmental causes. Infectious causes include pathogenic microbial antigenic triggers that have yet to be identified. However, it appears to be more likely that normal intestinal microflora as opposed to pathogenic organisms may have a significant role in development of IBD (Stevenson and Korzenik 2003). Defective colonic mucosa and abnormal intestinal epithelial permeability may increase the access of luminal dietary and bacterial products to the mucosa (Shanahan 2001). Environmental causes have been proposed in relationship to the prevalence of IBD (Bernstein et al 2006). However, there has been little evidence that any particular food components play a primary role in the etiology of the disease. Fecal stream has been shown to be a factor in promoting further mucosal inflammation and therefore the importance of the role of gut flora in IBD has been brought forward. While smoking has been related to increased risk for activity of disease in Crohn’s disease, it has demonstrated a protective effect in UC with the highest risk of developing UC in the first 2 years after cessation of smoking (Stevenson and Korzenik 2003).
Genetic studies have suggested that first-degree relatives have increased absolute risk of 7 percent, which is 4 to 20 times as higher than compared to the normal population (Orholm and Munkholm 1991). However, there is a low concordance rate in monozygotic twins, indicating that environmental factors are also involved (Bernstein et al 2006). Genetic linkage studies suggest an association between ulcerative colitis and the human leukocyte antigen (HLA) genes on chromosomes 12 and 16, but specific responsible genes are not well defined (Stevenson and Korzenik 2003). Genetic alteration experiments performed in germ-free environments have not yielded colitis, thus supporting the theory of a multifactorial cause of UC (Boden and Cho 2003).
Pathophysiology
The overall principle in the pathophysiolgy of ulcerative colitis is the dysregulation of the normal immune system against an antigenic trigger. The aggregate effect of genetic, environmental, and other processes is a sustained activation of the mucosal immune response (Podolosky 2002). A state of altered immune regulation leading to prolonged mucosal inflammatory response and thus resulting in the recruitment of leukocytes from the gut vasculature (Powrie 1995) exists in UC. The mucosa in patients with UC may be dominated by CD4+ non-T helper lymphocytes generating a humoral immune profile. Defective colonic mucosa allows the access of luminal dietary and bacterial products to the mucosa (Podolosky 2002). The possible antigenic triggers currently proposed include microbial pathogens, nonpathogenic microbial agents, dietary antigens and an autoimmune mechanism. Animal models using knock out genes that affect the mucosal immune system or epithelial integrity have resulted in intestinal mucosal inflammation in non germ-free environments (Strober et al. 1998). This therefore supports the fact that the presence of luminal bacteria and the absence of regulatory proteins in the mucosal immune system are generally required for development of intestinal inflammation. Furthermore, an uncontrolled immune activation with failure of suppression is a commonly explained mechanism of inflammatory bowel disease. Proinflammotory mediators, or cytokines, are also known to play an important role in UC, with evidence to support increased levels of IL-1, IL-6, IL-8 and tumor necrosis factor (TNF-a) (Fiocchi 1998).
Clinical presentation and diagnosis
The most common clinical course in patients with ulcerative colitis is chronic intermittent with approximately 10% of cases presenting as acute fulminating (Powell-Tuck and Truelove 1963) (Figure 1).
Figure 1.
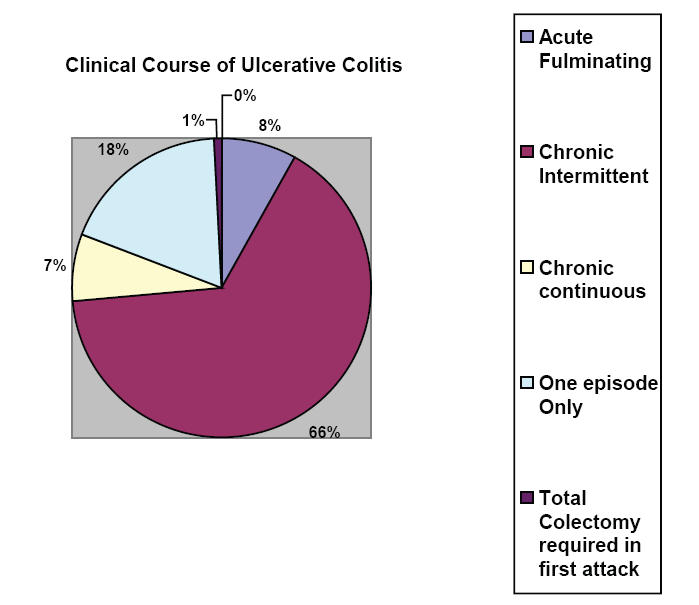
Clinical course of ulcerative colitis. Adapted from data: Powell-Tuck and Truelove (1963).
Between episodes, patient may be free of symptoms. Symptoms are related to the extent of the disease, with common clinical features being intermittent rectal bleeding, tenesmus, crampy pain, passage of mucous, and mild diarrhea. When the disease is severe, more systemic features can be seen. These include fevers, weight loss, severe abdominal pain, anemia, and malnutrition. Based on clinical and endoscopic findings the disease is characterized as to its severity and extent (Kornbluth and Sachar 2004). This allows clinicians to predict the extent of involvement and better assess the level of acuity of the patient. The most severe complication from an UC flare is toxic megacolon, which often requires surgery. If the disease is confined to the rectum, the patient may only complain of urgency and primarily tenesmus with bloody bowel movements and diarrhea.
Anatomic distribution of disease by endoscopic evaulation can be used to describe the degree of involvement and the state of activity. Almost all cases of UC have rectal involvement. Ulcerative proctitis is a disease limited to the rectum, whereas proctosigmoiditis extends to the mid sigmoid colon, left sided colitis extends to splenic flexure (30%) and extensive colitis extends beyond splenic flexure but not reaching cecum (20%) and pancolitis extends all the way to cecum. The various states of activity can be described as relapsing, mild/moderate active, severe active and remission, while the degree of severity of UC is described as mild, moderate, and severe and distinguished by frequency of bowel movements, abdominal symptoms, extent of colonic involvement, presence of fever or bleeding per rectum (Table 1). Mild is defined as less than 4 bowel movements a day, normal ESR and no signs of systemic toxicity and fulminant is defined as greater than 10 bowel movements a day, continuous bleeding, systemic toxicity, abdominal tenderness, transfusion requirement, and colonic dilation on x-ray. Therefore, once the diagnosis can be confirmed and the degree of involvement and activity can be determined, the specific treatment regimen is initiated.
Table 1.
Classification of ulcerative colitis
| Mild | Moderate | Severe | Fulminant |
|---|---|---|---|
| Less than four stools daily with or without blood No systemic signs of toxicity Normal ESR | More than four stools daily Minimal signs of toxicity | More than six bloody stools daily Evidence of toxicity demonstrated by fever, tachycardia, anemia, elevated ESR | More than ten bowel movements Continuous bleeding Toxicity Abdominal tenderness distension Blood transfusion requirement Colonic dilatation on abdominal film |
Adapted from (Kornbluth and Sachar 2004).
The diagnosis of UC is made by combining the clinical picture with the endoscopic appearance of mucosal ulceration, friable, edematous, erythematous granular appearing mucosa, often described as “sand sprinkled on a moist surface”. Biopsy results suggestive of UC include crypt abscesses, superficial microulcerations, and inflammatory infiltrate. Other histological findings include widespread crypt distortion and loss, and marked goblet cell mucin depletion. With chronic inflammation the architecture of the crypts are distorted, becoming branched, shortened and atrophied. One must remember that the clinical presentation and endoscopic findings are nonspecific and infectious and other acute self-limiting causes of colitis must always be excluded.
Treatment of ulcerative colitis
Introduction
The approach to therapy of UC has been dependent on severity of symptoms with frontline therapy being aminosalicylates. The intolerance of sulfasalazine being a restrictive factor, newer formulations have been developed which are free of the sulphur component and have better side-effect profile. These drugs are composed of 5-ASA, the active moiety of sulfasalazine, without the sulfapyridine carrier molecule that is usually the main cause of the side effects. Mesalamine is one of the 5-ASA based agents currently available and indicated for treatment of UC. This review will examine the evidence for 5-ASAs in the treatment of ulcerative colitis. The different 5-ASA formulations will be discussed and compared and the current data available regarding efficacy, dosages and side effect profile will be presented.
Sulfasalazine
Sulfasalazine (Pharmacia & Upjohn Company, Kalamazoo, MI, USA), first developed in 1942, has been the parent aminosalicylate in use for over 60 years (Hanauer 2004) and consists of 5-aminosalyclic acid linked by an azo bond to sulfapyridine (Figure 2). It combines an antibacterial agent (sulfapyridine) with an anti-inflammatory component (5-ASA). The sulfonamide moiety acts as a carrier to deliver the active component 5-ASA to the colon where it is released by bacterial action. Sulfasalazine is metabolized by colonic bacterial enzymes to produce the two active byproducts. Sulfapyraidine is metabolized by the liver and excreted in the urine whereas the 5-ASA component is acetylated by the colonic epithelium (Rochester and Abreu 2005). The original indication for 5-ASA was for rheumatoid arthritis, however it was subsequently found to be efficacious in ulcerative colitis. Misiewicz et al (1965) published the first placebo controlled maintenance trial in 1965 randomizing patients to receive sulfasalazine or placebo for one year. Seventy three percent of patients taking placebo relapsed compared to 21% taking the active drug, thus showing sulfasalazine to be highly efficacious for the treatment of ulcerative colitis.
Figure 2.
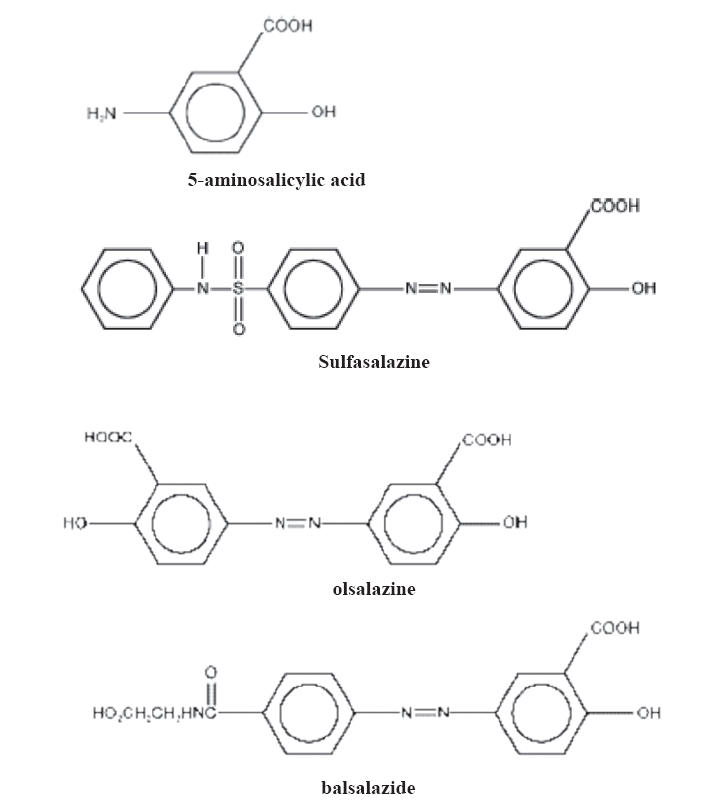
Chemical structures of 5-ASA preparations.
Due to the intolerance of sulfasalazine being a restrictive factor in optimizing the therapeutic dosage, more tolerable mesalamine-based drugs have been developed void of the sulphur component. These new formulations are composed of 5-ASA, the active moiety of sulfasalazine, without the poorly tolerated sulfapyridine carrier molecule. The newer generation aminosalicylates allow for targeted delivery with reduced side effects observed with sulfasalazine. These new formulations also allow for earlier release more proximally in the small intestine. An added benefit also includes variablility in the pH dependent site of release of various aminosalicylates (see Table 2).
Table 2.
Different preparations of mesalamine for UC therapy
| Delayed release | Slow release | Prodrugs | Prodrugs | Topicals | Sulfasalazine | |
|---|---|---|---|---|---|---|
| Formulation | Asacol | Pentasa | Olsalazine | Balsalazide | Mesalamine enema | |
| Preparation | Enteric coated 400 mg | Capsule 250 mg or 500 mg | Capsule 250 mg | Capsule 750 mg | 4 g/60 ml rectal suspension 1 g rectal suppository | |
| Solubility | pH > or equal 7 | Continuous release | pH independent | pH independent | ||
| Location of delivery | Terminal ileium | Small bowel, colon | Colon | Colon | Rectum | Small bowel, colon |
| Maintenance of remission | 2–4 g/day | 2–4 g/day | 1 g/day | 2.25 g TID | 4 g/day | 2 g/day |
| Mild to moderate | 2.4 to 4.8 g/daily | 2–4 g/daily | 2–3 g/daily | 6.75 g/day | 4 g/per rectum | 3–4 g/day |
| Active disease proctitis | TID dosing | QID dosing | BID dosing | TID dosing | BID 1g/BID Active disease: 1 g BID (suppository) or 4 g enema qd or BID Maintenance:1 g supp. Daily or prn symptoms | QID dosing |
A variety of different mechanisms have been proposed by which aminosalicylates work in inflammatory bowel disease. The main mechanism includes the inhibition of cyclooxygenase and lipoxygenase pathways to reduce the production of prostaglandins and leuokotrienes, respectively (Kaiser et al 1999). Mesalamine also reverses the antiproliferative effects of TNF-alpha thus disrupting the effect of cytokines by reducing intestinal cell transcription of inflammatory mediators (Kaiser et al 1999). Other processes described include inhibition of platelet activating factor and production of oxygen radicals and other anti-inflammatory factors (Egan et al 1999; Hanauer 2004). By reducing inflammatory prostaglandin production and the formation of other potent chemotactic substances including leukotriene B4 and certain hydroxy fatty acids (Grisham 1994), mesalamine plays a significant role in halting the perpetuation of a chronic inflammatory state.
Different preparations have been designed in accordance to delivery site depending where the highest activity of disease is located. There have been two major forms of delivery systems currently available. One method is coating 5-ASA with a pH sensitive resin or a semipermeable membrane. An alternative approach is a prodrug system which is to link the 5-ASA with another molecule by an azo bond (Figure 2). The enteric coated formulations include Asacol (Procter & Gamble Pharmaceuticals, Cincinatti OH, USA), coated with Eudragit S, which dissolves at pH 7.0 or above since pH reaches this level in the distal ileum and colon. Salofalk (Dr Falk Pharma GmbH, Feiburg, Germany), coated with Eudragit L, releases the active ingredient at pH 6.0 and above. Pentasa (Shire US, Inc., Wayne PA, USA) is a time released formulation containing a semipermeable membrane and releases the drug at pH greater than 6.0. Olsalazine (Dipentum; Celltech Parmaceuticals, Inc., Rochester NY, USA) and balsalazide (Salix Pharmaceuticals, Inc., Morrisville, NC, USA) are prodrugs, where one 5-ASA is bound covalently through an azo linkage to another 5-ASA and the release of the 5-ASA is by bacterial azo reduction in the colon. Variability may exist in the absorption of 5-ASA, most commonly due to the failing of the active drug to reach the appropriate pH in the small intestine. The therapeutic effect of these compounds is dependent on releasing of active drug in the colon which will therefore improve clinical response. Because of adherence issues, a newer high-strength 1.2 gram mesalamine formulation has been developed which utilizes a multimatrix (MMX) technology to produce a slow and homogenous delivery of mesalamine to the entire colon and rectum (Prantera et al 2005). Once dissolved above pH of 7.0, the core reveals a hydrophilic matrix that turns into a gel mass upon exposure to intestinal fluid, and pieces of the gel are distributed in different parts of the colon. With this higher concentration tablet and slow dissolution, the aim is to increase adherence by minimizing the number of tablets required. Another improved delivery method is a micropellet formulation, provided as individual sachets containing granules, with less frequent (2 packets twice a day) dosing. Studies have shown non-inferiority of the micropellet formulation compared to tablets in terms of clinical remission within 8 weeks (Cohen 2006).
5-ASA in active disease
Mesalamine
Although there is no evidence of greater clinical response of mesalamine versus other 5-ASA preparations, there is adequate data to support the effectiveness of mesalamine in mild/moderate active disease with response rates between 40%–70% and remission rates of 15%–20% (Snisky et al 1991; Kornbluth and Sachar 2004; Hanauer et al 2005). However, in studies comparing 5-ASA with sulfasalazine, there was no increased effectiveness of 5-ASA over sulfasalazine (Sutherland and MacDonald 2006). Two studies have shown that delayed – release mesalamine showed significant benefit compared with placebo (Schroeder et al 1987; Sninsky et al 1991). It was demonstrated that mesalamine 4.8 g/d had a higher complete response compared to those taking placebo (24% vs 5% p = 0.047) as well as higher partial response (50% vs 13%, p value not given) (Kane and Bjorkman 2003). The clinical response was based on predetermined criteria that included stool frequency, rectal bleeding, sigmoidoscopic appearance of the mucosa. A complete response was defined as total resolutions of all symptoms, whereas a partial response was a substantial but incomplete improvement in clinical parameters. The absolute benefit increase (ABI) based on overall response for mesalamine 4.8 g/d over placebo was 56% with a number needed to treat (NNT) of two. Responses have generally been dose-related with up to 80% of patients who receive daily doses of 4–6 g manifesting complete clinical remission or significant clinical improvement within 4 weeks (Kornbluth and Sachar 2004). Studies employing doses of 1.6 g/d and 2.4 g/d confirmed overall clinical response rates higher than placebo.
According to the American College of Gastroenterology practice guidelines on ulcerative colitis, mild-to moderate extensive colitis should be treated with oral sulfasalazine titrated up to 4–6 g per day or an alternative aminosalicylate dose up to 4.8 g per day (Grade A evidence) (Kornbluth and Sachar 2004). Patients with only moderately active ulcerative colitis treated with 4.8 g/d of mesalamine were significantly more likely to achieve overall improvement at week 6 compared to patients treated with 2.4 g/d (Hanauer et al 2005) (see Figure 3). This result was based on a double-blinded, controlled trial of 386 patients randomized to either 2.4 g/day or 4.8 g/day for 6 weeks. The primary endpoint was the proportion of patients in each arm achieving overall improvement from baseline at week 6. Overall improvement was defined as either complete resolution of signs and symptoms or a clinical response to therapy. Results demonstrated improvement in predetermined clinical parameters in 57% of patients treated with 2.4 g/day and 72.4% treated with 4.8 g/day (p = 0.0384).
Figure 3.
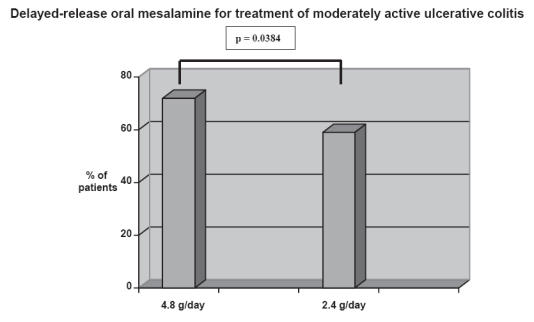
Double blinded controlled trial of 386 patients randomized to either 2.4 g/day or 4.8 g/day for 6 weeks. Overall improvement defined as either complete resolution of signs and symptoms or a clinical response to therapy. Results demonstrated improvement in clinical parameters in 72.4% of patients treated with 4.8 g/day and 57% treated with 4.8 g/day (p = 0.0384) Adapted from data: Hanuer et al (2005).
In patients with extensive mild/moderate active UC defined by involvement beyond the splenic flexure, Marteau et al (2005) demonstrated adding a mesalamine enema to an oral regimen would provide additional benefit for patients with extensive mild/moderate active UC. A randomized double blind study was conducted in 127 ambulatory patients receiving 4 g/day oral mesalamine. Patients were randomized to either receiving 1 g mesalamine enema nightly versus placebo and disease activity was assessed at 4 and 8 weeks. Disease activity was assessed using endoscopic signs of improvement and clinical features of response. Briefly, clinical and endoscopic features of response include decrease stool frequency, decrease episodes of rectal bleeding, physician global assessment, and decreased erythema, friability, and ulcerations on endoscopy. Objective scoring system was established for different stages of the clinical features and accordingly the endpoints of remission and clinical improvement were defined. The combination therapy of oral and enema mesalamine treatment was shown to be superior to oral alone in terms of both remission and improvement. Improvement was obtained in 89% of mesalamine enema group versus 62% of the placebo group at week 4 (p = 0.0008). At week 8, 86% of the mesalamine enema group versus 68% of the placebo group (p = 0.026) showed improvement (Marteau et al 2005) (see Figure 4).
Figure 4.
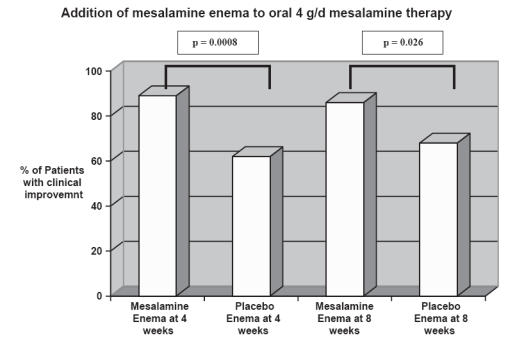
The combination therapy of oral and enema mesalamine treatment was shown to be superior to oral alone in terms of both remission and improvement. Improvement was defined by objective scoring method based on disease activity. Improvement was obtained in 89% of mesalamine group versus 62% of placebo group at week 4 (p = 0.0008). At week 8, 86% of mesalamine enema group versus 68% of placebo group (p = 0.026) showed improvement. Adapted from data: Marteau et al (2004).
There are approximately 10%–20% of patients who can be described to be in a chronically active inflammatory state, becoming steroid dependent. There is very little data evaluating the efficacy of 5-ASA for treatment of steroid dependent ulcerative colitis. Ardizzone et al (2005) compared the efficacy of 5-aminosalicylic acid and azathioprine in inducing remission of steroid dependent ulcerative colitis. Success in treatment was defined by clinical and endoscopic remission with steroid discontinuation, with azathioprine being more effective than 5-ASA in inducing clinical and endoscopic remission and avoiding long term steroid dependence. However in terms of side effects, dose reduction interventions took place in 14% of patients in the azathioprine group due to leukopenia and abnormal liver function tests.
Prodrugs
Balsalazide, which is pH-dependent, delayed release and slow release mesalamine preparation has also shown improvement in clinical symptoms in acute mild to moderate ulcerative colits (Pruitt et al 2002). Data has revealed that in newly diagnosed patients with less than 40 cm involvement improvement of symptoms occurred in a median time of 11 days. The authors did demonstrate that balsalazide patients showed improvement in sigmoidoscopic appearance, stool frequency, rectal bleeding, and physician’s global assessment score by 14 days. However, overall no statistically significance differences between mesalamine and balsalazide were detected at the end of week 8 of treatment. Mansfield et al (2002) compared the safety and efficacy of sulfasalazine 3 g with balsalazide 6.75 g in the initial daily treatment of mild to moderate ulcerative colitis and reported that balsalazide is effective as the sole treatment in this patient population with fewer withdrawals due to side-effects compared to the sulfasalazine group taking 3 g/day.
5-ASA in maintenance of remission
Seventy percent of patients with ulcerative colitis given no treatment can expect to experience a relapse over a 12-month period. As early as 1965 it was shown that sulfasalazine is effective in maintenance therapy in ulcerative colitis (Misiewicz et al 1965), and newer generation 5-ASAs including olsalazine and balsalazine have shown relapse prevention properties virtually the same as, but not greater than, those of equivalent doses of sulfasalazine (Kornbluth and Sachar 2004). A Cochrane Database analysis of 2,124 patients (Sutherland and MacDonald 2006) showed 5-ASA to be superior to placebo with regard to all measured outcome variables. Dose response trend for 5-ASA was again confirmed, and the odds ratio of 5-ASA compared to sulfasalazine was 0.83 (95% CI 0.60 to 1.13) for failure to induce global/clinical improvement or remission, and 0.66 (95% CI 0.42 to 1.04) for the failure to induce endoscopic improvement (Sutherland and Macdonald 2003). There was no statistical difference in maintenance of remission between 2.4 g/d compared to 1.2 g/d after one year (30). Although the increased dose did not appear to reduce the incidence of relapse of UC over a period of 1 year, the authors report that the increase dose was beneficial in overall delaying relapses. Most comparison studies of mesalamine have shown increased efficacy in remission with higher doses up to 4 g per day of aminosalicylates (Kornbluth and Sachar 2004).
Left sided ulcerative colitis/proctitis
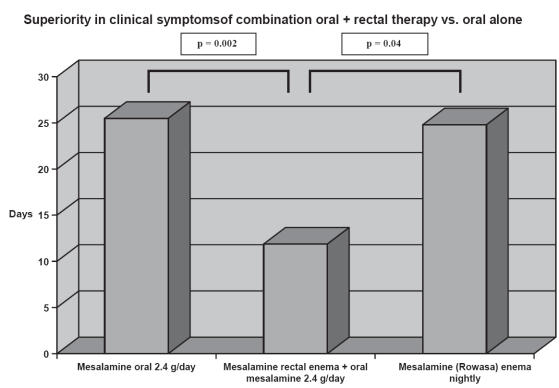
There is overwhelming data to support the use of topical formulations of 5-ASA in distal left sided colitis. The question arises whether topical therapy alone is efficacious or if topical therapy combined with oral mesalamine would produce higher response rates. A study to compare the efficacy of mesalamine rectal suspension enema, Rowasa (Solvay Pahramceuticals, Inc., Marietta, GA, USA), alone, oral mesalamine (Asacol) alone, and the combination of enema and tablet in patients with distal UC found that the combination of oral and rectal mesalamine therapy was well tolerated and produced earlier and more complete relief of rectal bleeding than oral or rectal therapy alone (Safdi et al 1997) (see Figure 5). However, it has not been clearly defined whether this is a dose response effect or independently a benefit of topical therapy.
Figure 5.
The combination of oral and rectal mesalamine therapy produced earlier and more complete relief of rectal bleeding than oral and rectal therapy alone. Pairwise analysis revealed that combination therapy resulted in significantly fewer days to cessation of rectal bleeding compared with either the mesalamine enema (p = 0.04 generalized Wilcoxon test) or mesalamine tablet group (p = 0.002, generalized Wilcoxon test) alone. Adapted from data: Safdi et al (1997).
Beclamethasone enemas in combination with 5-ASAs as well have favorable response in those patients who have failed treatment with oral 5-ASAs alone (D’Arienzo et al 1998). However, a comprehensive meta-analysis performed of 67 studies reported that the therapeutic approach in left-sided ulcerative colitis and proctitis was dose dependent and topical mesalamine was superior to oral therapies and topical steroids (Cohen et al 2000). The meta-analysis also found topical mesalamine was superior in achieving remission compared to topical steroids with overall decreased patient costs.
In the setting of left-sided distal colitis (proctitis), topical (rectal) formulations were found to be superior to oral aminosalicylates at inducing remission (Cohen et al 2000) and the therapeutic effect of mesalamine was strongly correlated with its mucosal concentration (Frieri et al 2005). In patients with high relapse rate (as measured by at least four moderate to severe relapses in the preceding 2 years), the continuous use of topical mesalamine (4 g/day) with high oral dosage (3.2 g–4.8 g/day) significantly decreased the total number of recurrences by increasing the mucosal concentration (Cohen et al 2000). A meta-analysis of five controlled trials comparing rectal mesalamine with placebo for distal colitis showed superiority of mesalamine over placebo (Marshall and Irvine 1995). Slow release 1 g mesalamine suppositories used three times per week was compared with placebo in maintaining remission in patients with proctitis and the data reveled that slow release suppositories were also effective for preventing relaspses in ulcerative proctitis (Marteau et al 1998).
Adverse effects of 5-ASAs
5-aminosalicylates have been shown to be in safe in short term use (Loftus et al 2003) with a dose-response efficacy without dose-related toxicity. There is sufficient data to demonstrate long-term safety with mesalamine at doses of up to 5 g daily (Cunliffe and Scott 2002). In clinical trials of active ulcerative colitis comparing mesalamine with placebo, the fraction of patients with adverse events ranged from 13% to 73% with mesalamine vs. 22% to 61% with placebo (Loftus et al 2003). The most commonly reported adverse events with the 5-ASA formulations include headache, GI symptoms such as diarrhea, bloating, nausea. Other rare side effects include interstitial nephritis, hepatitis, pericarditis, pancreatitis, pneumonitis, dermatitis, myocarditis, and hematological disturbances (Ransford and Langman 2002) (Table 3). On occasion, drug holidays may be initiated until diarrheal symptoms resolve. Nephrotoxicity can be seen with any of the 5-ASA compounds. Considering that IBD affects younger population, it is reasonable to also evaluate its safety profile in pregnancy. There has been no evidence of teratogenic effect or fetal toxicity with mesalamine (Diav-Citran et al 1998), placing it into a FDA category B for pregnancy.
Table 3.
Side effects of mesalamine
| Common | Rare |
|---|---|
| Headache | Interstitial nephritis |
| Diarrhea | Hepatitis |
| Bloating | Pericarditis |
| Nausea | Pancreatitis |
| Hypersensitivity | Pneumoninitis |
| Dermatitis | |
| Myocarditis | |
| Hematological disturbances | |
| Alopecia |
A prospective controlled cohort study of pregnancy exposure to mesalamine in women with IBD was performed. Although the mean daily dose used by the cohort was 2.4 g/d, lower than customarily used for active treatment, no major teratogenic effects were observed. Marteau et al (2004) establishing guideline for treatment in pregnancy recommended choosing alternative treatments or monitoring echogenecity of the fetal kidney when requiring mesalamine at doses greater than 3 g/day. This is in part due to case report of severe fetal nephropathy during renal morphogenesis (Colombel et al 1994). It is important to note that compared to a matched control group with nonteratogenic exposure, the cohort did have a higher incidence of preterm deliveries, decrease in mean maternal weight gain, and a decrease in mean birth weight. However this can be attributed to ongoing disease activity of UC as well (Diav – Citran et al 1998). No increase in these complications were noted when patients were in clinical remission during pregnancy.
5-ASAs in cancer prevention
Besides its therapeutic role in ulcerative colitis, 5-ASAs have been observed to have properties of chemoprevention for colon cancer. Since colorectal cancer has a higher incidence in patients with inflammatory bowel disease than the general population, there has been great interest in determining whether antinflammatory drugs used to treat IBD have an effect on reducing colorectal cancer rates (Bernstein et al 2001). Two prospective studies have reported favorable effects of 5-ASA therapy on surrogate markers of colorectal cancer, such as rectal cell proliferation and apoptotic index (Bus et al 1999; Reinacher-Schick et al 2000). Although there have been no randomized trials, pooled results of observational studies support a protective association for 5-ASAs and colorectal cancer (Velayos et al 2005). Van Staa et al (2005), using a study population of 18,969 patients from the General Practice Research Database in the UK, in a case control analysis, found that regular use of 5-ASA (1.6 g/d or higher) was associated with reduction in the risk of colorectal cancer in ulcerative colitis. They were also able to reproduce previous data which supports that mesalamine was associated with greater reduction in cancer risk compared to sulfasalazine. Rubin et al (2006) in a case control study of 26 case reported 5-ASAs use of 1.2 g/d or more was associated with a 72% reduction in the odds of dysplasia and colorectal cancer (odds ratio 0.28 95% CI 0.09–0.085) and as the total dose of aminosalicylates increased, the odds of dysplasia and cancer decreased (p = 0.056).
Conclusion
Aminosalicylate therapy remains the foundation for treating colitis and maintaining remission in mild to moderate ulcerative colitis. Over the years new formulations have been developed to make this product more tolerable and efficacious. Mesalamine, a 5-ASA is the active moiety of the precursor drug sulfasalazine, is significantly more tolerable than its predecessor. Considering that the efficacy of 5-ASA is dose dependent, 4.8 g/day and 2.4 g/day have been shown to be the optimal dosages for active disease and for maintenance therapy, respectively. As for left-sided distal disease, topical (rectal) formulation is superior in inducing remission.
Mesalamine is an excellent first-line therapy for a step-up approach in treating mild to moderate ulcerative colitis and for maintenance of remission. Furthermore, different modes of delivery have also been developed to enhance the therapeutic efficacy of these products. Overall treatment decisions should be based on the severity and extent of disease. A factor which will influence success of therapy is maximizing mucosal concentration of therapy by localizing the area of involvement and therefore utilizing the most appropriate delivery formulation. Compliance being a restrictive factor in treatment success, new formulations have been developed which will require less frequent dosing. Considering that UC is a chronic remitting active inflammatory condition, patients will most likely need lifelong therapy.
References
- Ardizzone S, Maconi G, Russo A, et al. Randomised controlled trial of azathioprine and 5-aminosalicylic acid for treatment of steroid dependent ulcerative colits. Gut. 2005;55:47–53. doi: 10.1136/gut.2005.068809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein CN, Blanchard JF, Kliewer, et al. Cancer risk in patients with inflammatory bowel disease: population based study. Cancer. 2001;91:854–62. doi: 10.1002/1097-0142(20010215)91:4<854::aid-cncr1073>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Bernstein CN, Rawsthorne P, Cheang M, et al. A population-based case control study of potential risk factors for IBD. Am J Gastroenterol. 2006;101:993–1002. doi: 10.1111/j.1572-0241.2006.00381.x. [DOI] [PubMed] [Google Scholar]
- Bonen DK, Cho JH. The genetics of inflammatory bowel disease. Gastroenterology. 2003;124:521–36. doi: 10.1053/gast.2003.50045. [DOI] [PubMed] [Google Scholar]
- Bus PJ, Nagtegaal ID, Verspaget, et al. Mesalazine-induced apoptosis of colorectal cancer: on the verge of a new chemopreventive era? Aliment Pharmacol Ther. 1999;13:1397–402. doi: 10.1046/j.1365-2036.1999.00652.x. [DOI] [PubMed] [Google Scholar]
- Cohen RD. Review article: evolutionary advances in the delivery of aminosalicylates for the treatment of ulcerative colitis. Aliment Pharmacol Ther. 2006;24:465–74. doi: 10.1111/j.1365-2036.2006.03010.x. [DOI] [PubMed] [Google Scholar]
- Cohen RD, Woseth DM, Thisted RA, et al. A Meta-analysis and overview of the Literature on Treatment Options for Left-Sided Ulcerative Colitis and Ulcerative Proctitis. Am J Gastroenterol. 2000;95:1263–76. doi: 10.1111/j.1572-0241.2000.01940.x. [DOI] [PubMed] [Google Scholar]
- Colombel JF, Brabant G, Gublrt MC, et al. Renal Insufficiency in infant: Side effect of prenatal exposure to meslazine? Lancet. 1994;344:620–1. doi: 10.1016/s0140-6736(94)92009-5. [DOI] [PubMed] [Google Scholar]
- Cunliffe RN, Scott BB, et al. Monitoring for drug side-effects in inflammatory bowel disease. Aliment Pharmacol Ther. 2002;16:647–62. doi: 10.1046/j.1365-2036.2002.01216.x. [DOI] [PubMed] [Google Scholar]
- D’Arienzo A, Manguso F, Cimino L, et al. Beclomethasone dipropionate (3 mg) enemas combined with oral 5-ASAs (2.4 g) in the treatment of ulcerative colitis not responsive to oral 5-ASA alone. Ital J Gastroenterol Hepatol. 1998;30:254–7. [PubMed] [Google Scholar]
- Diav-Citran O, Park YW, Veerasuntharan G, et al. The safety of Mesalamine in Human Pregnancy: A Prospective Controlled Cohort Study. Gastroenterology. 1998;114:23–8. doi: 10.1016/s0016-5085(98)70628-6. [DOI] [PubMed] [Google Scholar]
- Egan LJ, Mays DC, Huntoon CJ, et al. Inhibition of interleukin-1-stimulated NF-kappaB RelA/p65 phosphorylation by mesalamine is accompanied by decreased transcriptional activity. J Biol Chem. 1999;274:448–53. doi: 10.1074/jbc.274.37.26448. [DOI] [PubMed] [Google Scholar]
- Fiocchi C. Inflammatory bowel disease: etiology and pathogenesis. Gastroenterology. 1998;115:182–205. doi: 10.1016/s0016-5085(98)70381-6. [DOI] [PubMed] [Google Scholar]
- Frieri G, Pimpo M, Galletti, et al. Long-term oral plus topical mesalazine in frequently relapsing ulcerative colitis. Digestive and Liver Disease. 2005;37:92–6. doi: 10.1016/j.dld.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Grisham MD. Oxidants and free radicals in inflammatory bowel disease. Lancet. 1994;344:859–61. doi: 10.1016/s0140-6736(94)92831-2. [DOI] [PubMed] [Google Scholar]
- Hanauer SB. Review article: aminosalicylates in inflammatory bowel ulcerative colitis. Aliments Pharmacol Ther. 2004;20(Suppl 4):60–5. doi: 10.1111/j.1365-2036.2004.02048.x. [DOI] [PubMed] [Google Scholar]
- Hanauer SB, Sandborn WJ, Kornbluth A, et al. Delayed-release oral mesalamine at 4.8 g/day (800 mg tablet) for the treatment of moderately active disease. Ascend II Trial. American Journal of Gastroenterology. 2005;100:2478–85. doi: 10.1111/j.1572-0241.2005.00248.x. [DOI] [PubMed] [Google Scholar]
- Kaiser GC, Yan F, Polk DB, et al. Mesalamine blocks tumor necrosis factor growth inhibition and nuclear factor kappaB activation in mouse colonocytes. Gastroenterology. 1999;116:602–9. doi: 10.1016/s0016-5085(99)70182-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane SV, Bjorkman DJ. The efficacy of oral 5-ASAs in the treatment of active ulcerative colitis: A Systematic Review. Reviews in Gastroenterological Disorders. 2003;3:210–18. [PubMed] [Google Scholar]
- Kornbluth A, Sachar D. Ulcerative Colitis Practice Guidelines in adults (Update): American College of Gastroenterology, Practice Parameters Committee. American Journal of Gastroenerology. 2004:1372–85. doi: 10.1111/j.1572-0241.2004.40036.x. [DOI] [PubMed] [Google Scholar]
- Loftus EV, Silverstein Sandborn WJ, et al. Ulcerative colitis in Olmsted County, Minnesota, 1940–1993: incidence, prevalence, and survivial. Gut. 2000;46:336–43. doi: 10.1136/gut.46.3.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loftus EV, Kane SV, Bjorkman D, et al. Systematic review: short-term adverse effects of 5-aminosalicylic acid in the treatment of ulcerative colitis. Aliment Pharmacol Ther. 2003;19:179–89. doi: 10.1111/j.0269-2813.2004.01827.x. [DOI] [PubMed] [Google Scholar]
- Mansfield JC, Giaffer MH, Cann PA, et al. A double-blind comparison of balsalazide, 6.75 g and sulfasalazine, 3 g as sole therapy in the management of ulcerative colitis. Aliment Pharmacol Ther. 2002;16:69–77. doi: 10.1046/j.1365-2036.2002.01151.x. [DOI] [PubMed] [Google Scholar]
- Marshall JK, Irvine EJ. Rectal aminosalicylate therapy for distal ulcerative colitis: a meta analysis. Aliment Pharmacol Ther. 1995;9:293–300. doi: 10.1111/j.1365-2036.1995.tb00384.x. [DOI] [PubMed] [Google Scholar]
- Marteau PH, Probert CS, Lindgren M, et al. Combined oral and enema treatment with Pentasa (mesalazine) is superior to oral therapy alone in patients with extensive mild/moderate active ulcerative colitis: a randomized, double blind placebo controlled study. Gut. 2005;54:960–5. doi: 10.1136/gut.2004.060103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marteau PH, Crand J, Foucault M, et al. Use of mesalazine slow release suppositories 1 gm three times per week to maintain remission of ulcerative proctitis: a randomized double blind placebo controlled multicentre study. Gut. 1998;42:195–9. doi: 10.1136/gut.42.2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marteau PH, Seksik, Beaugerie, et al. Recommandations pour la pratique clinique pour le traitment de la rectocolite hemorragique. Gastroenterol Clin Biol. 2004;28:955–60. doi: 10.1016/s0399-8320(04)95172-x. [DOI] [PubMed] [Google Scholar]
- Misiewicz JJ, Lennard, Jones JE, et al. Controlled trial of sulphasalazine in maintenance therapy for ulcerative colitis. Lancet. 1965;1:185–8. [Google Scholar]
- Orholm M, Munkholm P, Langholz E, et al. Familial occurrence of inflammatory bowel disease. N Eng J Med. 1991;324:84–8. doi: 10.1056/NEJM199101103240203. [DOI] [PubMed] [Google Scholar]
- Paoluzi OA, Iacopini F, Pica P, et al. Comparison of two different daily dosages (2.4 vs 1.2 g) of oral mesalazine in maintenance of remission in ulcerative colitis patients: 1-year follow-up study. Aliment Pharmacol Ther. 2005;21:1111–9. doi: 10.1111/j.1365-2036.2005.02458.x. [DOI] [PubMed] [Google Scholar]
- Podolosky DK. Inflammatory Bowel Disease. N Eng J Med. 2002;347:417–29. doi: 10.1056/NEJMra020831. [DOI] [PubMed] [Google Scholar]
- Powell-Tuck J, Truelove SC. The course and prognosis of ulcerative colitis. Gut. 1963;4:299–315. doi: 10.1136/gut.4.4.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powrie F. T-cells in inflammatory bowel disease: protective and pathogenic roles. Immunity. 1995;3:171–4. doi: 10.1016/1074-7613(95)90086-1. [DOI] [PubMed] [Google Scholar]
- Prantera C, Viscido A, Biancone L, et al. A new oral delivery system for 5-ASA: preliminary clinical findings for MMX. Inflamm Bowel Dis. 2005;11:421–7. doi: 10.1097/01.mib.0000158386.25660.1e. [DOI] [PubMed] [Google Scholar]
- Pruitt R, Hanson J, Safdi M, et al. Balsalazide is superior to mesalamine in time to improvement of signs and symptoms of acute mild-to-moderate ulcerative colitis. Am J Gastroenterol. 2002;97:3078–86. doi: 10.1111/j.1572-0241.2002.07103.x. [DOI] [PubMed] [Google Scholar]
- Ransford RA, Langman MJ, et al. Sulphasalazine and mesalazine: serious adverse reactions re-evaluated on the basis of suspected adverse reaction reports to the Committee on Safety of Medicines. Gut. 2002;51:536–9. doi: 10.1136/gut.51.4.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochester J, Abreu MT. Ulcerative Colitis Therapy: Importance of Delivery Mechanisms. Reviews in Gastroenterological Disorders. 2005;5:215–22. [PubMed] [Google Scholar]
- Reinacher-Schick A, Seidensticker F, Petrash S, et al. Mesalazine changes apoptosis and proliferation in normal mucosa of patients with sporadic polyps of the large bowel. Endoscopy. 2000;32:245–54. doi: 10.1055/s-2000-135. [DOI] [PubMed] [Google Scholar]
- Rubin DT, LoSavio A, Yardon N, et al. Aminosalicylate Therapy in the Prevention of Dysplasia and Colorectal Cancer in Ulcerative Colitis. Clinical Gastroenterology and Hepatology. 2006;4:1346–50. doi: 10.1016/j.cgh.2006.08.014. [DOI] [PubMed] [Google Scholar]
- Safdi M, DeMicco M, Sninsky CA, et al. A double-blind comparison of oral versus rectal mesalamine combination therapy in treatment of distal ulcerative colitis. Am J Gastroenterol. 1997;92:1867–71. [PubMed] [Google Scholar]
- Schroeder KW, Tremaine WJ, Ilstrup, et al. Coated oral 5-aminosalicylic acid therapy for mild to moderately active ulcerative colitis. N Engl J Med. 1987;17:1625–9. doi: 10.1056/NEJM198712243172603. [DOI] [PubMed] [Google Scholar]
- Shanahan F. Inflammatory bowel disease: immunodiagnostics, immunotherapeutics, and ecotherapeutics. Gastroenterology. 2001;120:622–35. doi: 10.1053/gast.2001.22122. [DOI] [PubMed] [Google Scholar]
- Sninsky CA, Cort DH, Shanahan F, et al. Oral mesalamine (Asacol) for mildly to moderately active ulcerative colitis. A multicenter trial. Ann Intern Med. 1991;115:350–5. doi: 10.7326/0003-4819-115-5-350. [DOI] [PubMed] [Google Scholar]
- Stenson WF, Korzenik W. Inflammatory Bowel Disease. In: Yamata T, editor. Textbook of Gastroenterology. 4. Lippincott Williams and Wilkins; 2003. pp. 1699–759. [Google Scholar]
- Strober W, Ludviksson BR, Fuss IJ, et al. The pathogenesis of mucosal inflammation in murine models of inflammatory bowel disease and Crohn’s disease. Ann Intern Med. 1998;128:848–56. doi: 10.7326/0003-4819-128-10-199805150-00009. [DOI] [PubMed] [Google Scholar]
- Sutherland L, Macdonald JK. Oral 5-aminosalicylates for induction of remission in ulcerative colitis. Cochrane Database. 2006 doi: 10.1002/14651858.CD000543.pub2. [DOI] [PubMed] [Google Scholar]
- van Staa TP, Card T, Logan RF, et al. 5-Aminosalicylate use and colorectal cancer risk in inflammatory bowel disease: a large epidemiological study. Gut. 2005;54:1573–8. doi: 10.1136/gut.2005.070896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velayos FS, Terdiman JP, Walsh JM, et al. Effect of 5-aminosalicylastes use on colorectal cancer and dysplasia risk: a systematic review and meta-analysis of observational studies. Am J Gastroenterol. 2005;100:1345–53. doi: 10.1111/j.1572-0241.2005.41442.x. [DOI] [PubMed] [Google Scholar]