Abstract
Among adults in Western countries, chronic lymphocytic leukemia (CLL) is the most prevalent form of leukemia. CLL primarily affects the elderly and may be associated with multiple comorbidities. A cure has not been identified, and new treatment options are needed. Expression of Bcl-2 protein is associated with the pathogenesis of CLL and chemotherapy resistance. Oblimersen, a Bcl-2 antisense phosphorothioate oligonucleotide, is being evaluated in patients with CLL and other cancers; trials through Phase III have been completed. In the setting of relapsed/refractory CLL, single-agent oblimersen demonstrates modest activity, whereas the addition of oblimersen to fludarabine/cyclophosphamide significantly improves the rate of complete and nodular partial responses; moreover, these responses are durable and associated with clinical benefit. Oblimersen is more efficacious in relapsed rather than refractory patients. The side effect profile of oblimersen, alone or in combination with standard chemotherapy, is favorable compared with currently available chemotherapies. In the first cycle, an infusion reaction with or without tumor lysis syndrome is uncommon, and transient thrombocytopenia is observed. Catheter-related complications are associated with the need for continuous intravenous infusion of oblimersen over several days; other routes of administration are under clinical investigation. Oblimersen is a promising therapeutic approach for patients with relapsed CLL and should be further evaluated in the front-line setting.
Keywords: chronic lymphocytic leukemia, Bcl-2 antisense, G3139, Genasense®, oblimersen
Introduction
Among adults in Western countries, chronic lymphocytic leukemia (CLL) is the most commonly diagnosed form of leukemia and the most prevalent form of leukemia. More than 15,000 new cases will be reported in 2007 in the United States alone (Jemal et al 2007), primarily in middle-aged and elderly adults.
This malignancy is characterized by the progressive accumulation of small monoclonal B-cells in the blood, bone marrow, lymph nodes, and spleen with resultant organ enlargement. CLL may remain indolent for prolonged periods until patients require treatment for associated complications – primarily recurrent infections, anemia, thrombocytopenia, progressive lymphadenopathy or hepatosplenomegaly, and disease-related symptoms, such as fever, fatigue, and night sweats. The most frequent cause of death is recurrent infection due to the immunodeficiency associated with this disease (including hypogammaglobulinemia and impaired complement activation) and the immunodeficiency induced by therapies administered for this disease.
In the front-line setting, patients are typically treated with single-agent fludarabine or a fludarabine-based regimen. In fact, purine nucleosides such as fludarabine have replaced single-agent alkylating drugs as the treatment of choice for previously untreated patients and produce significantly higher response rates than such combination therapies as CAP or CHOP (Rai et al 2000; Leporrier et al 2001). Recent randomized studies have demonstrated that the combination of an alkylating agent and fludarabine significantly increases the rate of complete response (Flinn et al 2004; Eichhorst et al 2006); however, such regimens offer no demonstrable improvement in survival and are associated with increased myelosuppression when compared with single-agent fludarabine. With the availability of the monoclonal antibody rituximab, combination regimens such as fludarabine and rituximab with or without cyclophosphamide are becoming the standard of care for previously untreated patients with CLL.
Patients who relapse after initial therapy are typically treated again with fludarabine, administered either alone or in combination with other agents, including alkylating agents such as cyclophosphamide, monoclonal antibodies such as alemtuzumab or rituximab, and anthracyclines. Alemtuzumab, however, is increasingly being used in a setting of minimal residual disease (O’Brien et al 2003; Wendtner et al 2004; Moreton et al 2005).
Despite improvements in CLL therapy in the last 15 years with the introduction of fludarabine and rituximab, improvement in survival has not been prospectively confirmed (Byrd et al 2005). Treatment is often palliative given the toxicity of currently available therapies and the multiple comorbid conditions that may affect elderly patients with CLL.
Nonclinical pharmacology
Like other cancers, CLL is characterized, in part, by the failure of apoptotic (programmed cell-death) processes (Johnstone et al 2002; Reed 2006). Several molecules that block cell death have been identified, and some have been targeted for pharmacologic intervention. One such group of molecules is the Bcl-2 family, which includes 6 antiapoptotic proteins (Reed 2006). Overexpression of one or more of these antiapoptotic proteins, the most recognized of which is Bcl-2, essentially acts as a survival factor for cancer cells and is associated with resistance to chemotherapy in a number of hematologic cancers and solid tumors (Reed et al 1994; Klasa et al 2002; Reed 2006).
The BCL2 gene was originally described in association with the t(14;18) chromosomal abnormality identified in B-cell lymphoma, and the resultant Bcl-2 protein was found to inhibit cell death (Tsujimoto and Shimizu 2000). Data suggest that Bcl-2 negatively regulates the size or voltage potential of a mitochondrial-transmembrane pore by interacting with the pro-apoptotic protein Bax (Cheng et al 2001; Tsujimoto and Shimizu 2002). A Bcl-2/Bax-regulated pore is functionally similar to a switch that is either open or closed. In the open configuration, cytochrome C and calcium are released into the cytoplasm, thus initiating caspase activation and irreversibly committing the cell to death (Basañez et al 1999; Wang 2001; Kokoszka et al 2004; Sharpe et al 2004). By closing the pores through which cytochrome C is released, Bcl-2 negatively regulates apoptosis. Thus, the Bcl-2 protein is a fundamental contributor to both inherent and acquired resistance of cancer cells to chemotherapy (Johnstone et al 2002).
Expression of the Bcl-2 protein has been documented in virtually all patients with CLL (Schena et al 1992; Hanada et al 1993; Robertson et al 1996; Zaja et al 1998; Aviram et al 2000; Cheng et al 2001; Wang 2001; Klobusicka et al 2002; Menendez et al 2004; Tracey et al 2005). It is widely recognized that Bcl-2 upregulation plays a critical role in CLL and is associated with the pathogenesis of this malignancy (Schena et al 1992; Hanada et al 1993; Robertson et al 1996; Faderl et al 2002). The most frequent chromosomal deletion identified by fluorescence in situ hybridization in patients with CLL occurs in chromosome 13q14 (Döhner et al 2000). 13q14 deletions eliminate micro RNA (miRNA) regulators of Bcl-2 expression, resulting in enhanced Bcl-2 expression. Notably, re-introduction of miRNAs in a lymphoid cell line was observed to downregulate Bcl-2 and induce apoptosis (Cimmino et al 2005; Calin and Croce 2006).
Antisense DNA oligonucleotides that bind RNA can target specific proteins, induce enzymatic cleavage of the message, and thereby prevent protein translation (Curcio et al 1997). Oblimersen (Genasense®, Bcl-2 antisense, G3139) is a synthetic, 18-base, single-stranded phosphorothioate DNA oligonucleotide designed to downregulate bcl-2 mRNA expression (Klasa et al 2002). Oblimersen selectively hybridizes to the first 6 codons of the open reading frame that encodes the Bcl-2 protein. Formation of the RNA/antisense DNA duplex recruits RNase H, which degrades the RNA strand and releases the antisense DNA strand, allowing the drug to bind and cleave additional bcl-2RNA molecules, thus preventing translation of the Bcl-2 protein.
Multiple nonclinical models demonstrate the effects of oblimersen, including reduced Bcl-2 levels, reduced cell viability, increased activity of apoptotic mechanisms, reduction in tumor size, and enhanced activity of cytotoxic anticancer drugs (Klasa et al 2002). These findings have been documented following exposure to oblimersen alone and to oblimersen in combination with other therapeutic agents or modalities, including chemotherapy and radiotherapy, in tumor types that include B-cell neoplasias, myeloid leukemia, and many solid tumors (Gazitt et al 2001; Klasa et al 2002). A summary of findings in several nonclinical models of hematologic malignancies, including CLL, is provided in Table 1. Overall, given the prevalence of Bcl-2 overexpression in diverse cancers, nonclinical findings suggest that oblimersen may function in tumors from a broad range of genetic backgrounds. This observation underscores the importance of apoptosis as the mechanism of cell death induced by many anticancer therapies (Reed 2006).
Table 1.
Effects of oblimersen in nonclinical models of hematologic malignancies
| Malignancy | Tissues/species Methodology | Key finding(s) |
|---|---|---|
| Leukemia | Human CLL cells In vitro; exposure to oblimersen 2 μM for 72 hours; controls: fludarabine, dexamethasone (Auer et al 2001) Human CLL cells In vitro; exposure to oblimersen 5 μM for 24 hours; controls: G4126 (2-base mismatch oligonucleotide that [unlike oblimersen] lacks CpG motifs) (Baird et al 2001) |
|
| Human AML cells (Chiu et al 2006) |
|
|
| Lymphoma | Human diffuse large B-cell and transformed B-cell lymphoma cells in severe combined immune deficiency (SCID) beige mice In vitro; at least 72-hour exposure to oblimersen, bortezomib, and cyclophosphamide at varying doses and in various schedules alone and in combination In vivo; exposure to varying doses and various schedules of oblimersen, bortezomib, and cyclophosphamide alone and in combination (O’Connor et al 2006) Sweig lymphoblastoid cell line (LCL) in SCID mice Oblimersen 10 mg/kg × 5 with and without rituximab every 3 days for 12 days intraperitoneally (Loomis et al 2003) DoHH2 cells in SCID mice Oblimersen intraperitoneally 5.0 or 12.5 mg/kg/d with or without cyclophosphamide for 14 days (Klasa et al 2000) |
|
| DoHH2 human non-Hodgkin’s lymphoma cells in SCID mice and non-obese diabetic (NOD) SCID mice Oblimersen 14-day subcutaneous infusion (dose/concentration not specified) (Waters et al 2000) |
|
|
| Multiple myeloma | ARH-77, U266, HS-Sultan, 8226, and ARP-1 human myeloma cells with varying levels of Bcl-2 expression In vitro; exposure to oblimersen 0.2–2 μM for 1 to 4 days (Gazitt et al 2001) |
|
Abbreviations: CLL, chronic lymphocytic leukemia; LCL, lymphoblastoid cell line; NOD, non-obese diabetic; SCID, severe combined immune deficiency.
Clinical pharmacology
Pharmacokinetics
The pharmacokinetics of oblimersen have previously been reported (Demidov et al 2003; O’Brien et al 2005; Tolcher et al 2005; Rheingold et al 2007) and are consistent with those reported for other phosphorothioate oligonucleotides.
Oblimersen is rapidly metabolized, primarily by exonuclease-mediated cleavage that sequentially removes single nucleotides from the parent oligonucleotide, yielding a mononucleotide metabolite and the parent molecule shortened by 1 nucleotide (eg, N-1 [a 17-mer] and N-2 [a 16-mer]) (data on file, Genta Incorporated). The primary metabolites (N-1 and N-2) have been measured in pharmacokinetic studies and have been shown to be biologically active. In some published studies, pharmacokinetic parameters reported for oblimersen were based on the sum of oblimersen and its metabolites because the analytical methods used did not resolve the parent molecule from the chain-shortened metabolites.
Steady-state plasma concentrations of oblimersen and the N-1 and N-2 metabolites are rapidly achieved (within 6–10 hours) after initiation of Genasense administration by continuous intravenous infusion (data on file, Genta Incorporated). Exposure (based upon Css) to the N-1 and N-2 metabolites is approximately 38%–50% and 13%–42%, respectively, of the exposure to oblimersen over a dose range of 3–7 mg/kg/d (Demidov et al 2003; O’Brien et al 2005). At doses ranging from 3 to 7 mg/kg/d, steady-state plasma concentrations and oblimersen exposure increase with increasing doses, appear consistent with linear pharmacokinetics, and are not dependent on gender or age (Sekar et al 2005).
Oblimersen is rapidly cleared from the plasma after its administration (t½ ~ 2.4 hours) (O’Brien et al 2005; data on file, Genta Incorporated). Renal clearance of intact oblimersen represents only a small portion of the total clearance (O’Brien et al 2005; data on file, Genta Incorporated), as reported for other phosphorothioate oligonucleotides (Cossum et al 1993, 1994; Crooke et al 1994). Its rapid clearance from the plasma is consistent with data published on other phosphorothioate oligonucleotides and reflects rapid and wide distribution into tissues, as demonstrated preclinically (Raynaud et al 1997).
The pharmacokinetics of oblimersen are currently being studied in patients with hepatic impairment and patients with renal impairment. Preliminary data from 1 patient with moderately impaired hepatic function (ie, Child-Pugh Classification B) enrolled to date indicated no change in oblimersen clearance or metabolism, compared with findings in 2 patients with normal hepatic function (data on file, Genta Incorporated). Preliminary data from the study in patients with renal impairment are not yet available.
In an ongoing study of oblimersen administration by subcutaneous injection, administration of 225 mg to patients with solid tumors resulted in peak plasma levels of approximately 3 μg/mL, similar to steady-state levels achieved with administration of oblimersen 7 mg/kg/d by continuous intravenous infusion (data on file, Genta Incorporated).
Pharmacodynamics
Uptake of oblimersen into patient cells and downregulation of bcl-2 mRNA with a decrease in Bcl-2 protein has been demonstrated in clinical studies. Marcucci et al assessed the pharmacodynamics of oblimersen in patients with relapsed or refractory acute myeloid leukemia (Marcucci et al 2003). Patients received oblimersen as a 10-day continuous infusion, fludarabine and cytarabine starting on Day 6, and G-CSF starting on Day 5. Bone marrow aspirates from 12 patients were assessed for bcl-2 mRNA, of which 9 (75%) showed downregulation. Retained samples of bone marrow and peripheral blood mononuclear cells from 8 patients were subsequently assayed for intracellular oblimersen drug levels (Dai et al 2005). Significant cellular uptake of oblimersen was observed in mononuclear cells from both bone marrow and blood. Among the 8 patients, downregulation of bcl-2 mRNA in bone marrow was observed in 4 patients; bcl-2 mRNA was unchanged in 2 patients and increased in 2 patients.
These same investigators assessed the relationship between clinical response, plasma and intracellular oblimersen levels, and changes in bcl-2 mRNA and Bcl-2 protein levels in bone marrow mononuclear cells in 22 elderly patients treated with oblimersen and chemotherapy for previously untreated acute myeloid leukemia (Marcucci et al 2005). Patients who achieved a complete response showed decreases in median bcl-2 mRNA and protein levels relative to baseline. In contrast, nonresponding patients showed upregulation of bcl-2mRNA and no change in Bcl-2 protein levels.
Reduction of Bcl-2 protein levels in peripheral blood mononuclear cells was observed in pediatric patients with solid tumors after administration of oblimersen at doses of 3, 5, and 7 mg/kg/d (Rheingold et al 2007). Bcl-2 protein levels were reduced by Day 5 in 9 of 15 patients, including 6 of 9 patients treated with oblimersen 3 mg/kg/d, as shown in Figure 1.
Figure 1.
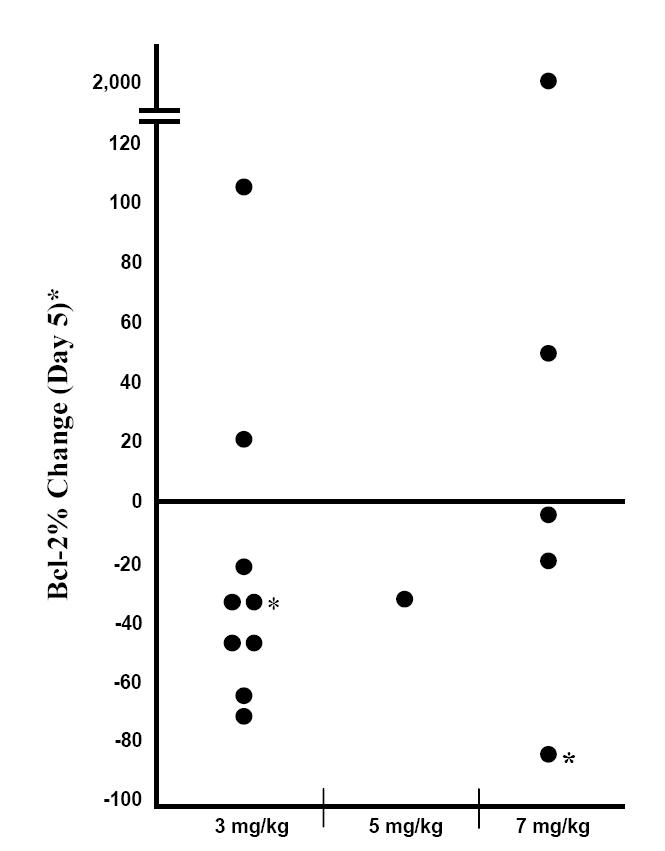
Bcl-2 response in peripheral blood mononuclear cells from pediatric patients with solid tumors by oblimersen dose: change from baseline (Day 0) on Day 5 (No Day 5 sample for 2 patients; response on Day 6 shown. Copyright © 2007. Reprinted with permission from the American Society of Clinical Oncology from Rheingold SR, Hogarty MD, Blaney SM, et al. 2007. A Phase I trial of G3139, a bcl-2 antisense oligonucleotide, combined with doxorubicin and cyclophosphamide in children with relapsed solid tumors: A Children’s Oncology Group study. J Clin Oncol, 25 1512–18.
Clinical studies
Oblimersen is undergoing clinical evaluation in a number of hematologic malignancies (eg, acute myeloid leukemia, CLL, multiple myeloma, and various lymphomas) and solid tumors (eg, advanced melanoma, breast cancer, gastrointestinal stromal tumors, nonsmall cell lung cancer, and prostate cancer). To date, 3 studies have been conducted with oblimersen in CLL (see Table 2), including administration as a single agent in a Phase I-II study (O’Brien et al 2005), in combination with fludarabine and rituximab in a Phase II study (Mavromatis et al 2005, 2006), and in combination with fludarabine and cyclophosphamide in a Phase III study (Rai et al 2005; O’Brien et al 2007). A total of 179 patients enrolled across the 3 studies have received oblimersen alone or in combination with chemotherapy. In each of the 3 studies, response was assessed according to the National Cancer Institute Working Group (NCI-WG) Guidelines (Cheson et al 1996).
Table 2.
Overview of clinical studies conducted in CLL
| Design | Oblimersen regimen and accompanying chemotherapy, if applicable Length of cycle; maximum number of cycles |
|---|---|
| Relapsed or refractory patients: Phase I/II multicenter study with dose finding/dose escalation in Phase I (O’Brien et al 2005) | Phase I: Oblimersen 3, 4, 5, and 7 mg/kg/d by continuous intravenous infusion for 5 days in Cycle 1 and for 7 days in cycles subsequent to Cycle 1 Phase II: Oblimersen 3 mg/kg/d by continuous intravenous infusion for 5 days in Cycle 1 and for 7 days in cycles subsequent to Cycle 1 21-day cycle; maximum of 12 cycles for responding patients; maximum of 6 cycles for all other patients |
| Previously treated and untreated patients: Phase II, multicenter (Mavromatis et al 2005, 2006) |
Part I: Cycle 1, oblimersen 1.5 mg/kg/d by continuous intravenous infusion for 7 days; rituximab 125 mg/m2by intravenous infusion on Day 4 and 250 mg/m2by intravenous infusion on Day 6; fludarabine 25 mg/m2by intravenous infusion on Days 6 through 8; Cycles 2–6, oblimersen 3 mg/kg/d by continuous intravenous infusion for 7 days; rituximab 375 mg/m2 by intravenous infusion on Day 5 followed by fludarabine 25 mg/m2 by intravenous infusion on Day 5; fludarabine 25 mg/m2by intravenous infusion on Days 6 and 7 Part II: All cycles, oblimersen 3 mg/kg/d by continuous intravenous infusion for 7 days; rituximab 375 mg/m2 by intravenous infusion on Day 5 followed by fludarabine 25 mg/m2 by intravenous infusion on Day 5; fludarabine 25 mg/m2 by intravenous infusion on Days 6, 7, 8, and 9 28-day cycle; for previously untreated patients, maximum of 6 cycles; for previously treated patients, maximum of 4 cycles with additional 2 cycles permitted at investigator’s discretion |
| Relapsed or refractory patients: Phase III multinational, multicenter, randomized, controlled, open-label study (Rai et al 2005; O’Brien et al 2007) | Oblimersen 3 mg/kg/d by continuous intravenous infusion for 7 days and fludarabine 25 mg/m2/d by intravenous infusion followed by cyclophosphamide 250 mg/m2/d by intravenous infusion on Days 5, 6, and 7 28-day cycle for up to 6 cycles |
Abbreviation: Flu/Cy, fludarabine/cyclophosphamide.
Phase I-II studies
In the Phase I-II study, 40 patients with relapsed or refractory CLL were treated with single-agent oblimersen at doses of 3 to 7 mg/kg/d (see Table 2), including 14 patients in Phase I and 26 patients in Phase II (O’Brien et al 2005). All but 9 patients received at least 2 cycles of treatment; the 9 patients included 7 who discontinued due to an adverse event, 3 of whom had received oblimersen in Phase I at a dose of 4, 5, or 7 mg/kg/d (data on file, Genta Incorporated). The maximum tolerated dose determined in Phase I was 3 mg/kg/d: the oblimersen dose was limited by the occurrence of an infusion reaction with or without tumor lysis at doses greater than 3 mg/kg/d. This reaction typically occurred in the first 24 to 48 hours of oblimersen administration and included high spiking fevers, rigors, and hypotension. The reaction is attributed to extensive apoptosis of B-lymphocytes and the resultant release of large quantities of cytokines directly into the systemic circulation. It has not been observed in patients with solid tumors, who are typically treated with oblimersen doses greater than 3 mg/kg/d and in whom cell destruction occurs more slowly than in patients with circulating tumor-cell populations.
A partial response was documented in 2 of 26 (8%) evaluable patients (1 in Phase I and 1 in Phase II) after 2 cycles (O’Brien et al 2005). It is noteworthy that both patients had been previously treated with at least 4 prior chemotherapy regimens (O’Brien et al 2005), and one had a history of Richter’s transformation prior to entering the study (data on file, Genta Incorporated). One of the 2 patients had disease progression by Cycle 6 and 1, disease progression by Cycle 7. Of the remaining 24 patients, 13 had stable disease for at least 2 months and 11 had progressive disease by the end of the second cycle (O’Brien et al 2005).
Evidence of single-agent oblimersen activity also included a ≥50% reduction from baseline in splenomegaly in 7/17 patients (41%), a ≥50% reduction from baseline in lymph node size in 7/22 patients (32%), and a ≥50% reduction from baseline in absolute lymphocyte count in 11/22 patients (50%) who had an absolute lymphocyte count >5,000/μL at baseline (O’Brien et al 2005).
The adverse event profile of oblimersen in patients with CLL was well defined in this study. Most events were Grade 1 or Grade 2 (ie, mild or moderate) in severity (National Cancer Institute Common Toxicity Criteria [NCI-CTC], Version 2.0) (data on file, Genta Incorporated). The most common events were pyrexia (33% of patients); fatigue (30%); cough and hypotension (20% each); and anemia, thrombocytopenia, nausea, and night sweats (18% each) (O’Brien et al 2005; data on file, Genta Incorporated). The single dose-limiting toxicity was an infusion reaction with or without tumor lysis, which occurred at doses greater than 3 mg/kg/d. No patient died due to an adverse event related to oblimersen (data on file, Genta Incorporated).
Catheter-related complications occurred in 6 (15%) patients and included erythema, bruising, bleeding, pain, cellulitis, and phlebitis at the intravenous administration site (data on file, Genta Incorporated). All but one of these events were Grade 1 or Grade 2 in severity (NCI-CTC); the one exception was a severe occurrence of phlebitis. None of these events result in discontinuation of study treatment.
In the Phase II trial, 24 patients, including 5 who had not previously been treated and 19 who had been previously treated, received oblimersen in combination with rituximab and fludarabine (see Table 2) (Mavromatis et al 2005, 2006). Oblimersen treatment was initiated at a dose of 1.5 mg/kg/d in Cycle 1 and adjusted to 3 mg/kg/d in subsequent cycles. The median number of cycles administered was 6.
Responses (complete, nodular partial, or partial) were observed in all 5 (100%) previously untreated patients and 13 (68%) of 19 previously treated patients (see Figure 2) (Mavromatis et al 2006). A complete response was achieved in 1 previously untreated patient and 1 previously treated patient; both patients had a negative flow-cytometric response and a negative polyclonal response for IgH gene rearrangement.
Figure 2.
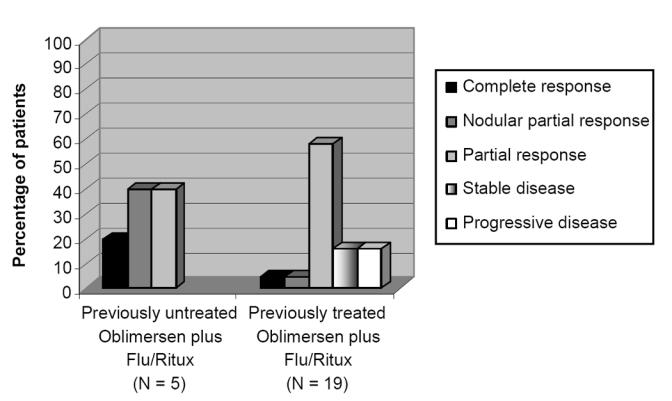
Overall response in previously untreated and previously treated patients with CLL: Phase II study.
Baseline characteristics among the responding patients included elevated CD38 expression in CLL cells in 3 of the 5 previously untreated patients and 6 of the 13 previously treated patients (Mavromatis et al 2006). Also among the 13 previously treated patients who responded, 5 patients had elevated Zap-70 (including 1 patient with a molecular CR [defined as absence of IgH gene rearrangement based on flow cytometry and polymerase chain reaction analysis]), 2 patients had poor-risk cytogenetics (11q deletion) at baseline, and 9 patients were ‘sensitive’ to the previously administered therapy (ie, they had achieved a response to prior therapy that had lasted for at least 6 months). These prior therapies included fludarabine and rituximab administered alone or in combination with other agents.
Adverse events tended to be Grade 1 or Grade 2 in severity (NCI-CTC) (data on file, Genta Incorporated). The most frequently reported complications among the 24 treated patients included rigors (58% of patients); neutropenia (54%); nausea (50%); fatigue and pyrexia (46% each); drug hypersensitivity (32%); thrombocytopenia (33%); anemia (29%); diarrhea, insomnia, and rash (25% each); and headache (21%). Many of these events were consistent with those reported in the Phase I-II study of single-agent oblimersen; the exceptions (eg, drug hypersensitivity, rash, diarrhea, insomnia) are events reported with one or both of the coadministered chemotherapy agents (fludarabine or rituximab). The drug hypersensitivity reactions were clearly associated with rituximab administration. No patient had an adverse event with an outcome of death (data on file, Genta Incorporated).
Catheter-related complications occurred in 5 (24%) patients (data on file, Genta Incorporated). These included swelling of the extremity secondary to the intravenous infusion, pain or soreness at the administration site, loss of line patency, and line occlusion. All of these events were Grade 1 or Grade 2 in severity (NCI-CTC), and none resulted in discontinuation of study treatment.
Of interest was the absence of tumor lysis syndrome and infusion reactions in the first treatment cycle, during which oblimersen was administered at the reduced dose of 1.5 mg/kg/d. The only infusion reaction occurred in Cycle 3 and was confirmed to be associated with rituximab administration (data on file, Genta Incorporated). The patient continued in the study, receiving oblimersen and fludarabine in the remaining 3 cycles without recurrence of this event.
Phase III study
In the Phase III study, 241 patients with relapsed or refractory CLL were stratified according to 3 criteria: responsive versus refractory to prior fludarabine therapy, number of prior regimens (1 or 2 versus ≥3), and duration of response to last prior therapy (>6 months versus ≤6 months) (Rai et al 2005; O’Brien et al 2007). Patients were centrally randomized to treatment with oblimersen, fludarabine, and cyclophosphamide (oblimersen plus Flu/Cy group, N = 120) or the fludarabine-cyclophosphamide regimen alone (Flu/Cy group, N =121; see Table 2).
Primary eligibility criteria included measurable and active disease (NCI-WG Guidelines; Cheson et al 1996); intermediate- or high-risk stage (modified Rai criteria; Cheson et al 1996); history of relapse from or no response to ≥1 prior chemotherapy regimen, at least one of which must have included 2 or more cycles of fludarabine; and ECOG performance status ≤2.
Response and disease progression were centrally determined (O’Brien et al 2007). Bone marrow specimens were assessed by an independent hematopathologist blinded to study treatment and clinical information, and clinical response and progression were determined by a CLL expert (Dr Kanti Rai) blinded to study treatment. For patients followed by CT/ultrasound from baseline (which is allowed but not required by NCI-WG Guidelines; Cheson et al 1996), these radiographic studies were required to have normalized before bone marrow biopsy was performed to confirm response.
The primary efficacy endpoint was response rate (complete response plus nodular partial response) in the intent-to-treat population, as determined by the blinded CLL expert. In determining sample size, it was assumed that the response rate would be 24% for the chemotherapy-only group versus 44% for the oblimersen plus Flu/Cy group. A minimum of 200 patients (100 per treatment arm) was needed to provide a power of 80% at an overall alpha of 0.05 (two-sided), accounting for the protocol-specified interim analysis using a Chi square test.
The 2 groups generally were well balanced at baseline (see Table 3) (O’Brien et al 2007). There was an approximate 1-year difference between treatment groups in the median time from initial diagnosis to randomization, with patients in the oblimersen plus Flu/Cy group having had CLL for a longer period than those in the Flu/Cy group. Approximately 75% of patients in both groups had abnormal radiographic findings at baseline.
Table 3.
Demographic and disease characteristics at baseline by treatment group: Phase III study, intent-to-treat population
| Characteristic | Oblimersen plus Flu/Cy (N = 120) |
Flu/Cy (N = 121) |
|---|---|---|
| Mean age, years | 62.5 | 62.0 |
| Median (minimum, maximum) | 62.5 (35, 86) | 63.0 (42, 82) |
| Age group, n (%) | ||
| <65 years | 67 (56) | 69 (57) |
| ≥65 years | 53 (44) | 52 (43) |
| ≥75 years | 17 (14) | 10 (8) |
| Gender, n (%) | ||
| Male | 89 (74) | 89 (74) |
| Female | 31 (26) | 32 (26) |
| Race, n (%) | ||
| White, not of Hispanic origin | 105 (88) | 109 (90) |
| Black, not of Hispanic origin | 6 (5) | 7 (6) |
| Hispanic | 8 (7) | 4 (3) |
| Other | 1 (1) | 1 (1) |
| Time from initial diagnosis to randomization, months | ||
| Median (minimum, maximum) | 70.2 (2.4, 383.8) | 58.1 (2.2, 249.6) |
| Modified Rai Stage, n (%) | ||
| 0–I | 29 (24) | 29 (24) |
| II–IV | 91 (76) | 91 (75) |
| Unknown | 0 (0) | 1 (1) |
| Lymphocyte immunophenotype, n (%) | ||
| CD5 | 120 (100) | 121 (100) |
| CD19 | 120 (100) | 119 (98) |
| CD20 | 101 (84) | 97 (80) |
| Number of prior regimens, n (%) | ||
| 1 or 2 | 60 (50) | 63 (52) |
| ≥3 | 60 (50) | 58 (48) |
Abbreviations: Flu/Cy, fludarabine/cyclophosphamide. (Copyright © 2007. Reprinted with permission from the American Society of Clinical Oncology from O’Brien S, Moore JO, Boyd TE, et al. Randomized Phase 3 trial of fludarabine plus cyclophosphamide with or without oblimersen sodium (Bcl-2 antisense) in patients with relapsed or refractory chronic lymphocytic leukemia. J Clin Oncol, 25:1114–20. data on file, Genta Incorporated).
In both groups, 115 patients received protocol therapy, and the median number of cycles administered was 4 (O’Brien et al 2007). In addition, fludarabine and cyclophosphamide were administered at or close to the doses specified by protocol in both treatment groups. Across all cycles, the actual dose of fludarabine administered compared with the planned dose averaged 91% in the oblimersen plus Flu/Cy group and 93% in the Flu/Cy group; corresponding percentages for cyclophosphamide were 90 and 93, respectively (data on file, Genta Incorporated). The most common reason for early discontinuation was adverse event/toxicity (36% and 35% of patients in the oblimersen plus Flu/Cy group and Flu/Cy group, respectively).
The primary endpoint of the study was achieved: the addition of oblimersen to the standard chemotherapy significantly increased the rate of complete plus nodular partial responses. Twenty (17%) of 120 patients in the oblimersen plus Flu/Cy group achieved a complete or nodular partial response versus 8 (7%) of 121 patients in the Flu/Cy group (P = 0.025; see Table 4) (Rai et al 2005; O’Brien et al 2007). Importantly, the rate of complete response was more than tripled in the oblimersen plus Flu/Cy group (11 [9%] patients versus 3 [2%] patients in the Flu/Cy group [P = 0.03]) (O’Brien et al 2007). Consistent with the observation in the Phase II study (Mavromatis et al 2006), patients who remained sensitive to fludarabine were those most apt to respond to the addition of oblimersen to standard chemotherapy (see Table 4). This finding was not apparent among responding patients treated with Flu/Cy alone. Also consistent with the Phase II study, responses were achieved in patients with cytogenetic factors associated with an adverse prognosis (oblimersen plus Flu/Cy group, 2 patients with 17p or 11q deletions among the 9 of 20 responding patients with cytogenetic data at baseline) (O’Brien et al 2007).
Table 4.
Complete and partial nodular responses by prior treatment history and treatment group: Phase III study, intent-to-treat population
| Oblimersen plus Flu/Cy (N = 120) n/N (%) |
Flu/Cy (N = 121) n/N (%) |
|
|---|---|---|
| Response | ||
| Complete or nodular | ||
| partial response | 20/120 (17)a | 8/121 (7)a |
| Complete response | 11/120 (9)b | 3/121 (3)b |
| Fludarabine sensitivity | ||
| Relapsed | 13/51 (25) | 3/50 (6) |
| Refractory | 7/69 (10) | 5/71 (7) |
| Number of prior regimens | ||
| 1–2 | 14/60 (23) | 4/63 (6) |
| 3 or more | 6/60 (10) | 4/58 (7) |
| Response to last therapy | ||
| >6 months | 12/54 (22) | 3/52 (6) |
| ≤6 months | 8/66 (12) | 5/69 (7) |
Abbreviation: Flu/Cy, fludarabine/cyclophosphamide.
P, 0.025, continuity-corrected Chi square test
P, 0.03, Fisher’s exact test
Copyright © 2007. Reprinted with permission from the American Society of Clinical Oncology from O’Brien S, Moore JO, Boyd TE, et al. Randomized Phase 3 trial of fludarabine plus cyclophosphamide with or without oblimersen sodium (Bcl-2 antisense) in patients with relapsed or refractory chronic lymphocytic leukemia. J Clin Oncol, 25:1114–20.
All complete and nodular responses were durable, lasting 6 or more months (see Figure 3) (O’Brien et al 2007). Follow-up through a minimum of 2 years showed a relapse rate of 25% (5 of 20 patients in the oblimersen plus Flu/Cy group) versus 75% (6 of 8 patients) in the Flu/Cy group. Median duration of CR/nPR was 20 months in the Flu/Cy group and not reached in the oblimersen plus Flu/Cy group, in which it is estimated that duration will exceed 31 months. Moreover, based on an intent-to-treat analysis of response duration, a highly significant difference was apparent that favored the oblimersen plus Flu/Cy group (hazard ratio = 0.62; P = 0.002; see Figure 4.
Figure 3.
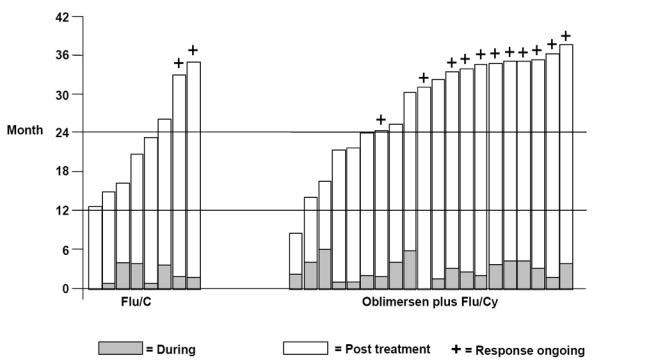
Duration of response (from date of first response) during and following protocol therapy among patients achieving a CR or nPR by treatment group: Phase III Study (Copyright © 2007. Reprinted with permission from the American Society of Clinical Oncology from O'Brien S, Moore JO, Boyd TE, et al. Randomized Phase 3 trial of fludarabine plus cyclophosphamide with or without oblimersen sodium (Bcl-2 antisense) in patients with relapsed or refractory chronic lymphocytic leukemia. J Clin Oncol, 25:1114–20).
Figure 4.
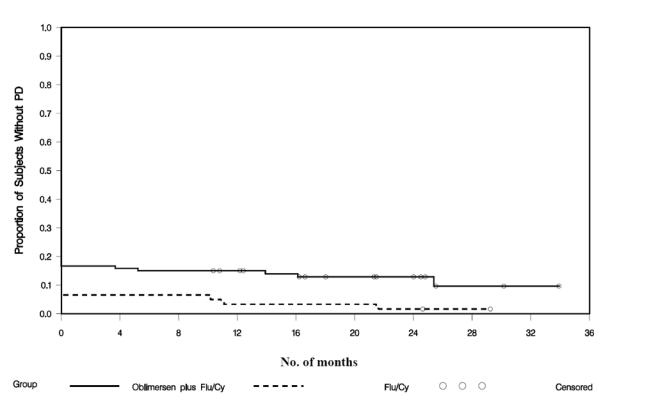
Duration of response from date of best response by treatment group: Phase III study, intent-to-treat population (data on file, Genta Incorporated).
Time to progression was correlated with response (P < 0.0001) (O’Brien et al 2007). Among patients with a complete or nodular partial response, 70% of patients (14 of 20) in the oblimersen plus Flu/Cy group versus 50% (4 of 8) in the Flu/Cy group were without disease progression 2 or more years after randomization. Similarly, survival was correlated with response (P < 0.0001; see Figure 5) (O’Brien et al 2007). Among patients with a complete or nodular partial response, 70% of patients in the oblimersen plus Flu/Cy group versus 38% in the Flu/Cy group were alive 3 years after randomization. Based on an intent-to-treat analysis, the estimated 3-year survival rate was 46% and 37% in the oblimersen plus Flu/Cy and Flu/Cy groups, respectively.
Figure 5.
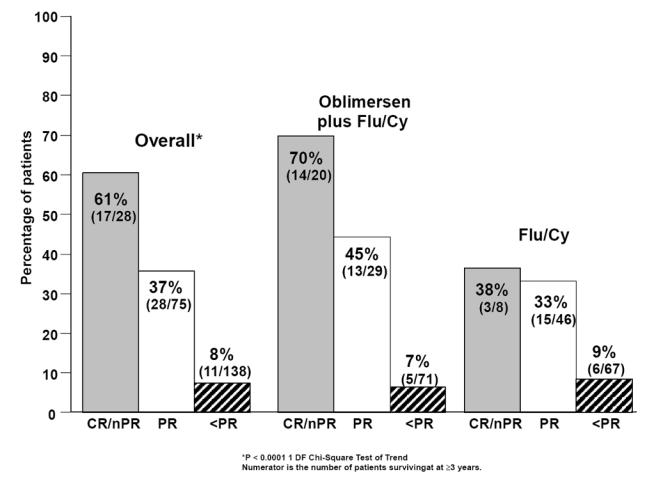
Survival with 3 years of follow-up by response and treatment group: Phase III study, intent-to-treat population (Copyright © 2007. Reprinted with permission from the American Society of Clinical Oncology from O’Brien S, Moore JO, Boyd TE, et al. Randomized Phase 3 trial of fludarabine plus cyclophosphamide with or without oblimersen sodium (Bcl-2 antisense) in patients with relapsed or refractory chronic lymphocytic leukemia. J Clin Oncol, 25:1114–20).
Additional clinical benefit afforded with the achievement of a complete or nodular partial response was the disappearance of CLL symptoms for ≥180 days (P < 0.0001 for CR/nPR versus PR versus < PR) (O’Brien et al 2007). As specified by protocol, these symptoms included fever, night sweats, fatigue, abdominal discomfort or early satiety due to hepatosplenomegaly, impaired cosmesis or impaired mobility due to lymphadenopathy, and ‘other.’ Among patients who were symptomatic at baseline regardless of treatment group, 17 (94%) of patients with a complete or nodular partial response versus 36 (59%) with a partial response were symptom-free for at least 180 days prior to disease progression or initiation of new therapy. Patients with a complete or nodular partial response were afforded a minimum of approximately 11 additional symptom-free months compared with patients who had a partial response.
The oblimersen plus Flu/Cy regimen was superior across all prospectively defined strata. Analysis showed that certain patients disproportionately benefited from the addition of oblimersen to standard chemotherapy (Rai et al 2005; O’Brien et al 2007). In particular, fludarabine-responsive patients, ie, those who had achieved a partial response or better for at least 6 months after fludarabine therapy and then relapsed (oblimersen plus Flu/Cy group, N = 51; Flu/Cy group, N = 50), derived maximal benefit from the addition of oblimersen to fludarabine and cyclophosphamide (O’Brien et al 2007). The beneficial effect of oblimersen in the fludarabine-responsive population was apparent both for CR and nPR (oblimersen plus Flu/Cy group, 25%; Flu/Cy group, 6%; P = 0.016).
Importantly, in this group of fludarabine-responsive patients, there was a statistically significant benefit in survival for the oblimersen plus Flu/Cy group (O’Brien et al 2007). The median overall survival with 3 years of follow-up for all patients was not reached in the oblimersen plus Flu/Cy group and was 33 months in the Flu/Cy group (hazard ratio = 0.53; P = 0.05; see Figure 6).
Figure 6.
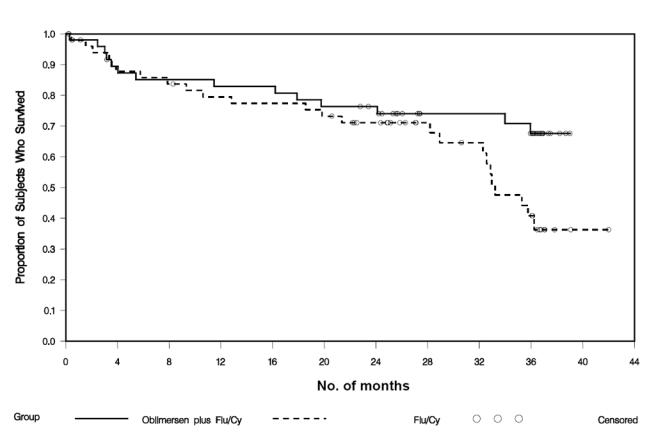
Kaplan-meier survival curves by treatment group: Phase III study, intent-to-treat population, fludarabine-responsive patients (Copyright © 2007. Reprinted with permission from the American Society of Clinical Oncology from O’Brien S, Moore JO, Boyd TE, et al. Randomized Phase 3 trial of fludarabine plus cyclophosphamide with or without oblimersen sodium (Bcl-2 antisense) in patients with relapsed or refractory chronic lymphocytic leukemia. J Clin Oncol, 25:1114–20).
This randomized, controlled study allowed identification of those adverse events that may be exacerbated when oblimersen is added to standard chemotherapy. The most frequently reported adverse events (all grades) among the 115 patients treated with oblimersen plus Flu/Cy included nausea (72% of patients), thrombocytopenia (49%), pyrexia (48%), fatigue (44%), anemia (39%), and vomiting (30%) (see Table 5) (O’Brien et al 2007; data on file, Genta Incorported). Except for anemia and neutropenia, more patients treated with oblimersen plus Flu/Cy than Flu/Cy alone experienced these frequently reported events.
Table 5.
Frequently reported adverse events by treatment group: Phase III study, all treated patients
| All | Grade 3 + Grade 4 | |||
|---|---|---|---|---|
| Adverse event a | Oblimersen plus Flu/Cy (N = 115) % |
Flu/Cy (N = 115) % |
Oblimersen plus Flu/Cy (N = 115) % |
Flu/Cy (N = 115) % |
| Nausea | 72 | 48 | 8 | 2 |
| Thrombocytopenia | 49 | 40 | 33 | 20 |
| Pyrexia | 49 | 29 | 3 | 3 |
| Fatigue | 44 | 31 | 6 | 4 |
| Anemia | 39 | 42 | 15 | 15 |
| Vomiting | 30 | 23 | 6 | 1 |
| Cough | 28 | 22 | 1 | 0 |
| Constipation | 26 | 19 | 2 | 1 |
| Neutropenia | 24 | 33 | 19 | 24 |
| Headache | 23 | 14 | 1 | 3 |
| Diarrhea | 22 | 14 | 1 | 1 |
| Dyspnea | 21 | 17 | 5 | 2 |
Abbreviation: Flu/Cy, fludarabine/cyclophosphamide.
Includes events reported in ≥20% of patients in either treatment group
(Copyright © 2007. Reprinted with permission from the American Society of Clinical Oncology from O’Brien S, Moore JO, Boyd TE, et al. Randomized Phase 3 trial of fludarabine plus cyclophosphamide with or without oblimersen sodium (Bcl-2 antisense) in patients with relapsed or refractory chronic lymphocytic leukemia. J Clin Oncol, 25:1114–20.; data on file, Genta Incorporated).
With the exception of thrombocytopenia and neutropenia, most adverse events were Grade 1 or Grade 2 in severity (NCI-CTC) (data on file Genta Incorporated). Consistent with an increased incidence of Grade 3 or Grade 4 thrombocytopenia in the oblimersen plus Flu/Cy group (see Table 5), platelet transfusions were administered to more patients in the oblimersen plus Flu/Cy group than in the Flu/Cy group. For the most part, these transfusions were administered primarily in Cycle 1 to patients with baseline platelet counts of >10,000 but <25,000 (see Table 6) (data on file, Genta Incorporated). Importantly, as shown in Table 6, most transfusions were not associated with a bleeding event, and the incidence of Grade 3–4 bleeding events was low in both groups (4% and 2%, respectively). Given that the Flu/Cy standard of care is highly immunosuppressive and myelosuppressive, it is noteworthy that the addition of Genasense to the Flu/Cy regimen did not increase the incidence of neutropenia or anemia (all grades, as well as Grade 3–4 occurrences).
Table 6.
Platelet count at baseline and at time of first transfusion by treatment group: Phase III study, all treated patients
| <100,000 at baseline | ≥100,000 at baseline | |||
|---|---|---|---|---|
| Oblimersen plus Flu/Cy (n = 41) No. of patients |
Flu/Cy (n = 39) No. of patients |
Oblimersen plus Flu/Cy (n = 74) No. of patients |
Flu/Cy (n = 76) No. of patients |
|
| Patients with platelet count at time of first transfusiona | 9 | 2 | 6 | 2 |
| Platelet count ≤10,000 | 2 | 1b | 1 | 0 |
| Platelet count >10,000 – <25,000 | 7c | 1 | 5d | 2e |
Abbreviations: Flu/Cy, fludarabine/cyclophosphamide; no., number.
The lower of either the last platelet count prior to transfusion or the first platelet count after transfusion
This patient had rectal bleeding (Grade 1).
One patient had hematuria (Grade 2).
One patient had a subepidermal hemorrhage (Grade 3).
One patient had a hemorrhagic disorder (Grade 3).
Catheter-related complications, all of which were Grade 1 or 2 in severity, were reported for 16% and 3% of patients in the oblimersen plus Flu/Cy and Flu/Cy groups, respectively (O’Brien et al 2007). These events mainly consisted of tenderness, bruising, redness, swelling, and seepage at the catheter site. None of these events resulted in discontinuation of study treatment (data on file, Genta Incorporated).
Of particular concern in patients with CLL is the occurrence of opportunistic infections and second malignancies, neither of which was increased with the addition of oblimersen to chemotherapy (opportunistic infections: oblimersen plus Flu/Cy group, 4%; chemotherapy-only group, 7%; second malignancies: 1% and 4%, respectively) (O’Brien et al 2007). Also noteworthy in the first cycle of therapy in the oblimersen plus Flu/Cy group is the low incidence of infusion-related events, which were limited to tumor lysis syndrome in two (2%) patients and a cytokine-release-type reaction in one (1%) patient.
Treatment-related adverse events with an outcome of death were uncommon in both groups (oblimersen plus Flu/Cy group, 5 [4%]; Flu/Cy group, 2 [2%]). Causes of death in the two treatment groups were fairly similar except for two deaths due to tumor lysis/cytokine release syndrome in the oblimersen plus Flu/Cy group.
In the fludarabine-responsive treated population (oblimersen plus Flu/Cy group, N = 47; chemotherapy-only group, N = 49), adverse events were quantitatively and qualitatively similar to those reported for all treated patients in the Phase III study (see Table 7) (data on file, Genta Incorporated). Only 3 events of Grade 3–4 severity occurred in more than 10% of patients in either group – thrombocytopenia, neutropenia, and anemia. The incidence of both neutropenia and anemia was significantly increased in the Flu/Cy group compared with the oblimersen plus Flu/Cy group (P = 0.03). No infusion-related reactions occurred in either group in the fludarabine-responsive population. The benefits associated with the use of oblimersen in combination with standard chemotherapy in the fludarabine-responsive population clearly outweighed the risks associated with administration of the combination regimen.
Table 7.
Comparison of key safety findings: Phase III study, all treated patients and fludarabine-relapsed treated patients
| Oblimersen plus Flu/Cy % | Flu/Cy % | P value | |
|---|---|---|---|
| All treated patients | N = 115 | N = 115 | |
| Grade 3–4 adverse events | |||
| Thrombocytopenia | 33 | 20 | 0.03 |
| Neutropenia | 19 | 24 | NS |
| Anemia | 15 | 15 | NS |
| Treatment-related discontinuations due to adverse events | 25 | 24 | NS |
| Treatment-related deaths | 4 | 2 | NS |
| Fludarabine-relapsed treated patients | N = 47 | N = 49 | |
| Grade 3–4 adverse events | |||
| Thrombocytopenia | 32 | 22 | NS |
| Neutropenia | 13 | 31 | 0.03 |
| Anemia | 4 | 18 | 0.03 |
| Treatment-related discontinuations due to adverse events | 30 | 27 | NS |
| Treatment-related deaths | 0 | 4 | NS |
Abbreviations: Flu/Cy, fludarabine/cyclophosphamide; NS, not significant.(data on file, Genta Incorporated).
Role of oblimersen in the treatment of CLL
New drugs are needed to treat patients with CLL in both the front-line and relapsed/refractory setting. Drugs with novel mechanisms of action are particularly desirable given the limitations of agents currently used to treat this malignancy. The phosphorothioate oligonucleotide oblimersen is particularly well designed for the treatment of patients with CLL, having been engineered to prevent translation of the Bcl-2 protein that is associated with both the pathogenesis of CLL and resistance to chemotherapy.
Uptake of oblimersen into patient cells with subsequent decreases in bcl-2 RNA and Bcl-2 protein has been documented in patients with acute myeloid leukemia (Marcucci et al 2005), but has not yet been assessed in patients with CLL. Results suggest a correlation between clinical outcome and the antisense activity of oblimersen as measured by bcl-2 mRNA downregulation and Bcl-2 protein levels.
The 3 clinical studies of oblimersen in CLL confirm that oblimersen is an effective therapeutic agent in this disease. Results of the Phase III trial, the only randomized comparative study in CLL to date, are especially compelling and include a statistically significant increase in the rate of complete and nodular partial responses. These responses were durable and highly correlated with time to progression, survival, and duration of symptomatic relief (P < 0.0001) and afford patients true clinical benefit. The addition of oblimersen to fludarabine/cyclophosphamide therapy significantly increased the rate of complete and nodular partial responses from 7% (with standard chemotherapy) to 17%. In the population of patients who remained sensitive to fludarabine, the difference was much greater (6% versus 25%, respectively), and survival clearly indicated a beneficial, statistically significant effect with the oblimersen plus Flu/Cy regimen. As cytogenetic data were not required for entry in this study, the efficacy of oblimersen in high-risk patients with CLL has yet to be documented.
Although the response rates achieved in the Phase III study were lower than those reported in the literature with fludarabine/cyclophosphamide alone (Wierda et al 2006), important differences in this study include the multicenter study design, central determination of hematologic and clinical response, heavily pretreated patient population, and the requirement for re-imaging by CT/ultrasound to determine response in patients with abnormalities identified at baseline by these radiographic methods (O’Brien et al 2007). Such radiographic imaging has been shown to reduce the response rate by approximately one third (Eichhorst et al 2006).
Although a complete or nodular partial response was achieved in some patients refractory to fludarabine, a disproportionately greater number of responding patients were those who remained sensitive to this agent, that is, the relapsed patient population. This finding is consistent with the chemosensitizing effect of oblimersen and was similarly evident in the Phase II trial conducted in previously treated and previously untreated patients.
Considering current CLL therapies, the addition of oblimersen to chemotherapy raises no new safety concerns. Several findings that occurred most often during the first treatment cycle – thrombocytopenia and, rarely, infusion reactions – are similarly reported with available therapies, familiar to the oncologist, and managed by careful patient monitoring and scrupulous attention to the patient’s hydration status. The increased incidence of catheter-related complications associated with the current route of administration for oblimersen may be eliminated if other routes of administration for oblimersen currently under clinical evaluation (subcutaneous injection, short intravenous infusion) prove effective.
Oblimersen is a promising new agent that may add to the efficacy of other treatments in the management of patients with CLL. Further study in the untreated CLL population is warranted, particularly in patients with high-risk cytogenetic factors, and continued study in other hematologic malignancies, particularly those of B-cell origin, is appropriate.
References
- Auer RL, Corbo M, Fegan CD, et al. Bcl-2 antisense (Genasense™) induces apoptosis and potentiates activity of both cytotoxic chemotherapy and rituximab in primary CLL cells. Blood; Proceedings of the 43rd Annual Meeting, American Society of Hematology; Dec 7–11; Orlando, FL, United States. 2001. p. 808a. abstract 3358. [Google Scholar]
- Aviram A, Rabizadeh E, Zimra Y, et al. Expression of bcl-2 and bax in cells isolated from B-chronic lymphocytic leukemia patients at different stages of the disease. Eur J Haematol. 2000;64:80–4. doi: 10.1034/j.1600-0609.2000.90042.x. [DOI] [PubMed] [Google Scholar]
- Baird ME, Lucas DM, Mone AP, et al. G3139 promotes release of IL-8 by chronic lymphocytic leukemia cells cultured in vitro: potential implications for therapeutic use. Blood; Proceedings of the 43rd Annual Meeting, American Society of Hematology; 2001 Dec 7–11; Orlando, FL. 2001. p. 281b. abstract 4863. [Google Scholar]
- Basañez G, Nechushtan A, Drozhinin O, et al. Bax, but not Bcl-xL, decreases the lifetime of planar phospholipid bilayer membranes at subnanomolar concentrations. Proc Natl Acad Sci U S A. 1999;96:5492–7. doi: 10.1073/pnas.96.10.5492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd JC, Rai K, Peterson BL, et al. Addition of rituximab to fludarabine may prolong progression-free survival and overall survival in patients with previously untreated chronic lymphocytic leukemia: an updated retrospective comparative analysis of CALGB 9712 and CALGB 9011. Blood. 2005;105:49–53. doi: 10.1182/blood-2004-03-0796. [DOI] [PubMed] [Google Scholar]
- Calin GA, Croce CM. Genomics of chronic lymphocytic leukemia microRNAs as new players with clinical significance. Semin Oncol. 2006;33:167–73. doi: 10.1053/j.seminoncol.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Cheng EH-YA, Wei MC, Weiler S, et al. BCL-2, BCL-X(L) sequester BH3 domain-only molecules preventing BAX- and BAK-mediated mitochondrial apoptosis. Mol Cell. 2001;8:705–11. doi: 10.1016/s1097-2765(01)00320-3. [DOI] [PubMed] [Google Scholar]
- Cheson BD, Bennett JM, Grever M, et al. National Cancer Institute-sponsored working group guidelines for chronic lymphocytic leukemia: revised guidelines for diagnosis and treatment. Blood. 1996;87:4990–7. [PubMed] [Google Scholar]
- Chiu SJ, Liu S, Perrotti D, et al. Efficient delivery of a Bcl-2-specific antisense oligodeoxyribonucleotide (G3139) via transferrin receptor-targeted liposomes. J Control Release. 2006;112:199–207. doi: 10.1016/j.jconrel.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Cimmino A, Calin GA, Fabbri M, et al. miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc Natl Acad Sci U S A. 2005;102:13944–9. doi: 10.1073/pnas.0506654102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossum PA, Sasmor H, Dellinger D, et al. Disposition of the 14C-labeled phosphorothioate oligonucleotide ISIS 2105 after intravenous administration to rats. J Pharmacol Exp Ther. 1993;267:1181–90. [PubMed] [Google Scholar]
- Cossum PA, Truong L, Owens SR, et al. Pharmacokinetics of a 14C-labeled phosphorothioate oligonucleotide, ISIS 2105, after intradermal administration to rats. J Pharmacol Exp Ther. 1994;269:89–94. [PubMed] [Google Scholar]
- Crooke ST, Grillone LR, Tendolkar A, et al. A pharmacokinetic evaluation of 14C-labeled afovirsen sodium in patients with genital warts. Clin Pharmacol Ther. 1994;56:641–6. doi: 10.1038/clpt.1994.189. [DOI] [PubMed] [Google Scholar]
- Curcio LD, Bouffard DY, Scanlon KJ. Oligonucleotides as modulators of cancer gene expression. Pharmacol Ther. 1997;74:317–32. doi: 10.1016/s0163-7258(97)00005-3. [DOI] [PubMed] [Google Scholar]
- Dai G, Chan KK, Liu S, et al. Cellular uptake and intracellular levels of the Bcl-2 antisense G3139 in cultured cells and treated patients with acute myeloid leukemia. Clin Cancer Res. 2005;11:2998–3008. doi: 10.1158/1078-0432.CCR-04-1505. [DOI] [PubMed] [Google Scholar]
- Data on file. Genta Incorporated. Berkeley Heights, New Jersey
- Demidov LV, Kharkevitch G, Itri LM, et al. A pharmacokinetic (PK) study of oblimersen sodium (G3139) + dacarbazine (DTIC) in patients with metastatic melanoma (MM). J Clin Oncol; Proceedings of the 39th Annual Meeting, American Society of Clinical Oncologists; 2003 May 31–Jun 3; Chicago, Illinois, United States. 2003. p. 156. abstract 623. [Google Scholar]
- Döhner H, Stilgenbauer S, Benner A, et al. Genomic abberations and survival in chronic lymphocytic leukemia. N Engl J Med. 2000;343:1910–16. doi: 10.1056/NEJM200012283432602. [DOI] [PubMed] [Google Scholar]
- Eichhorst BF, Busch R, Hopfinger, et al. Fludarabine plus cyclophosphamide versus fludarabine alone in first-line therapy of younger patients with chronic lymphocytic leukemia. Blood. 2006;107:885–91. doi: 10.1182/blood-2005-06-2395. [DOI] [PubMed] [Google Scholar]
- Faderl S, Keating MJ, Do K-A, et al. Expression profile of 11 proteins and their prognostic significance in patients with chronic lymphocytic leukemia. Leukemia. 2002;16:1045–52. doi: 10.1038/sj.leu.2402540. [DOI] [PubMed] [Google Scholar]
- Flinn IW, Kumm E, Grever MR, et al. Fludarabine and cyclophosphamide produces a higher complete response rate and more durable remissions than fludarabine in patients with previously untreated CLL: Intergroup Trial E2997. Blood; Proceedings of the 46th Annual Meeting, American Society of Hematology; 2004 Dec 4–7; San Diego CA, United States. 2004. p. 139a. abstract 475. [Google Scholar]
- Gazitt Y, Liu Q, Vesole D. Bcl-2 antisense oligonucleotides (ASO) enhances apoptosis and cytotoxicity in drug-resistant myeloma cells. Blood; Proceedings of the 43rd Annual Meeting, American Society of Hematology; 2001 Dec 7–11; Orlando, FL, United States. 2001. p. 641a. abstract 2688. [Google Scholar]
- Hanada M, Delia D, Aiello A, et al. bcl-2 gene hypomethylation and high-level expression in B-cell chronic lymphocytic leukemia. Blood. 1993;82:1820–8. [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- Johnstone RW, Ruefli AA, Lowe SW. Apoptosis: a link between cancer genetics and chemotherapy. Cell. 2002;108:153–64. doi: 10.1016/s0092-8674(02)00625-6. [DOI] [PubMed] [Google Scholar]
- Klasa RJ, Bally MB, Ng R, et al. Eradication of human non-Hodgkin’s lymphoma in SCID mice by BCL-2 antisense oligonucleotides combined with low-dose cyclophosphamide. Clin Cancer Res. 2000;6:2492–500. [PubMed] [Google Scholar]
- Klasa RJ, Gillum AM, Klem RE. Oblimersen Bcl-2 antisense: facilitating apoptosis in anticancer treatment. Antisense Nucleic Acid Drug Dev. 2002;12:193–213. doi: 10.1089/108729002760220798. [DOI] [PubMed] [Google Scholar]
- Klobusicka M, Kusenda J, Babusikova O. Immunocytochemical detection of bcl-2 and p53 proteins in B-chronic lymphocytic leukemia patients. Neoplasma. 2002;49:387–93. [PubMed] [Google Scholar]
- Kokoszka JE, Waymire KG, Levy SE, et al. The ADP/ATP translocator is not essential for the mitochondrial permeability transition pore. Nature. 2004;427:461–5. doi: 10.1038/nature02229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leporrier M, Chevret S, Cazin B, et al. Randomized comparison of fludarabine, CAP, and CHOP in 938 previously untreated stage B and C chronic lymphocytic leukemia patients. Blood. 2001;98:2319–25. doi: 10.1182/blood.v98.8.2319. [DOI] [PubMed] [Google Scholar]
- Loomis R, Carbone R, Reiss M, et al. Bcl-2 antisense (G3139, Genasense) enhances the in vitro and in vivo response of Epstein-Barr virus-associated lymphoproliferative disease to rituximab. Clin Cancer Res. 2003;9:1931–9. [PubMed] [Google Scholar]
- Marcucci G, Byrd JC, Dai G, et al. Phase I and pharmacodynamic studies of G3139, a Bcl-2 antisense oligonucleotide, in combination with chemotherapy in refractory or relapsed acute leukemia. Blood. 2003;101:425–32. doi: 10.1182/blood-2002-06-1899. [DOI] [PubMed] [Google Scholar]
- Marcucci G, Stock W, Dai G, et al. Phase I study of oblimersen sodium, an antisense to Bcl-2, in untreated older patients with acute myeloid leukemia: pharmacokinetics, pharmacodynamics, and clinical activity. J Clin Oncol. 2005;23:3404–11. doi: 10.1200/JCO.2005.09.118. [DOI] [PubMed] [Google Scholar]
- Mavromatis B, Rai KA, Wallace PK, et al. Efficacy and safety of the combination of Genasense™ (oblimersen sodium, Bcl-2 antisense oligonucleotide), fludarabine and rituximab in previously treated and untreated subjects with chronic lymphocytic leukemia. Blood; Proceedings of the 47th Annual Meeting, American Society of Hematology; 2005 Dec 10–13; Atlanta, Georgia, United States. 2005. p. 602a. abstract 2129. [Google Scholar]
- Mavromatis B, Rai K, Wallace PK, et al. Impact of prognostic markers on outcomes in patients with advanced chronic lymphocytic leukemia treated with the regimen of fludarabine/rituximab plus oblimersen (Bcl-2 antisense). J Clin Oncol; Proceedings of the 42nd Annual Meeting, American Society of Clinical Oncologists; 2006 Jun 2–6; Atlanta, Georgia, United States. 2006. p. 363s. abstract 6609. [Google Scholar]
- Menendez P, Vargas A, Bueno C, et al. Quantitative analysis of bcl-2 expression in normal and leukemic human B-cell differentiation. Leukemia. 2004;18:491–8. doi: 10.1038/sj.leu.2403231. [DOI] [PubMed] [Google Scholar]
- Moreton P, Kennedy B, Lucas G, et al. Eradication of minimal residual disease in B-cell chronic lymphocytic leukemia after alemtuzumab therapy is associated with prolonged survival. J Clin Oncol. 2005;23:2971–9. doi: 10.1200/JCO.2005.04.021. [DOI] [PubMed] [Google Scholar]
- O’Brien SM, Cunningham CC, Golenkov AK, et al. Phase I to II multicenter study of oblimersen sodium, a Bcl-2 antisense oligonucleotide, in patients with advanced chronic lymphocytic leukemia. J Clin Oncol. 2005;23:7697–702. doi: 10.1200/JCO.2005.02.4364. [DOI] [PubMed] [Google Scholar]
- O’Brien SM, Kantarjian HM, Thomas DA, et al. Alemtuzumab as treatment for residual disease after chemotherapy in patients with chronic lymphocytic leukemia. Cancer. 2003;98:2657–63. doi: 10.1002/cncr.11871. [DOI] [PubMed] [Google Scholar]
- O’Brien S, Moore JO, Boyd TE, et al. Randomized Phase 3 trial of fludarabine plus cyclophosphamide with or without oblimersen sodium (Bcl-2 antisense) in patients with relapsed or refractory chronic lymphocytic leukemia. J Clin Oncol. 25:1114–20. doi: 10.1200/JCO.2006.07.1191. [DOI] [PubMed] [Google Scholar]
- O’Connor OA, Smith EA, Toner LE, et al. The combination of the proteasome inhibitor bortezomib and the Bcl-2 antisense molecule oblimersen sensitizes human B-cell lymphomas to cyclophosphamide. Clin Cancer Res. 2006;12:2902–11. doi: 10.1158/1078-0432.CCR-05-0308. [DOI] [PubMed] [Google Scholar]
- Rai KR, Moore JO, Boyd TE, et al. Chemotherapy with or without oblimersen sodium (Bcl-2 antisense; Genasense® G3139) in advanced chronic lymphocytic leukemia: report of a large randomized study. Leuk Lymphoma. 2005;46(Suppl 1):S16. [Google Scholar]
- Rai KR, Peterson BL, Appelbaum FR, et al. Fludarabine compared with chlorambucil as primary therapy for chronic lymphocytic leukemia. N Engl J Med. 2000;343:1750–7. doi: 10.1056/NEJM200012143432402. [DOI] [PubMed] [Google Scholar]
- Raynaud FI, Orr RM, Goddard PM, et al. Pharmacokinetics of G3139, a phosphorothioate oligodeoxynucleotide antisense to bcl-2, after intravenous administration or continuous subcutaneous infusion to mice. J Pharmacol Exp Ther. 1997;281:420–7. [PubMed] [Google Scholar]
- Reed JC. Drug insight: cancer therapy strategies based on restoration of endogenous cell death mechanisms. Nat Clin Pract Oncol. 2006;3:388–98. doi: 10.1038/ncponc0538. [DOI] [PubMed] [Google Scholar]
- Reed JC, Kitada S, Takayama S, et al. Regulation of chemoresistance by the bcl-2 oncoprotein in non-Hodgkin’s lymphoma and lymphocytic leukemia cell lines. Ann Oncol. 1994;5:61S–65S. doi: 10.1093/annonc/5.suppl_1.s61. [DOI] [PubMed] [Google Scholar]
- Rheingold SR, Hogarty MD, Blaney SM, et al. A Phase I trial of G3139, a bcl-2 antisense oligonucleotide, combined with doxorubicin and cyclophosphamide in children with relapsed solid tumors: A Children’s Oncology Group study. J Clin Oncol. 2007;25:1512–18. doi: 10.1200/JCO.2006.09.5125. [DOI] [PubMed] [Google Scholar]
- Robertson LE, Plunkett W, McConnell K, et al. Bcl-2 expression in chronic lymphocytic leukemia and its correlation with the induction of apoptosis and clinical outcome. Leukemia. 1996;10:456–9. [PubMed] [Google Scholar]
- Schena M, Larsson L-G, Gottardi D, et al. Growth- and differentiation-associated expression of bcl-2 in B-chronic lymphocytic leukemia cells. Blood. 1992;79:2981–9. [PubMed] [Google Scholar]
- Sekar VJ, Barrett J, Chandula R, et al. Exploratory analysis of exposure-toxicity relationships with oblimersen sodium: Potential for patient management. American Society of Clinical Pharmacology and Therapeutics Annual Meeting; March, 2005; Orlando, FL. 2005. [Google Scholar]
- Sharpe JC, Arnoult D, Youle RJ. Control of mitochondrial permeability by Bcl-2 family members. Biochim Biophys Acta. 2004;1644:107–13. doi: 10.1016/j.bbamcr.2003.10.016. [DOI] [PubMed] [Google Scholar]
- Tolcher AW, Chi K, Kuhn J. A Phase II, pharmacokinetic, and biological correlative study of oblimersen sodium and docetaxel in patients with hormone-refractory prostate cancer. Clin Cancer Res. 2005;11:3854–61. doi: 10.1158/1078-0432.CCR-04-2145. [DOI] [PubMed] [Google Scholar]
- Tracey L, Perez-Rosado A, Artiga MJ, et al. Expression of the NF-kappaB targets BCL2 and BIRC5/Survivin characterizes small B-cell and aggressive B-cell lymphomas, respectively. J Pathol. 2005;206:123–34. doi: 10.1002/path.1768. [DOI] [PubMed] [Google Scholar]
- Tsujimoto Y, Shimizu S. Bcl-2 family: life-or-death switch. FEBS Lett. 2000;466:6–10. doi: 10.1016/s0014-5793(99)01761-5. [DOI] [PubMed] [Google Scholar]
- Tsujimoto Y, Shimizu S. The voltage-dependent anion channel: an essential player in apoptosis. Biochimie. 2002;84:187–93. doi: 10.1016/s0300-9084(02)01370-6. [DOI] [PubMed] [Google Scholar]
- Wang X. The expanding role of mitochondria in apoptosis. Genes Dev. 2001;15:2922–33. [PubMed] [Google Scholar]
- Waters JS, Clarke PA, Cunningham D, et al. BCL-2 antisense oligodeoxynucleotide (ODN) (G3139) therapy exerts its anti-tumour action through a sequence-specific antisense effect, and NOT a cell-mediated immune response. J Clin Oncol; Proceedings of the 36th Annual Meeting, American Society of Clinical Oncologists; 2000 May 20–23; New Orleans, LA, United States. 2000. p. 14a. abstract 48. [Google Scholar]
- Wendtner C-M, Ritgen M, Schweighofer CD, et al. Consolidation with alemtuzumab in patients with chronic lymphocytic leukemia (CLL) in first remission – experience on safety and efficacy within a randomized multicenter phase III trial of the German CLL Study Group (GCLLSG) Leukemia. 2004;18:1093–1101. doi: 10.1038/sj.leu.2403354. [DOI] [PubMed] [Google Scholar]
- Wierda W, O’Brien S, Faderl S, et al. A retrospective comparison of three sequential groups of patients with recurrent/refractory chronic lymphocytic leukemia treated with fludarabine-based regimens. Cancer. 2006;106:337–45. doi: 10.1002/cncr.21554. [DOI] [PubMed] [Google Scholar]
- Zaja F, di Loreto C, Amoroso V, et al. BCL-2 immunohistochemical evaluation in B-cell chronic lymphocytic leukemia and hairy cell leukemia before treatment with fludarabine and 2-chloro-deoxy-adenosine. Leuk Lymphoma. 1998;28:567–72. doi: 10.3109/10428199809058365. [DOI] [PubMed] [Google Scholar]


