Abstract
In rodents, extended access to cocaine produces an escalation in cocaine self-administration that has face and construct validity for human compulsive drug intake. Here we report that rats with six-hour access (long access, LgA) to cocaine self-administration produced a higher breakpoint for cocaine using a progressive-ratio schedule than rats with one-hour access (short access, ShA), and prazosin (α1 receptor antagonist) reduced the higher breakpoint for cocaine in LgA rats. Additionally, the number of neurons with α1-adrenergic receptor-like immunoreactivity in the bed nucleus of stria terminalis (BNST) was found to be much lower in LgA rats than in ShA and drug-naive rats. In contrast, UK14304 (α2 receptor agonist) and betaxolol (β1 receptor antagonist) had no effect on cocaine self-administration in either group. The data suggest that activation of the α1-noradrenergic system, perhaps in the BNST, is associated with increased motivation for cocaine in rats with extended access.
Keywords: cocaine, self-administration, escalation, alpha-1 noradrenergic receptor, rats, bed nucleus of stria terminalis
1. Introduction
Cocaine dependence is associated with compulsive use and loss of control over intake (American Psychiatric Association, 2000). In rodents, extended access to cocaine and methamphetamine produced an escalation in self-administration of the drugs (Ahmed and Koob, 1998; Kitamura et al., 2006), which has face validity for compulsive drug intake in humans. Therefore, the investigation of neuropharmacological changes during escalation in cocaine self-administration in rodents may provide key information about the neural mechanisms underlying the development of drug dependence in humans.
Cocaine increases extracellular norepinephrine (NE), dopamine, and serotonin by blocking monoamine reuptake transporters (Reith et al., 1997; Rothman et al., 2001). These findings prompted researchers to investigate the role of monoamines in cocaine self-administration. Focusing on norepinephrine, early studies using a self-administration paradigm found no convincing evidence of the involvement of NE in the acute reinforcing effects of psychostimulants. For example, direct and indirect noradrenergic receptor agonists did not function as positive reinforcers in monkeys or dogs (Risner and Jones, 1976; Wee and Woolverton, 2004; Woolverton, 1987) although the reinforcing effect of clonidine, an α2-adrenergic receptor agonist, has been noted (Davis and Smith, 1977; Woolverton et al., 1982, respectively). Moreover, pretreatment with noradrenergic receptor ligands did not affect amphetamine or cocaine self-administration under a fixed-ratio (FR) schedule in rats or monkeys (Wilson and Schuster, 1974; Woolverton, 1987; Yokel and Wise, 1976; Tella, 1995; Yokel and Wise, 1978). Depleting central nervous system NE did not alter cocaine self-administration under an FR schedule in rats (Roberts et al., 1977).
However, some studies using paradigms other than self-administration seem to support the involvement of NE in the acute rewarding effects of psychostimulants. In conditioned place preference, NE depletion in the medial prefrontal cortex blocked the development of cocaine-associated place preference in mice (Ventura et al., 2007). Alpha1b noradrenergic receptor knockout mice also showed reduced cocaine preference compared to wild type mice under a two-bottle choice paradigm (Drouin et al., 2002). In contrast, dopamine β-hydroxylase knockout mice (NE depletion) showed enhanced sensitivity to cocaine-induced place preference, suggesting the development of compensatory neural responses in knockout mice (Weinshenker et al., 2002). Taken together, the role of noradrenergic activity in the acute reinforcing/rewarding effects of psychostimulants remains speculative.
One possibility is that extended access to cocaine self-administration may alter the noradrenergic system by repeatedly stimulating the system, which may in turn contribute to the development of drug dependence. It was shown that repeated administration of amphetamine produced a sensitized response of NE release in the prefrontal cortex (Salomon et al., 2006). An increased number of norepinephrine transporters in the brain were also found in rhesus monkeys after 100 days of cocaine self-administration compared to 5 days of cocaine self-administration (Macey et al., 2003; Beveridge et al., 2005). Moreover, previous cocaine exposure by repeated passive administration increased a breakpoint for cocaine self-administration on a progressive-ratio (PR) schedule, which was blocked by administration of prazosin in rats (Zhang and Kosten, 2007).
The purpose of the present study was to test the hypothesis that neuroadaptation in the central noradrenergic system has a role in the increased cocaine self-administration in rats with extended access. Our hypothesis was that increased cocaine self-administration under a PR schedule in rats with extended access may be mediated by an altered noradrenergic system. To test this hypothesis, cocaine self-administration in rats with one-hour and six-hour access was tested with pretreatment by prazosin (a selective α1-noradrenergic receptor antagonist; Boyajian and Leslie, 1987), UK14304 (a selective α2-noradrenergic receptor agonist; Andorn et al., 1988; Ernsberger et al., 1997) and betaxolol (a β1-noradrenergic receptor antagonist; Smith and Teitler, 1999). Additionally, the density of α1-noradrenergic receptors in brain regions that are associated with drug dependence (Koob, 2003) was compared between groups.
2. Experimental procedures
All animal use procedures were approved by The Scripps Research Institute Animal Care and Use Committee and were in accordance with the National Institutes of Health guidelines.
Self-administration
Animals and apparatus
Twenty-seven male Wistar rats (Charles River, Hollister, CA), each weighing between 250 and 275 g at the beginning of the study, served as subjects. They were housed in groups of three in plastic cages with a 12 h:12 h light/dark cycle with lights off at 8:00AM. Food and water were available ad libitum. During experimental sessions, each rat was placed in a standard operant chamber, which was placed in a light- and sound-attenuating cubicle (28 × 26 × 20 cm; Med Associates Inc., St Albans, VT). A drug injection was delivered by a syringe pump (Razel Scientific Instruments, Georgia, VT) located on top of the cubicle. Experimental sessions were controlled and recorded by a PC computer with custom interface and software in the experimental room.
Procedure
Rats were implanted with a silastic catheter (0.3 mm i.d. × 0.64 mm o.d.; Dow Corning, Midland, MI) into the right external jugular vein under aseptic conditions. The catheter was flushed daily with heparinized saline (30 units/ml). The patency of catheters in the rats was tested using an ultra short-acting barbiturate, Brevital® (methohexital sodium, 10 mg/ml, 2 mg/rat) whenever a catheter failure was suspected during the study. Generally, a total loss of muscle tone within 3 s after a Brevital injection indicated the patency of a catheter. Detailed procedures of animal care and surgery have been previously described (Wee et al., 2007).
Experimental sessions were conducted once a day during the dark (active) cycle. The start of a session was signaled by the presentation of two response levers into the chamber. Responding on the right lever resulted in the delivery of 0.1 ml of a drug injection over 4 s. During an injection, stimulus lights above both levers were illuminated and lasted throughout a time-out period (20 s) that followed each injection. Pressing the left lever was counted but had no other programmed consequences. Self-administration procedure
After surgery, rats were trained to self-administer cocaine at a high dose of 1 mg/kg/injection for three days under an FR1 schedule of reinforcement, where one lever press resulted in the delivery of drug injection. The dose of cocaine was then reduced to 0.5 mg/kg/injection, and the rats were allowed to self-administer 0.5 mg/kg/injection of cocaine for six more sessions under an FR1 schedule.
After the baseline sessions, the rats were divided into two groups balanced by the number of injections/session in the last baseline session. During an escalation period, one group of rats (long access, LgA) self-administered 0.5 mg/kg/injection of cocaine in 6 h sessions, whereas the other group (short access, ShA) did so in 1 h sessions. Escalation sessions lasted for a minimum of 14 sessions and until there was no trend of increased or decreased responding for three consecutive sessions. After escalation sessions, the dose-response function of cocaine was determined under a PR schedule of reinforcement. For the PR schedule, the response requirement began at 1 response/injection and increased according to the following equation: responses/injection=[5 × e(injection number × 0.2)]−5 (Richardson and Roberts, 1996). When a rat failed to achieve the response requirement within 1 h, the session ended. A session length under a PR schedule was set at 12 h, and PR sessions lasted an average of 3 h across rats. Test PR sessions were held on Tuesdays and Fridays. Escalation sessions (LgA, 6 h session; ShA, 1 h session under an FR1 schedule) separated two test sessions. After the determination of the cocaine dose-response function, the effect of pretreatment with UK14304 (a selective α2-noradrenergic receptor agonist, 0.03–0.3 mg/kg, i.p.), prazosin (a selective α1-noradrenergic receptor antagonist, 1–3 mg/kg, i.p.) or betaxolol (a β1-noradrenergic receptor antagonist, 0.3–10 mg/kg, s.c.) on cocaine self-administration was examined under a PR schedule. Eleven of the 27 rats were examined with all three drugs. The rest of the rats were tested with either one or two drugs. Prazosin was tested first in 11 rats and last in the other 11 rats. UK14304 was tested first in 16 rats. Betaxolol was tested between prazosin and UK14304. After testing each noradrenergic drug, the rats were removed from cocaine self-administration for 7–8 days, then 12 days of successive escalation sessions were reestablished (ShA, 1 h session; LgA, 6 h session). Consequently, a total of 19–20 days separated the pretreatment periods of two noradrenergic drugs in all rats. Doses of each drug were tested in a counterbalanced manner across rats. Ten mg/kg of betaxolol was tested only in four rats of each group because of the high cost of the drug. The pretreatment time for UK14304, prazosin, and betaxolol was, respectively, 10, 10, and 30 min before a test session. The injection volume was always 1 ml/kg.
Data analysis
The data were expressed as the mean number of injections/session and the mean number of injections in the first hour of the session for each group of rats. Cocaine self-administration was compared across daily sessions over the initial 14 escalation sessions using a two-way repeated-measures analysis of variance (ANOVA) with the Bonferroni post hoc tests (access × daily session; Prism 4.0, GraphPad, San Diego, CA). Responding for doses of cocaine under a PR schedule was expressed as the number of injections/session and compared between LgA and ShA rats using a two-way repeated-measures ANOVA (access × cocaine dose). With a significant interaction and main effects of access and cocaine dose, Student’s t-test was performed on the number of injections/session between LgA and ShA groups at each cocaine dose under the PR schedule. The effect of noradrenergic drugs on cocaine self-administration was compared using a two-way repeated-measures ANOVA with the Bonferroni post hoc test (access × doses of a test drug; Prism 4.0, GraphPad, San Diego, CA). All data under a PR schedule followed the Gaussian distribution when tested using the D’Agostine and Pearson omnibus normality test (Prism 4.0, GraphPad, San Diego, CA).
Immunohistochemistry
After the self-administration study, ShA, LgA, and age-matched drug-naive rats were anesthetized with chloral hydrate (35%, 3 ml/450 g body weight, i.p.) and perfused transcardially with cold 0.1 M phosphate buffered saline (PBS), and then with 4% paraformaldehyde in 0.1 M PBS (pH 7.4) for 20 min at a rate of 15 ml/min. Brains were cryoprotected in 30% sucrose and sectioned coronally on a freezing microtome (American Optical Corporation, Buffalo, NY) at 40 μm, and sections were stored in 0.1% NaN3 in 0.1 M PBS at 4°C before processing for immunohistochemistry.
Immunostaining of α1 noradrenergic receptors was measured in the medial prefrontal cortex (mPFC), orbitofrontal cortex (OFC), bed nucleus of stria terminalis (BNST), and ventral tegmental area (VTA). Rabbit polyclonal anti-α1 noradrenergic receptor antibody (cat# PA1-047; Affinity BioReagents, Golden, CO; 1:1000, raised against the peptide sequence 339–349 of the human α1 adrenergic receptor) was used for labeling α1 noradrenergic receptors. This antibody was non-selective for subtypes of α1 receptors as was prazosin (see Lomasney et al., 1991; Wang et al., 2007). The left and right hemispheres of sections (bregma 4.20 for OFC, 3.7 and 3.2 for mPFC [including the anterior cingulate, prelimbic and infralimbic cortices], −0.26 and −0.30 mm for BNST, −5.30 and −5.60 mm for VTA; Paxinos and Watson, 1997) through the rat brain were slide-mounted and dried overnight prior to immunohistochemistry. All incubations were performed at room temperature unless otherwise indicated. Slide-mounted sections were subjected to an antigen unmasking pretreatment step as described previously (Mandyam et al., 2004). Slides were incubated with 0.3% H2O2 for 30 min to remove any endogenous peroxidase activity. Nonspecific binding was blocked with 5% serum and 0.5% Tween-20 in 0.1 M PBS for 60 min and incubated with the primary antibody (in 5% serum and 0.5% Tween-20) for 48 h at 4°C. After washing with 0.1 M PBS, the sections were exposed to biotinylated secondary immunoglobulin-G for 1 h (Vector Laboratories, Burlingame, CA; 1:200). After secondary antibody incubation, slides were incubated in avidin-biotin complex for 1 h (cat# PK-6100; Vector Laboratories, Burlingame, CA), and staining was visualized with 3,3-diaminobenzidine (DAB, cat# 34065; Pierce Laboratories, Rockford, IL). Sections were counterstained with Fast Red (Vector Laboratories, Burlingame, CA). Omission or dilution of the primary antibody resulted in lack of specific staining, thus serving as a negative control for antibody experiments.
Data analysis
Analysis was performed with the experimenter blinded to the group analyzed, to localize and quantify immunoreactivity (IR). DAB staining of the coded slides was visualized and quantified with a Zeiss Axiophot photomicroscope. Staining was examined, and X-IR neurons from the left and right hemispheres that were localized in the counting frame (mPFC, OFC and BNST: 0.078 mm2; VTA: 0.09 mm2) were visually quantified (Paxinos and Watson, 1997) (Figure 6). The total number of IR neurons both in the left and right hemispheres was counted and expressed as the total number of IR neurons/mm2 in each rat and averaged across rats within a group. A difference in the total number of IR neurons/mm2 across groups was found using one-way ANOVA with the Student-Neuman-Keul’s post hoc test (Prism 4.0, GraphPad, San Diego, CA).
Figure 6.

Quantification ofα1-noradrenergic receptors in the frontal cortex (FC), bed nucleus of the stria terminalis (BNST), and ventral tegmental area (VTA) across drug-naive, short access (ShA), and long access (LgA) rats (n=5 in each group). A–F: Schematic of a coronal section through the striatum (A) indicating the areas examined for quantitative analysis (area 1 is dorsal-mediolateral BNST; area 2 is ventral-mediolateral BNST), α1-adrenergic neurons in the dorsal-mediolateral BNST from a drug naive (B) and LgA rat (C). Schematic of a coronal section through the hippocampus (D) used for quantitative analysis (area 3 is the VTA), α1-noradrenergic neurons in the VTA from a drug naive (E) and LgA rat (F). Quantitative neuron counts are expressed as mean ± SEM of the total number of immunoreactive neurons/mm2. #p<0.01 compared to the dorsal BNST of the drug-naive group in (G) and #p<0.01 compared to the mPFC of the drug naive group in (H). *p<0.001 compared to drug-naive and ShA groups within each brain region. Scale bar in C is 10 μm (applies to B–C, E–F).
Drugs
The National Institute on Drug Abuse (Rockville MD) provided (−)-cocaine hydrochloride. Prazosin hydrochloride, UK14304, and betaxolol hydrochloride were purchased from Sigma-Aldrich Co. (St Louis, MO). Cocaine was dissolved in sterile 0.9% saline for self-administration. Prazosin, UK14304, and betaxolol were dissolved in 20% dimethylsulfoxide (DMSO) in water, 10% DMSO in water, and sterile water, respectively. Doses of cocaine for self-administration were prepared by varying the concentration of cocaine in drug solutions while the injection volume was kept constant at 0.1 ml/injection. Each drug solution was prepared for each rat based on its body weight and was updated every two or three days. Doses of drugs were expressed as salt. Rabbit polyclonal anti-α1 adrenergic receptor antibody was purchased from Affinity BioReagents (Golden, CO). Biotinylated secondary IgG was purchased from Vector Laboratories (Burlingame, CA).
3. Results
Cocaine self-administration in LgA rats significantly increased over 14 sessions starting in session 2 and 4 compared to session 1, respectively, within a session and during the first hour of each session (Figure 1). A two-way ANOVA found a significant interaction between access and daily session in escalation of cocaine self-administration within a session as well as during the first hour of each session [session, F(13,325)= 14.74, p<0.001; first hour, F(13,325)=5.60, p<0.001]. Cocaine self-administration in ShA rats was stable over 14 sessions.
Figure 1.

Self-administration of cocaine by rats under a fixed-ratio schedule during the escalation period. Data are expressed as the number of injections on the left axis and mg/kg on the right axis. The upper panel represents data from entire sessions, and the lower panel represents data from the first hour of sessions. Error bars are SEM values. Filled circles indicate self-administration by rats in 6 h sessions (long access, LgA), and open circles indicate self-administration by rats in 1 h sessions (short access, ShA). *p<0.05 compared to session 1.
Under a PR schedule, a significant interaction between access and cocaine dose was found in responding under the PR schedule [two-way ANOVA: F(2,40)=3.72, p<0.05, Figure 2]. Additionally, there were main effects of cocaine dose and access on responding under the PR schedule [cocaine dose, F(2,40)=59.65, p< 0.001; access, F(1,40)=6.16, p<0.05]. Responding for saline did not differ between LgA and ShA rats (Student t-test, p > >0.05). However, LgA rats produced a significantly higher break -point for cocaine than ShA rats at doses of 0.5 mg/kg and 1 mg/kg/injection (Student t-test, p<0.05).
Figure 2.
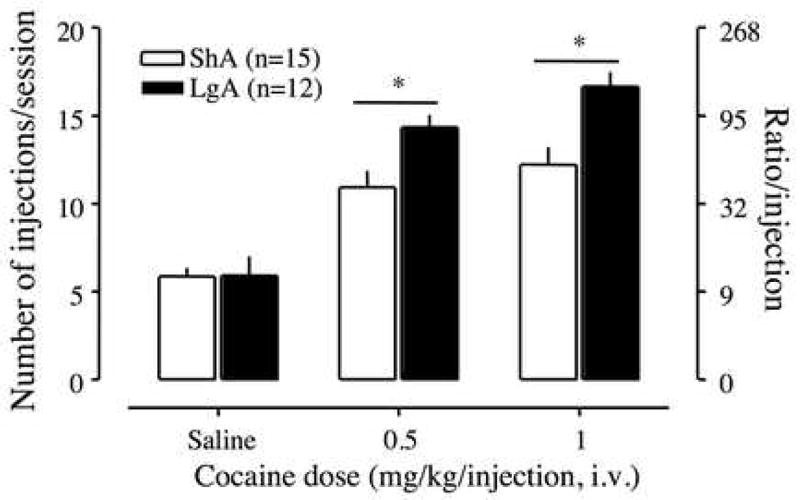
Dose-response function of cocaine by rats responding under a PR schedule. Test sessions under a PR schedule ended when rats did not achieve reinforcement within 1 h. Data are expressed as the number of injections/session on the left axis and the ratio per injection on the right axis. Error bars are SEM values. Open bars indicate responding by short access (ShA) rats, and filled bars indicate responding by long access (LgA) rats. *p<0.05 compared to ShA rats at each dose of cocaine.
Prazosin significantly decreased the breakpoint for 0.5 mg/kg/injection of cocaine in LgA rats at 3 mg/kg (Figure 3). However, the drug had no effect on cocaine self-administration in ShA rats. A two-way ANOVA between prazosin pretreatment and access showed a significant interaction between these two factors and a main effect of prazosin on cocaine self-administration [F(2,40)=3.29, p<0.05; F(2,40)=4.59, p<0.05, respectively].
Figure 3.
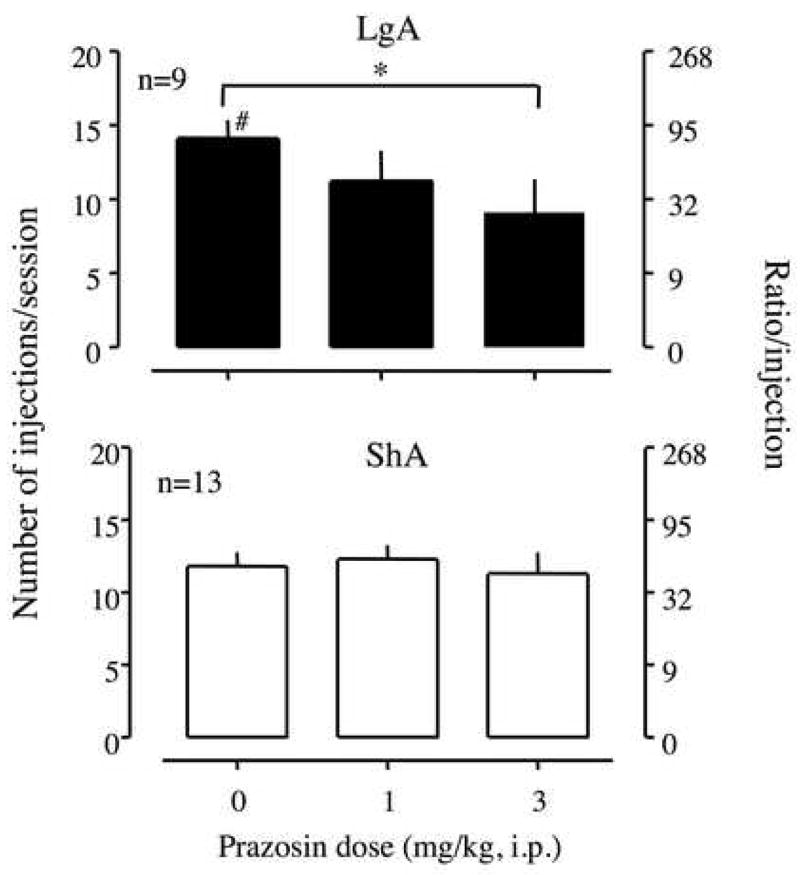
Effect of prazosin, an α1-noradrenergic receptor antagonist, on the break-point for 0.5 mg/kg/injection of cocaine under a PR schedule. Prazosin was intraperitoneally injected 10 min before a session. Data are expressed as the number of injections/session on the left axis and the ratio per injection on the right axis. Error bars are SEM values. The upper panel represents data from long access (LgA) rats, and the lower panel represents data from short access (ShA) rats. *p<0.05 compared to vehicle treatment. #p<0.05 compared to ShA rats.
UK14,304 and betaxolol did not produce any effect on cocaine self-administration of 0.5 mg/kg/injection in either LgA or ShA rats [two-way ANOVA; UK14304, F(3,42)=0.84, p>0.05, Figure 4; betaxolol, F(3,42)=1.07, p>0.05, Figure 5]. When the dose of UK14304 was increased to 1 mg/kg, all rats lost their muscle tone immediately after the injection.
Figure 4.
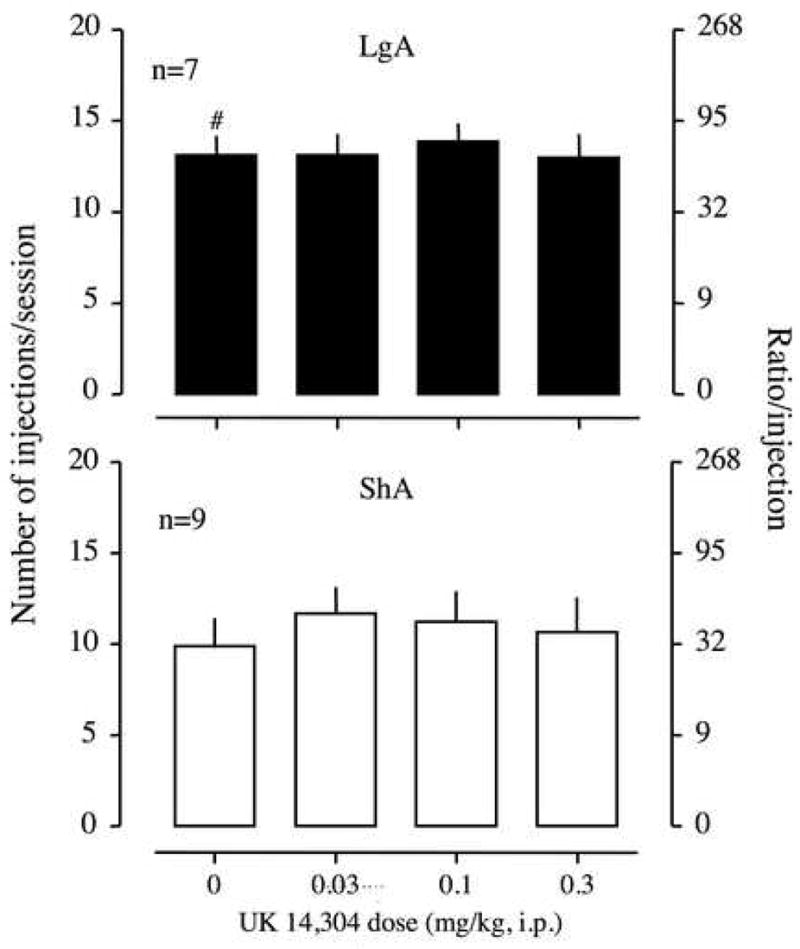
Effect of UK14304, an α2-noradrenergic receptor agonist, on the break-point for 0.5 mg/kg/injection of cocaine under a PR schedule. UK14304 was intraperitoneally injected 10 min before a session. Data are expressed as the number of injections/session on the left axis and the ratio per injection on the right axis. Error bars are SEM values. The upper panel is the data from long access (LgA) rats, and the lower panel is the data from short access (ShA) rats.
Figure 5.
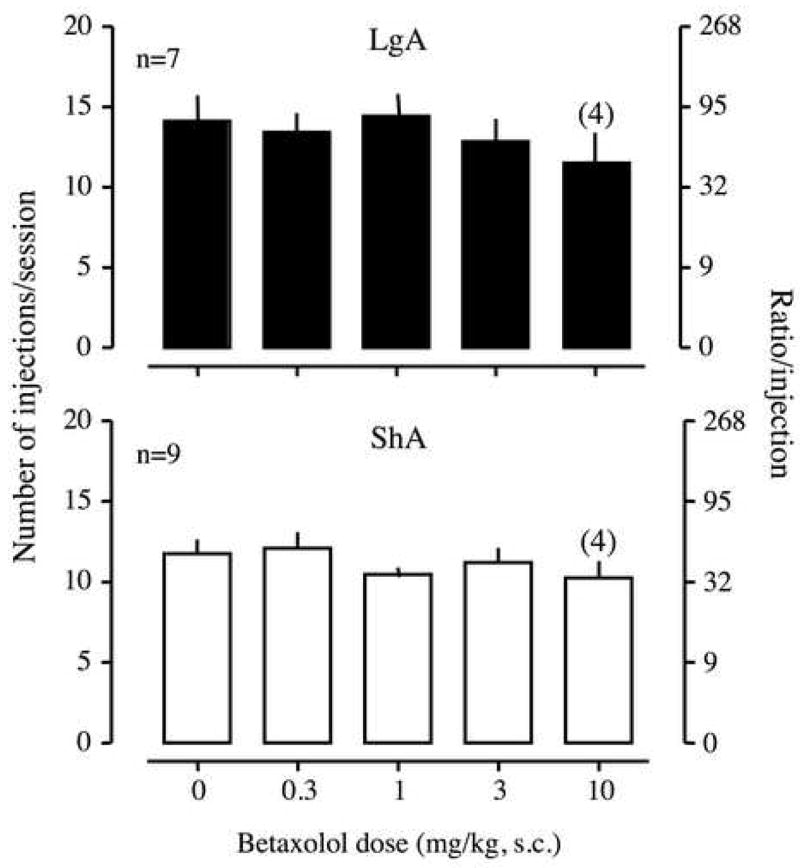
Effect of betaxolol, a β1-noradrenergic receptor antagonist, on the break-point for 0.5 mg/kg/injection of cocaine under a PR schedule. Betaxolol was subcutaneously injected 30 min before a session. Data are expressed as the number of injections/session on the left axis and the ratio per injection on the right axis. Error bars are SEM values. The upper panel is the data from long access (LgA) rats, and the lower panel is the data from short access (ShA) rats. The number in parentheses above 10 mg/kg of betaxolol indicates the number of rats that were tested with the dose of the drug.
The distribution of α1 noradrenergic receptor staining was similar to the pattern of mRNA expression previously reported in the striatum and thalamus (Domyancic and Morilak, 1997). α1 noradrenergic receptor staining was mostly cytoplasmic, and, in a few neurons, staining also was visible in the processes [Figure 6B and E (drug-naive), C and F (LgA)]. Quantitative analysis in the drug-naive control rats indicated the highest number of neurons with α1 noradrenergic receptor-like IR neurons in the dorsal BNST compared with the ventral BNST, the VTA, and the FC [F(6,38)=68.07, p<0.001; Figure 6G, gray striped bars]. Both the dorsal and ventral BNST of LgA rats showed a significantly decreased number of IR neurons to 46% and 68% the level of drug-naïve rats, respectively [dorsal BNST, F(2,14)=15.3, p<0.001; ventral BNST, F(2,14)=10.4, p<0.01; Figure 6 G]. However, no significant change in the number of IR neurons was found in the VTA and in subregions of the FC across groups.
4. Discussion
In agreement with previous studies (Ahmed and Koob, 1998, 1999), cocaine self-administration increased with 6 h access in LgA rats compared to ShA rats. Additionally, responding for doses of cocaine was higher in LgA than in ShA rats under a PR schedule. An upward shift of the cocaine dose-response function was previously found in LgA rats under an FR and a PR schedule (Ahmed and Koob, 1998; Paterson and Markou, 2003). Additionally, it was reported that LgA rats exhibited tolerance to the locomotor-stimulating effect of cocaine challenge (Ben-Shahar et al., 2004, 2005). In humans, compulsive drug use is accompanied by increased drug intake and tolerance (American Psychiatric Association, 2000), lending some face validity to the rodent model of self-administration with extended access.
Acute administration of cocaine is well documented to enhance noradrenergic neurotransmission by inhibiting NE reuptake (Iversen, 1973; Rothman et al., 2001; Wee et al., 2006). Additionally, increased levels of norepinephrine transporters was observed in rhesus monkeys after 100 days of cocaine self-administration compared with 5 days of cocaine self-administration (Macey et al., 2003; Beveridge et al., 2005). Therefore, we hypothesized that cocaine self-administration with extended access would alter noradrenergic activity in LgA rats to a greater extent than in ShA rats, and that these changes may in turn play a role in the increased cocaine self-administration in LgA rats. Under the present conditions, prazosin pretreatment reduced cocaine self-administration under a PR schedule in LgA rats, but not in ShA rats. Moreover, prazosin decreased self-administration of cocaine in LgA rats to the level of ShA rats. This suggests that α1 noradrenergic receptor activation may be related to increased motivation for cocaine in LgA rats with extended access. Results with experimenter-administered cocaine have shown that passive administration of cocaine (10 mg/kg/day) for five days produced the increased breakpoint for cocaine self-administration in rats under a PR schedule, and co-pretreatment of prazosin (0.3 mg/kg) with cocaine (10 mg/kg/day) inhibited the increase of the breakpoint for cocaine (Zhang and Kosten, 2007). Moreover, similar to the present results, prazosin had no effect on the breakpoint for cocaine in non-cocaine-pretreated rats. No effect of prazosin on non-escalated cocaine self-administration was previously reported in monkeys under FR and fixed-interval schedules (Woolverton, 1987; Howell and Byrd, 1991). Thus, the present and previous findings suggest a relationship between changes in α1 noradrenergic neurotransmission and the neuroplasticity associated with the increased cocaine intake.
The number of neurons with α1 noradrenergic receptor-like IR neurons was significantly higher in the BNST than in the VTA and cortices in drug-naive rats, consistent with the literature that the density of noradrenergic terminals is very high in the BNST (Brownstein and Palkovits, 1984). In addition, the number of IR neurons in the BNST was found to be lower by 40–60% in LgA rats than in ShA and drug-naive rats, suggesting a decrease in the number of α1 noradrenergic receptors during extended access to cocaine self-administration. We hypothesize that extended access to cocaine self-administration causes a downregulation of α1 noradrenergic receptors as a compensatory response to a prolonged overflow of extracellular NE by cocaine. A decreased number of α1 noradrenergic receptors, perhaps reduced spare receptors, in LgA rats may make noradrenergic signal transmission easily affected by the blockade of the receptors by prazosin, a hypothesis supported by the self-administration data showing that cocaine self-administration in LgA rats was sensitive to the inhibition of α1 noradrenergic receptors by prazosin. However, this conclusion should be taken with a caveat, and the direct causal relationship between decreased α1 receptors in the BNST and the effect of prazosin on increased cocaine self-administration in LgA needs further research. The increased norepinephrine transporters in the BNST after 100 days of cocaine self-administration in monkeys (Macey et al., 2003) is consistent with the present results because the increased norepinephrine transporters implies a compensatory response of increased clearance of extracellular NE.
An alternative explanation of the present finding may be that that there is an intake-dependent emergence of α1 noradrenergic regulation of cocaine self-administration down stream from the BNST that makes activity in ascending noradrenergic neurotransmission relevant in LgA rats. The potential recruitment of α1 noradrenergic pathways in an intake-dependent manner may have resulted in prazosin effects on increased cocaine self-administration in LgA rats regardless of the level of α1 noradrenergic receptor expression.
It should be noted that we did not observe any significant changes in the number of IR neurons in the VTA and FC that receive noradrenergic projections from the locus coeruleus comprising the dorsal noradrenergic bundle (Moore and Bloom, 1979; Weinshenker and Schroeder, 2006). In contrast, the ventral noradrenergic bundle projects from the pons and medulla to the hypothalamus and the extended amygdala (for review, see Moore and Bloom, 1979), and the BNST is a key component of the extended amygdala. Thus, the present results may suggest that the noradrenergic changes in the ventral noradrenergic projection rather than in the dorsal noradrenergic projection are related to increased cocaine self-administration, thereby increased motivation for cocaine in LgA rats.
One question would be the nonspecific effect of the doses of prazosin (1, 3 mg/kg) on operant behavior. However, the doses of prazosin did not reduce the rate of self-administration in ShA rats under the present conditions. Zhang and Kosten (2005) reported that 0.5 mg/kg of prazosin disrupted the rate of responding for food in Sprague-Dawley rats. In contrast, in Long Evans rats, 3.2 mg/kg of prazosin produced only “small decreases” in the rate of responding under a drug discrimination paradigm (Berthold et al., 1992). Similarly, 2 mg/kg of prazosin (i.p.) did not alter locomotor activity in rats (Wellman et al., 2002). Thus, prazosin in the present study is likely to have decreased cocaine self-administration in LgA rats via a more specific motivational interaction.
In contrast to prazosin, betaxolol pretreatment did not affect cocaine self-administration in any groups under the present conditions. It was shown that pretreatment with propranolol, a nonselective β-noradrenergic receptor antagonist, decreased cocaine self-administration under an FR schedule in rats and squirrel monkeys (Harris et al., 1996; Goldberg and Gonzalez, 1976). However, in a drug discrimination paradigm, Kleven and Koek (1997) implicated the involvement of the β2 rather than β1 noradrenergic system in the discriminative stimulus effects of cocaine in rats. Additionally, Leri et al. (2002) found that the mixture of β1 and β2 noradrenergic receptor antagonists attenuated stress-induced, but not cocaine-induced, reinstatement of responding for cocaine. Therefore, we conclude that the β1-noradrenergic system is not involved in cocaine self-administration.
With respect to the α2-noradrenergic system, clonidine has been shown to have positive reinforcing properties in rats and rhesus monkeys (Davis and Smith, 1977; Woolverton et al., 1982). However, clonidine is a mixed α2-noradrenergic and imidazoline-1 receptor agonist, whereas UK14304 is a selective α2 receptor agonist (Andorn et al., 1988; Ernsberger et al., 1997; Georges et al., 2005; Georges and Aston-Jones, 2003). Therefore, the action of clonidine may be mediated by imidazoline receptors. On the other hand, the α2 noradrenergic receptor antagonist, yohimbine, was shown to reinstate responding for psychostimulants in monkeys and rats (Lee et al., 2004; Shepard et al., 2004) presumably through disinhibition of ascending noradrenergic neurotransmission. NE reuptake inhibitors have also been shown to reinstate cocaine seeking (Platt et al., 2007). Whether stress-induced reinstatement produced by noradrenergic agents is related to the noradrenergic role in cocaine intake escalation remains to be explored.
An important question is why the effects of α1 receptor blockade and α2 receptor stimulation differed in the present study. Forray and colleagues (1999) demonstrated that the local perfusion of UK14304 and prazosin into the BNST produced asymmetric effects on the basal and K+ evoked release of the extracellular NE and glutamate in the BNST in rats. Although both drugs increased basal and K+ evoked NE release in the BNST, UK14304 also increased basal glutamate release whereas prazosin did not. Additionally, UK14304 and prazosin, respectively, increased and decreased K+ evoked glutamate release. Recently, Egli and colleagues (2005) reported that NE modulates glutamate system in the BNST. Therefore, it appears that the stimulation of α2 receptors may not simply be considered to be equal to the blockade of α1 receptors.
One might speculate that pretreatment time and/or drug doses contributed to the lack of effects of betaxolol and UK14304 on cocaine self-administration. However, betaxolol (0.01–10 mg/kg, i.p.) was effective on water-maintained responding and responding under a drug discrimination paradigm with 30 or 35 min pretreatment in rats (Zhang et al., 2001; O’Donnell, 1997). Likewise, we observed that 1 mg/kg of UK14304 produced a loss of muscle tone and sedation, indicating that behaviorally active doses were tested. Moreover, UK14304 (0.1 mg/kg) decreased firing rates of rat locus coeruleus neurons immediately after an intraperitoneal injection in in vivo cell recordings, suggesting that the drug easily crossed the blood-brain barrier (Georges and Aston-Jones, 2003). UK14304 pretreatment (0.05 mg/kg, i.p.) also increased immediate-early gene expression in the rat cerebral cortex when sacrificed 90 min after injection, indicating that UK14304 was long-acting (Shen and Gundlach, 2000).
In summary, the present data demonstrated that increased cocaine self-administration in LgA rats under a PR schedule was sensitive to α1 noradrenergic receptor inhibition. Additionally, the number of neurons with α1-adrenergic receptor-like IR was significantly lower in the BNST of LgA rats than in ShA and drug-naive rats. Therefore, the data suggest that altered α1-noradrenergic neurotransmission may be related to increased motivation for cocaine self-administration in rats with extended access to the drug, and changes in the noradrenergic projections to the extended amygdala, such as the BNST, may underlie altered motivation for cocaine during cocaine self-administration with extended access. In contrast, the present study did not find any relationship between the α2- and β1-noradrenergic systems and cocaine self-administration in any groups under the present conditions.
Acknowledgments
We gratefully acknowledge the technical assistance of Mike Pham, JoAnn Lee, Stephanie Chan, and Hanan Jammal, who were undergraduate students of the University of California, San Diego, in the self-administration and the immunohistochemistry studies. We also thank Mike Arends for editorial assistance. This is publication number 18632 from The Scripps Research Institute.
Role of the funding source
National Institute on Drug Abuse grant DA004398 (G.F.K) supported the study; the NIDA had no further role in study design, in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Conflict of Interest
All the authors (S.W., C.D.M., D.M.L., G.F.K.) have no conflict of interest to declare.
Author Disclosures
Author S. Wee performed the self-administration experiment and wrote the first draft of the manuscript. Author C.D. Maydyam performed immunohistochemistry experiment. Authors S. Wee, C.D. Mandyam and G.F. Koob contributed to the design of the study, analysis of the data and the final manuscript. D.M. Lekic performed part of the self-administration study. All the authors approved the final manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282:298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Koob GF. Long-lasting increase in the set point for cocaine self-administration after escalation in rats. Psychopharmacology. 1999;146:303–312. doi: 10.1007/s002130051121. [DOI] [PubMed] [Google Scholar]
- Andorn AC, Carlson MA, Gilkeson RC. Specific [3H]UK 14,304 binding in human cortex occurs at multiple high affinity states with alpha 2-adrenergic selectivity and differing affinities for GTP. Life Sci. 1988;43:1805–1812. doi: 10.1016/0024-3205(88)90279-2. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, IV-TR. American Psychiatry Press; Washington, D: 2000. [Google Scholar]
- Aston-Jones G, Delfs JM, Druhan J, Zhu Y. The bed nucleus of the stria terminalis. A target site for noradrenergic actions in opiate withdrawal. Ann NY Acad Sci. 1999;877:486–498. doi: 10.1111/j.1749-6632.1999.tb09284.x. [DOI] [PubMed] [Google Scholar]
- Ben-Shahar O, Ahmed SH, Koob GF, Ettenberg A. The transition from controlled to compulsive drug use is associated with a loss of sensitization. Brain Res. 2004;995:46–54. doi: 10.1016/j.brainres.2003.09.053. [DOI] [PubMed] [Google Scholar]
- Ben-Shahar O, Moscarello JM, Jacob B, Roarty MP, Ettenberg A. Prolonged daily exposure to i.v. cocaine results in tolerance to its stimulant effects. Pharmacol Biochem Behav. 2005;82:411–416. doi: 10.1016/j.pbb.2005.09.013. [DOI] [PubMed] [Google Scholar]
- Berthold CW, 3rd , Gonzales RA, Moerschbaecher JM. Prazosin attenuates the effects of cocaine on motor activity but not on schedule-controlled behavior in the rat. Pharmacol Biochem Behav. 1992;43:111–115. doi: 10.1016/0091-3057(92)90646-w. [DOI] [PubMed] [Google Scholar]
- Beveridge TJ, Smith HR, Nader MA, Porrino LJ. Effects of chronic cocaine self-administration on norepinephrine transporters in the nonhuman primate brain. Psychopharmacology. 2005;180:781–788. doi: 10.1007/s00213-005-2162-1. [DOI] [PubMed] [Google Scholar]
- Boyajian CL, Leslie FM. Pharmacological evidence for alpha-2 adrenoceptor heterogeneity: differential binding properties of [3H]rauwolscine and [3H]idazoxan in rat brain. J Pharmacol Exp Ther. 1987;241:1092–1098. [PubMed] [Google Scholar]
- Brownstein MJ, Palkovits M. Catecholamines, serotonin, acetylcholine and gamma-amonobutyric acid in the rat brain: biochemical studies. In: Bjorklund A, Hokfelt T, editors. Classical transmitters in the CNS: Part I (series title: Handbook of Chemical Neuroanatomy, vol. 2) Elsevier; Amsterdam: 1984. pp. 23–54. [Google Scholar]
- Davis WM, Smith SG. Catecholaminergic mechanisms of reinforcement: direct assessment by drug-self-administration. Life Sci. 1977;20:483–492. doi: 10.1016/0024-3205(77)90391-5. [DOI] [PubMed] [Google Scholar]
- Delfs JM, Zhu Y, Druhan JP, Aston-Jones G. Noradrenaline in the ventral forebrain is critical for opiate withdrawal-induced aversion. Nature. 2000;403:430–434. doi: 10.1038/35000212. [DOI] [PubMed] [Google Scholar]
- Domyancic AV, Morilak DA. Distribution of alpha1A adrenergic receptor mRNA in the rat brain visualized by in situ hybridization. J Comp Neurol. 1997;386:358–378. doi: 10.1002/(sici)1096-9861(19970929)386:3<358::aid-cne3>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Drouin C, Darracq L, Trovero F, Blanc G, Glowinski J, Cotecchia S, Tassin JP. Alpha1b-adrenergic receptors control locomotor and rewarding effects of psychostimulants and opiates. J Neurosci. 2002;22:2873–2884. doi: 10.1523/JNEUROSCI.22-07-02873.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egli RE, Kash TL, Choo K, Savchenko V, Matthews RT, Blakely RD, Winder DG. Norepinephrine modulates glutamatergic transmission in the bed nucleus of the stria terminalis. Neuropsychopharmacology. 2005;30:657–668. doi: 10.1038/sj.npp.1300639. [DOI] [PubMed] [Google Scholar]
- Ernsberger P, Friedman JE, Koletsky RJ. The I1-imidazoline receptor: from binding site to therapeutic target in cardiovascular disease. J Hypertens Suppl. 1997;15:S9–S23. doi: 10.1097/00004872-199715011-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georges F, Caille S, Vouillac C, Le Moine C, Stinus L. Role of imidazoline receptors in the anti-aversive properties of clonidine during opiate withdrawal in rats. Eur J Neurosci. 2005;22:1812–1816. doi: 10.1111/j.1460-9568.2005.04356.x. [DOI] [PubMed] [Google Scholar]
- Georges F, Aston-Jones G. Prolonged activation of mesolimbic dopaminergic neurons by morphine withdrawal following clonidine: participation of imidazoline and norepinephrine receptors. Neuropsychopharmacology. 2003;28:1140–1149. doi: 10.1038/sj.npp.1300161. [DOI] [PubMed] [Google Scholar]
- Goldberg SR, Gonzalez FA. Effects of propranolol on behavior maintained under fixed-ratio schedules of cocaine injection or food presentation in squirrel monkeys. J Pharmacol Exp Ther. 1976;198:626–634. [PubMed] [Google Scholar]
- Harris GC, Hedaya MA, Pan WJ, Kalivas P. Beta-adrenergic antagonism alters the behavioral and neurochemical responses to cocaine. Neuropsychopharmacology. 1996;14:195–204. doi: 10.1016/0893-133X(95)00089-V. [DOI] [PubMed] [Google Scholar]
- Howell LL, Byrd LD. Characterization of the effects of cocaine and GBR 12909, a dopamine uptake inhibitor, on behavior in the squirrel monkey. J Pharmacol Exp Ther. 1991;258:178–185. [PubMed] [Google Scholar]
- Iversen LL. Catecholamine uptake processes. Br Med Bull. 1973;29:130–135. doi: 10.1093/oxfordjournals.bmb.a070982. [DOI] [PubMed] [Google Scholar]
- Kitamura O, Wee S, Specio SE, Koob GF, Pulvirenti L. Escalation of methamphetamine self-administration in rats: a dose-effect function. Psychopharmacology. 2006;186:48–53. doi: 10.1007/s00213-006-0353-z. [DOI] [PubMed] [Google Scholar]
- Kleven MS, Koek W. Discriminative stimulus properties of cocaine: enhancement by beta-adrenergic receptor antagonists. Psychopharmacology. 1997;131:307–312. doi: 10.1007/s002130050297. [DOI] [PubMed] [Google Scholar]
- Koob GF. Neuroadaptive mechanisms of addiction: studies on the extended amygdala. Eur Neuropsychopharmacol. 2003;13:442–452. doi: 10.1016/j.euroneuro.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Lee B, Tiefenbacher S, Platt DM, Spealman RD. Pharmacological blockade of alpha2-adrenoceptors induces reinstatement of cocaine-seeking behavior in squirrel monkeys. Neuropsychopharmacology. 2004;29:686–693. doi: 10.1038/sj.npp.1300391. [DOI] [PubMed] [Google Scholar]
- Leri F, Flores J, Rodaros D, Stewart J. Blockade of stress-induced but not cocaine-induced reinstatement by infusion of noradrenergic antagonists into the bed nucleus of the stria terminalis or the central nucleus of the amygdala. J Neurosci. 2002;22:5713–5718. doi: 10.1523/JNEUROSCI.22-13-05713.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macey DJ, Smith HR, Nader MA, Porrino LJ. Chronic cocaine self-administration upregulates the norepinephrine transporter and alters functional activity in the bed nucleus of the stria terminalis of the rhesus monkey. J Neurosci. 2003;23:12–16. doi: 10.1523/JNEUROSCI.23-01-00012.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandyam CD, Norris RD, Eisch AJ. Chronic morphine induces premature mitosis of proliferating cells in the adult mouse subgranular zone. J Neurosci Res. 2004;76:783–794. doi: 10.1002/jnr.20090. [DOI] [PubMed] [Google Scholar]
- Moore RY, Bloom FE. Central catecholamine neuron systems: anatomy and physiology of the norepinephrine and epinephrine systems. Annu Rev Neurosci. 1979;2:113–168. doi: 10.1146/annurev.ne.02.030179.000553. [DOI] [PubMed] [Google Scholar]
- O’Donnell JM. Pharmacological characterization of the discriminative stimulus effects of clenbuterol in rats. Pharmacol Biochem Behav. 1997;58:813–818. doi: 10.1016/s0091-3057(97)00038-5. [DOI] [PubMed] [Google Scholar]
- Paterson NE, Markou A. Increased motivation for self-administered cocaine after escalated cocaine intake. Neuroreport. 2003;14:2229–2232. doi: 10.1097/00001756-200312020-00019. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 3. Academic Press; San Diego: 1997. [DOI] [PubMed] [Google Scholar]
- Pertovaara A, Haapalinna A, Sirvio J, Virtanen R. Pharmacological properties, central nervous system effects, and potential therapeutic applications of atipamezole, a selective alpha2-adrenoceptor antagonist. CNS Drug Rev. 2005;11:273–288. doi: 10.1111/j.1527-3458.2005.tb00047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt DM, Rowlett JK, Spealman RD. Noradrenergic Mechanisms in Cocaine-Induced Reinstatement of Drug Seeking in Squirrel Monkeys. J Pharmacol Exp Ther. 2007 doi: 10.1124/jpet.107.121806. in press. [DOI] [PubMed] [Google Scholar]
- Reith ME, Li MY, Yan QS. Extracellular dopamine, norepinephrine, and serotonin in the ventral tegmental area and nucleus accumbens of freely moving rats during intracerebral dialysis following systemic administration of cocaine and other uptake blockers. Psychopharmacology. 1997;134:309–317. doi: 10.1007/s002130050454. [DOI] [PubMed] [Google Scholar]
- Richardson NR, Roberts DC. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Risner M, Jones BE. Role of noradrenergic and dopaminergic processes in amphetamine self-administration. Pharmacol Biochem Behav. 1976;5:477–482. doi: 10.1016/0091-3057(76)90113-1. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Corcoran ME, Fibiger HC. On the role of ascending catecholaminergic systems in intravenous self-administration of cocaine. Pharmacol Biochem Behav. 1977;6:615–620. doi: 10.1016/0091-3057(77)90084-3. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH, Dersch CM, Romero DV, Rice KC, Carroll FI, Partilla JS. Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin. Synapse. 2001;39:32–41. doi: 10.1002/1098-2396(20010101)39:1<32::AID-SYN5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Salomon L, Lanteri C, Glowinski J, Tassin JP. Behavioral sensitization to amphetamine results from an uncoupling between noradrenergic and serotonergic neurons. Proc Natl Acad Sci. 2006;103:7476–7481. doi: 10.1073/pnas.0600839103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen PJ, Gundlach AL. Differential modulatory effects of alpha- and beta-adrenoceptor agonists and antagonists on cortical immediate-early gene expression following focal cerebrocortical lesion-induced spreading depression. Brain Res Mol Brain Res. 2000;83:133–144. doi: 10.1016/s0169-328x(00)00216-3. [DOI] [PubMed] [Google Scholar]
- Shepard JD, Bossert JM, Liu SY, Shaham Y. The anxiogenic drug yohimbine reinstates methamphetamine seeking in a rat model of drug relapse. Biol Psychiatry. 2004;55:1082–1089. doi: 10.1016/j.biopsych.2004.02.032. [DOI] [PubMed] [Google Scholar]
- Smith C, Teitler M. Beta-blocker selectivity at cloned human beta 1- and beta 2-adrenergic receptors. Cardiovasc Drugs Ther. 1999;13:123–126. doi: 10.1023/a:1007784109255. [DOI] [PubMed] [Google Scholar]
- Tella SR. Effects of monoamine reuptake inhibitors on cocaine self-administration in rats. Pharmacol Biochem Behav. 1995;51:687–692. doi: 10.1016/0091-3057(94)00438-o. [DOI] [PubMed] [Google Scholar]
- Torok TL, Nagykaldi Z, Saska Z, Kovacs T, Nada SA, Zilliikens S, Magyar K, Sylvester Vizi E. Presynaptic alpha2-receptors regulate reverse Na+/Ca2+-exchange and transmitter release in Na+-loaded peripheral sympathetic nerves. Neurochem Int. 2004;45:699–711. doi: 10.1016/j.neuint.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Ventura R, Morrone C, Puglisi-Allegra S. Prefrontal/accumbal catecholamine system determines motivational salience attribution to both reward- and aversion-related stimuli. Proc Natl Acad Sci. 2007;104:5181–5186. doi: 10.1073/pnas.0610178104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villegier AS, Drouin C, Bizot JC, Marien M, Glowinski J, Colpaert F, Tassin JP. Stimulation of postsynaptic alpha1b- and alpha2-adrenergic receptors amplifies dopamine-mediated locomotor activity in both rats and mice. Synapse. 2003;50:277–284. doi: 10.1002/syn.10267. [DOI] [PubMed] [Google Scholar]
- Wee S, Specio SE, Koob GF. Effects of dose and session duration on the rate of cocaine self-administration. J Pharmacol Exp Ther. 2007;320:1134–1143. doi: 10.1124/jpet.106.113340. [DOI] [PubMed] [Google Scholar]
- Wee S, Wang Z, He R, Zhou J, Kozikowski AP, Woolverton WL. Role of the increased noradrenergic neurotransmission in drug self-administration. Drug Alcohol Depend. 2006;82:151–157. doi: 10.1016/j.drugalcdep.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Wee S, Woolverton WL. Evaluation of the reinforcing effects of atomoxetine in monkeys: comparison to methylphenidate and desipramine. Drug Alcohol Depend. 2004;75:271–276. doi: 10.1016/j.drugalcdep.2004.03.010. [DOI] [PubMed] [Google Scholar]
- Weinshenker D, Schroeder JP. There and Back Again: A Tale of Norepinephrine and Drug Addiction. Neuropsychopharmacology. 2006 doi: 10.1038/sj.npp.1301263. in press. [DOI] [PubMed] [Google Scholar]
- Weinshenker D, Miller NS, Blizinsky K, Laughlin ML, Palmiter RD. Mice with chronic norepinephrine deficiency resemble amphetamine-sensitized animals. Proc Natl Acad Sci. 2002;99:13873–13877. doi: 10.1073/pnas.212519999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellman P, Ho D, Cepeda-Benito A, Bellinger L, Nation J. Cocaine-induced hypophagia and hyperlocomotion in rats are attenuated by prazosin. Eur J Pharmacol. 2002;455:117–126. doi: 10.1016/s0014-2999(02)02616-x. [DOI] [PubMed] [Google Scholar]
- Wilson MC, Schuster CR. Aminergic influences on intravenous cocaine self-administration by Rhesus monkeys. Pharmacol Biochem Behav. 1974;2:563–571. doi: 10.1016/0091-3057(74)90021-5. [DOI] [PubMed] [Google Scholar]
- Woolverton WL. Evaluation of the role of norepinephrine in the reinforcing effects of psychomotor stimulants in rhesus monkeys. Pharmacol Biochem Behav. 1987;26:835–839. doi: 10.1016/0091-3057(87)90618-6. [DOI] [PubMed] [Google Scholar]
- Woolverton WL, Wessinger WD, Balster RL. Reinforcing properties of clonidine in rhesus monkeys. Psychopharmacology. 1982;77:17–23. doi: 10.1007/BF00436094. [DOI] [PubMed] [Google Scholar]
- Yokel RA, Wise RA. Amphetamine- type reinforcement by dopaminergic agonists in the rat. Psychopharmacology. 1978;58:289–296. doi: 10.1007/BF00427393. [DOI] [PubMed] [Google Scholar]
- Yokel RA, Wise RA. Attenuation of intravenous amphetamine reinforcement by central dopamine blockade in rats. Psychopharmacology. 1976;48:311–318. doi: 10.1007/BF00496868. [DOI] [PubMed] [Google Scholar]
- Zhang XY, Kosten TA. Previous exposure to cocaine enhances cocaine self-administration in an alpha 1-adrenergic receptor dependent manner. Neuropsychopharmacology. 2007;32:638–645. doi: 10.1038/sj.npp.1301120. [DOI] [PubMed] [Google Scholar]
- Zhang XY, Kosten TA. Prazosin, an alpha-1 adrenergic antagonist, reduces cocaine-induced reinstatement of drug-seeking. Biol Psychiatry. 2005;57:1202–1204. doi: 10.1016/j.biopsych.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Zhang HT, Frith SA, Wilkins J, O’Donnell JM. Comparison of the effects of isoproterenol administered into the hippocampus, frontal cortex, or amygdala on behavior of rats maintained by differential reinforcement of low response rate. Psychopharmacology. 2001;159:89–97. doi: 10.1007/s002130100889. [DOI] [PubMed] [Google Scholar]


