Abstract
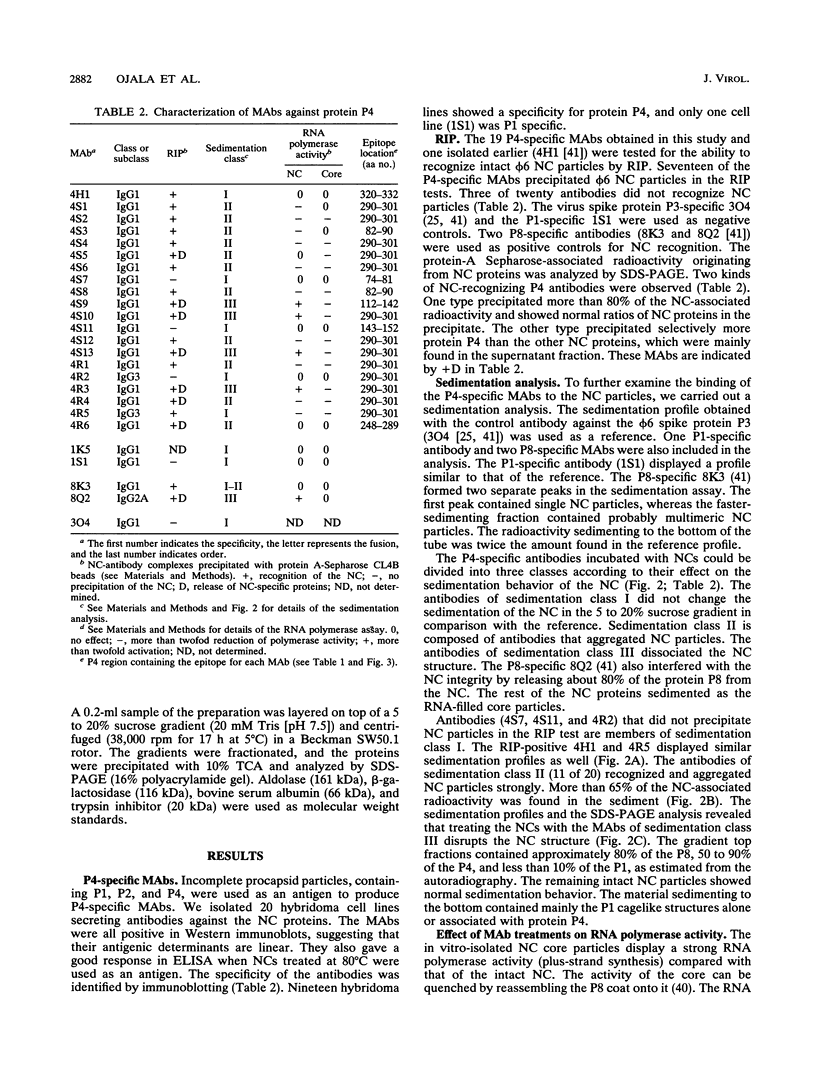
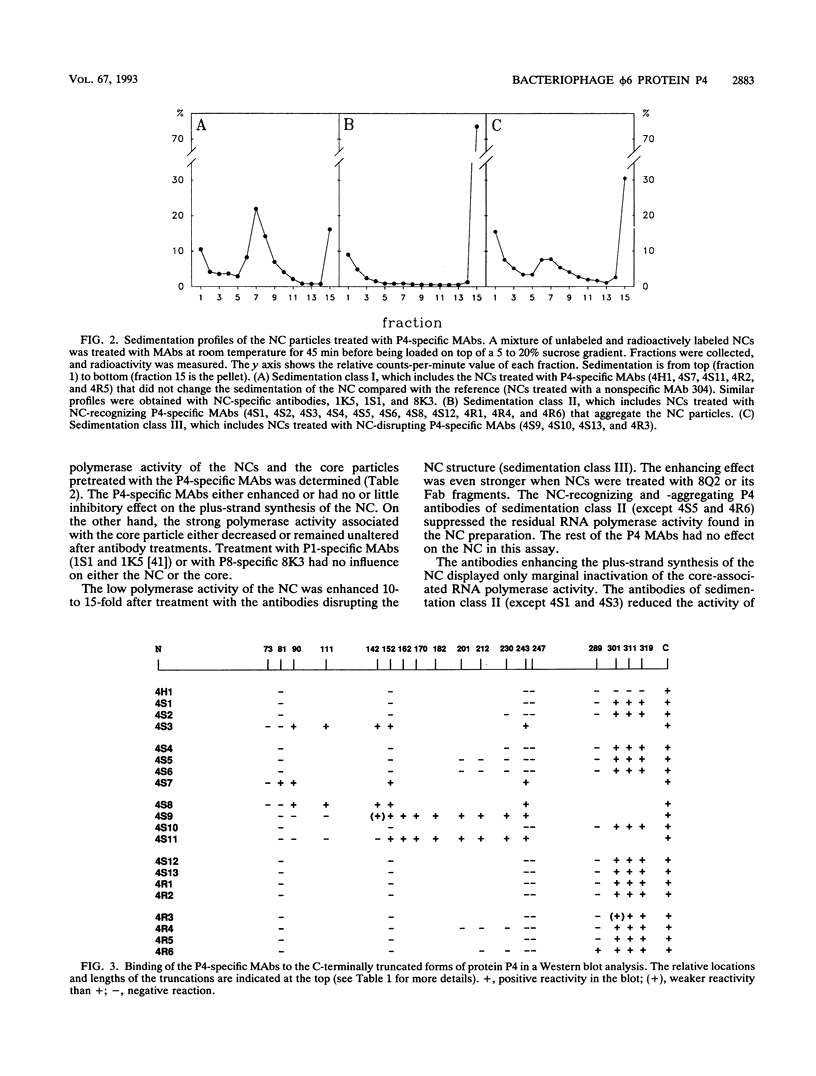
Protein P4, an early protein of double-stranded RNA bacteriophage phi 6, is a component of the virion-associated RNA polymerase complex and possesses a nucleoside triphosphate (NTP) phosphohydrolase activity. We have produced and characterized a panel of 20 P4-specific monoclonal antibodies. Epitope mapping using truncated molecules of recombinant P4 revealed seven linear epitopes. The accessibility of the epitopes on the phi 6 nucleocapsid (NC) surface showed that at least the C terminus and an internal domain, containing the consensus sequence for NTP binding, protrude the NC shell. Four of the NC-binding antibodies distorted the integrity of the NC by releasing protein P4 and the major NC surface protein P8. This finding suggests a close contact between these two proteins. The dissociation of the NC led to the activation of the virion-associated RNA polymerase. The multimeric status of the recombinant P4 was similar to that of the virion-associated P4, indicating that no accessory virus proteins are needed for its multimerization.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Annunziato M. E., Marciani D. J. Efficient purification of mouse monoclonal antibodies from ascites fluid by medium-performance anion exchange chromatography. Gene Anal Tech. 1987 Jan-Feb;4(1):1–4. doi: 10.1016/0735-0651(87)90005-7. [DOI] [PubMed] [Google Scholar]
- Bamford D. H., Palva E. T. Structure of the lipid-containing bacteriophage phi 6. Disruption by Triton X-100 treatment. Biochim Biophys Acta. 1980 Sep 18;601(2):245–259. doi: 10.1016/0005-2736(80)90530-1. [DOI] [PubMed] [Google Scholar]
- Bamford D. H. lipid-containing bacterial viruses: disruption studies on phi 6. Prog Clin Biol Res. 1981;64:477–489. [PubMed] [Google Scholar]
- Borsa J., Sargent M. D., Lievaart P. A., Copps T. P. Reovirus: evidence for a second step in the intracellular uncoating and transcriptase activation process. Virology. 1981 May;111(1):191–200. doi: 10.1016/0042-6822(81)90664-4. [DOI] [PubMed] [Google Scholar]
- Bruenn J. A. Relationships among the positive strand and double-strand RNA viruses as viewed through their RNA-dependent RNA polymerases. Nucleic Acids Res. 1991 Jan 25;19(2):217–226. doi: 10.1093/nar/19.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol Relat Areas Mol Biol. 1978;47:45–148. doi: 10.1002/9780470122921.ch2. [DOI] [PubMed] [Google Scholar]
- Cohen J., Laporte J., Charpilienne A., Scherrer R. Activation of rotavirus RNA polymerase by calcium chelation. Arch Virol. 1979;60(3-4):177–186. doi: 10.1007/BF01317489. [DOI] [PubMed] [Google Scholar]
- Cohen J. Ribonucleic acid polymerase activity associated with purified calf rotavirus. J Gen Virol. 1977 Sep;36(3):395–402. doi: 10.1099/0022-1317-36-3-395. [DOI] [PubMed] [Google Scholar]
- Cuppels D. A., Van Etten J. L., Burbank D. E., Lane L. C., Vidaver A. K. In vitro translation of the three bacteriophage phi 6 RNAs. J Virol. 1980 Jul;35(1):249–251. doi: 10.1128/jvi.35.1.249-251.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day L. A., Mindich L. The molecular weight of bacteriophage phi 6 and its nucleocapsid. Virology. 1980 Jun;103(2):376–385. doi: 10.1016/0042-6822(80)90196-8. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emini E. A., Hughes J. V., Perlow D. S., Boger J. Induction of hepatitis A virus-neutralizing antibody by a virus-specific synthetic peptide. J Virol. 1985 Sep;55(3):836–839. doi: 10.1128/jvi.55.3.836-839.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emori Y., Iba H., Okada Y. Semi-conservative transcription of double-stranded RNA catalyzed by bacteriophage phi 6 RNA polymerase. J Biochem. 1980 Dec;88(6):1569–1575. doi: 10.1093/oxfordjournals.jbchem.a133131. [DOI] [PubMed] [Google Scholar]
- Etten J. V., Lane L., Gonzalez C., Partridge J., Vidaver A. Comparative properties of bacteriophage phi6 and phi6 nucleocapsid. J Virol. 1976 May;18(2):652–658. doi: 10.1128/jvi.18.2.652-658.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frilander M., Gottlieb P., Strassman J., Bamford D. H., Mindich L. Dependence of minus-strand synthesis on complete genomic packaging in the double-stranded RNA bacteriophage phi 6. J Virol. 1992 Aug;66(8):5013–5017. doi: 10.1128/jvi.66.8.5013-5017.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gefter M. L., Margulies D. H., Scharff M. D. A simple method for polyethylene glycol-promoted hybridization of mouse myeloma cells. Somatic Cell Genet. 1977 Mar;3(2):231–236. doi: 10.1007/BF01551818. [DOI] [PubMed] [Google Scholar]
- Gottlieb P., Strassman J., Bamford D. H., Mindich L. Production of a polyhedral particle in Escherichia coli from a cDNA copy of the large genomic segment of bacteriophage phi 6. J Virol. 1988 Jan;62(1):181–187. doi: 10.1128/jvi.62.1.181-187.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb P., Strassman J., Frucht A., Qiao X. Y., Mindich L. In vitro packaging of the bacteriophage phi 6 ssRNA genomic precursors. Virology. 1991 Apr;181(2):589–594. doi: 10.1016/0042-6822(91)90892-f. [DOI] [PubMed] [Google Scholar]
- Gottlieb P., Strassman J., Mindich L. Protein P4 of the bacteriophage phi 6 procapsid has a nucleoside triphosphate-binding site with associated nucleoside triphosphate phosphohydrolase activity. J Virol. 1992 Oct;66(10):6220–6222. doi: 10.1128/jvi.66.10.6220-6222.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb P., Strassman J., Qiao X. Y., Frucht A., Mindich L. In vitro replication, packaging, and transcription of the segmented double-stranded RNA genome of bacteriophage phi 6: studies with procapsids assembled from plasmid-encoded proteins. J Bacteriol. 1990 Oct;172(10):5774–5782. doi: 10.1128/jb.172.10.5774-5782.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb P., Strassman J., Qiao X., Frilander M., Frucht A., Mindich L. In vitro packaging and replication of individual genomic segments of bacteriophage phi 6 RNA. J Virol. 1992 May;66(5):2611–2616. doi: 10.1128/jvi.66.5.2611-2616.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantula J., Bamford D. H. Chemical crosslinking of bacteriophage phi 6 nucleocapsid proteins. Virology. 1988 Aug;165(2):482–488. doi: 10.1016/0042-6822(88)90592-2. [DOI] [PubMed] [Google Scholar]
- Huismans H., van Dijk A. A., Els H. J. Uncoating of parental bluetongue virus to core and subcore particles in infected L cells. Virology. 1987 Mar;157(1):180–188. doi: 10.1016/0042-6822(87)90327-8. [DOI] [PubMed] [Google Scholar]
- Jameson B. A., Wolf H. The antigenic index: a novel algorithm for predicting antigenic determinants. Comput Appl Biosci. 1988 Mar;4(1):181–186. doi: 10.1093/bioinformatics/4.1.181. [DOI] [PubMed] [Google Scholar]
- Kenney J. M., Hantula J., Fuller S. D., Mindich L., Ojala P. M., Bamford D. H. Bacteriophage phi 6 envelope elucidated by chemical cross-linking, immunodetection, and cryoelectron microscopy. Virology. 1992 Oct;190(2):635–644. doi: 10.1016/0042-6822(92)90901-z. [DOI] [PubMed] [Google Scholar]
- Koonin E. V., Gorbalenya A. E., Chumakov K. M. Tentative identification of RNA-dependent RNA polymerases of dsRNA viruses and their relationship to positive strand RNA viral polymerases. FEBS Lett. 1989 Jul 31;252(1-2):42–46. doi: 10.1016/0014-5793(89)80886-5. [DOI] [PubMed] [Google Scholar]
- Ktistakis N. T., Lang D. The dodecahedral framework of the bacteriophage phi 6 nucleocapsid is composed of protein P1. J Virol. 1987 Aug;61(8):2621–2623. doi: 10.1128/jvi.61.8.2621-2623.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Köhler G., Howe S. C., Milstein C. Fusion between immunoglobulin-secreting and nonsecreting myeloma cell lines. Eur J Immunol. 1976 Apr;6(4):292–295. doi: 10.1002/eji.1830060411. [DOI] [PubMed] [Google Scholar]
- Ludert J. E., Michelangeli F., Gil F., Liprandi F., Esparza J. Penetration and uncoating of rotaviruses in cultured cells. Intervirology. 1987;27(2):95–101. doi: 10.1159/000149726. [DOI] [PubMed] [Google Scholar]
- Mindich L. Bacteriophage phi 6: a unique virus having a lipid-containing membrane and a genome composed of three dsRNA segments. Adv Virus Res. 1988;35:137–176. doi: 10.1016/s0065-3527(08)60710-1. [DOI] [PubMed] [Google Scholar]
- Mindich L., MacKenzie G., Strassman J., McGraw T., Metzger S., Romantschuk M., Bamford D. cDNA cloning of portions of the bacteriophage phi 6 genome. J Bacteriol. 1985 Jun;162(3):992–999. doi: 10.1128/jb.162.3.992-999.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mindich L., Nemhauser I., Gottlieb P., Romantschuk M., Carton J., Frucht S., Strassman J., Bamford D. H., Kalkkinen N. Nucleotide sequence of the large double-stranded RNA segment of bacteriophage phi 6: genes specifying the viral replicase and transcriptase. J Virol. 1988 Apr;62(4):1180–1185. doi: 10.1128/jvi.62.4.1180-1185.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mindich L., Sinclair J. F., Cohen J. The morphogenesis of bacteriophage phi6: particles formed by nonsense mutants. Virology. 1976 Nov;75(1):224–231. doi: 10.1016/0042-6822(76)90021-0. [DOI] [PubMed] [Google Scholar]
- Ojala P. M., Romantschuk M., Bamford D. H. Purified phi 6 nucleocapsids are capable of productive infection of host cells with partially disrupted outer membranes. Virology. 1990 Oct;178(2):364–372. doi: 10.1016/0042-6822(90)90333-m. [DOI] [PubMed] [Google Scholar]
- Olkkonen V. M., Bamford D. H. Quantitation of the adsorption and penetration stages of bacteriophage phi 6 infection. Virology. 1989 Jul;171(1):229–238. doi: 10.1016/0042-6822(89)90530-8. [DOI] [PubMed] [Google Scholar]
- Olkkonen V. M., Bamford D. H. The nucleocapsid of the lipid-containing double-stranded RNA bacteriophage phi 6 contains a protein skeleton consisting of a single polypeptide species. J Virol. 1987 Aug;61(8):2362–2367. doi: 10.1128/jvi.61.8.2362-2367.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olkkonen V. M., Gottlieb P., Strassman J., Qiao X. Y., Bamford D. H., Mindich L. In vitro assembly of infectious nucleocapsids of bacteriophage phi 6: formation of a recombinant double-stranded RNA virus. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9173–9177. doi: 10.1073/pnas.87.23.9173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olkkonen V. M., Ojala P. M., Bamford D. H. Generation of infectious nucleocapsids by in vitro assembly of the shell protein on to the polymerase complex of the dsRNA bacteriophage phi 6. J Mol Biol. 1991 Apr 5;218(3):569–581. doi: 10.1016/0022-2836(91)90702-8. [DOI] [PubMed] [Google Scholar]
- Olkkonen V. M., Pekkala P. M., Bamford D. H. Monoclonal antibodies to the major structural proteins of bacteriophage phi 6. Virology. 1988 Jul;165(1):317–320. doi: 10.1016/0042-6822(88)90693-9. [DOI] [PubMed] [Google Scholar]
- Romantschuk M., Olkkonen V. M., Bamford D. H. The nucleocapsid of bacteriophage phi 6 penetrates the host cytoplasmic membrane. EMBO J. 1988 Jun;7(6):1821–1829. doi: 10.1002/j.1460-2075.1988.tb03014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent M. D., Long D. G., Borsa J. Functional analysis of the interactions between reovirus particles and various proteases in vitro. Virology. 1977 May 1;78(1):354–358. doi: 10.1016/0042-6822(77)90110-6. [DOI] [PubMed] [Google Scholar]
- Semancik J. S., Vidaver A. K., Van Etten J. L. Characterization of segmented double-helical RNA from bacteriophage phi6. J Mol Biol. 1973 Aug 25;78(4):617–625. doi: 10.1016/0022-2836(73)90283-0. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Moffatt B. A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986 May 5;189(1):113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- Sturzenbecker L. J., Nibert M., Furlong D., Fields B. N. Intracellular digestion of reovirus particles requires a low pH and is an essential step in the viral infectious cycle. J Virol. 1987 Aug;61(8):2351–2361. doi: 10.1128/jvi.61.8.2351-2361.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1074–1078. doi: 10.1073/pnas.82.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usala S. J., Brownstein B. H., Haselkorn R. Displacement of parental RNA strands during in vitro transcription by bacteriophage phi 6 nucleocapsids. Cell. 1980 Apr;19(4):855–862. doi: 10.1016/0092-8674(80)90076-8. [DOI] [PubMed] [Google Scholar]
- Van Etten J. L., Burbank D. E., Cuppels D. A., Lane L. C., Vidaver A. K. Semiconservative synthesis of single-stranded RNA by bacteriophage phi 6 RNA polymerase. J Virol. 1980 Feb;33(2):769–773. doi: 10.1128/jvi.33.2.769-773.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Etten J. L., Vidaver A. K., Koski R. K., Burnett J. P. Base composition and hybridization studies of the three double-stranded RNA segments of bacteriophage phi 6. J Virol. 1974 Jun;13(6):1254–1262. doi: 10.1128/jvi.13.6.1254-1262.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidaver A. K., Koski R. K., Van Etten J. L. Bacteriophage phi6: a Lipid-Containing Virus of Pseudomonas phaseolicola. J Virol. 1973 May;11(5):799–805. doi: 10.1128/jvi.11.5.799-805.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J. E., Saraste M., Runswick M. J., Gay N. J. Distantly related sequences in the alpha- and beta-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1982;1(8):945–951. doi: 10.1002/j.1460-2075.1982.tb01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]



