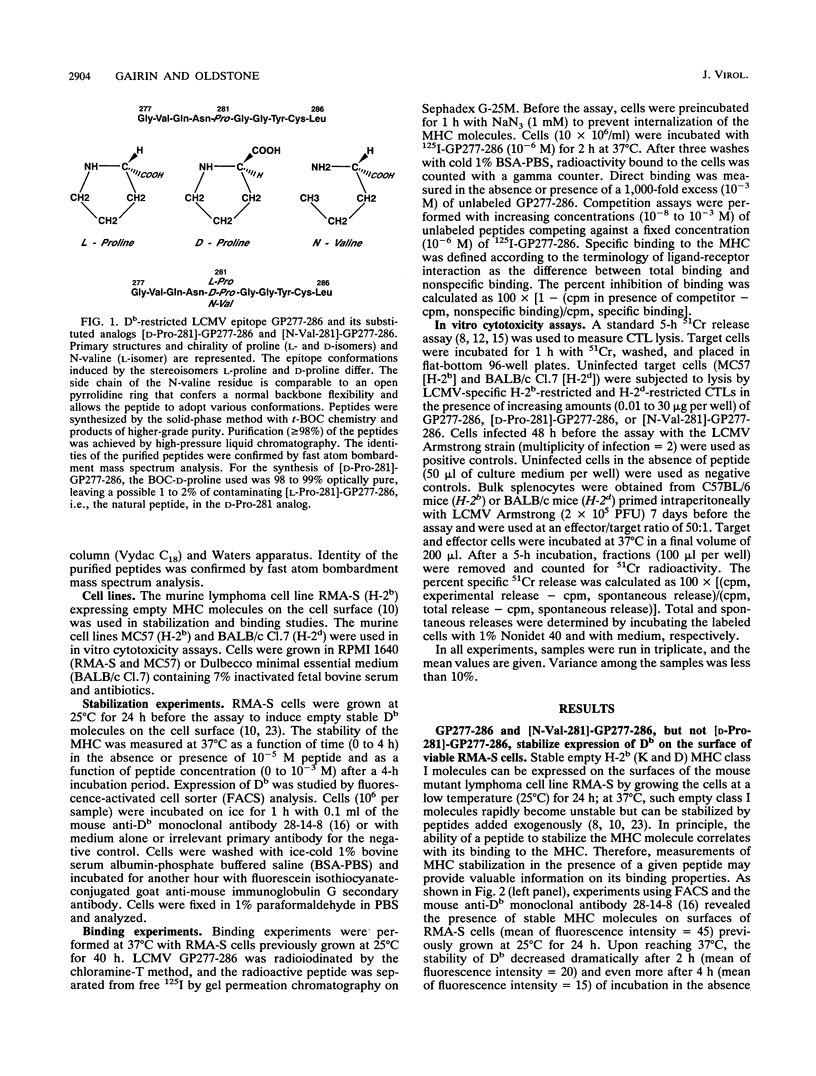
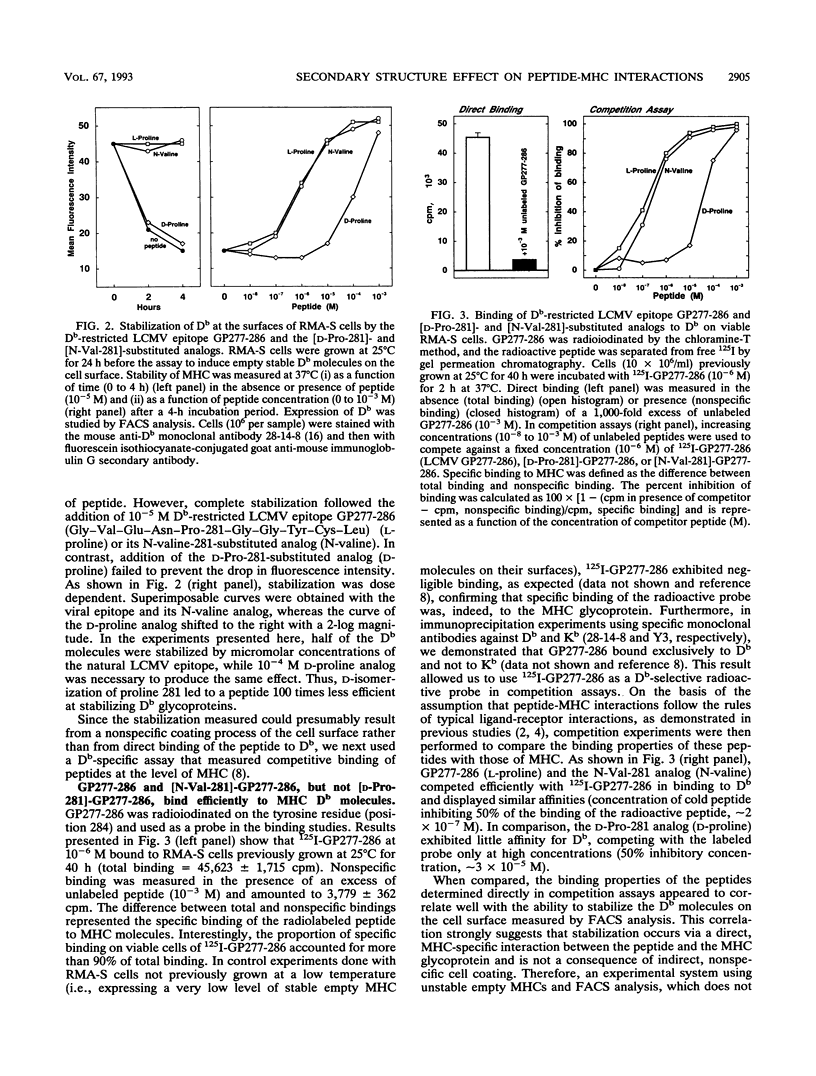
Abstract
Viral antigens are presented to cytotoxic T lymphocytes (CTLs) by H-2-restricted major histocompatibility complex (MHC) glycoproteins. Binding of the endogenously processed viral peptides (epitopes) to their specific MHC molecules is an early intracellular event in the recognition process and is necessary for subsequent killing of virus-infected cells by virus-specific CTLs. It is now well established that interaction between a viral antigenic peptide and MHC is dependent on the primary structure (length and amino acid sequence) of that antigen. Here we show, using the H-2Db-restricted epitope GP277-286 of lymphocytic choriomeningitis virus as a model, that the secondary structure (conformation) of the viral sequence also plays a crucial role in the binding of a viral antigen to MHC glycoprotein and in its subsequent presentation to virus-specific CTLs.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barinaga M. Getting some "backbone": how MHC binds peptides. Science. 1992 Aug 14;257(5072):880–881. doi: 10.1126/science.1502554. [DOI] [PubMed] [Google Scholar]
- Cerundolo V., Elliott T., Elvin J., Bastin J., Rammensee H. G., Townsend A. The binding affinity and dissociation rates of peptides for class I major histocompatibility complex molecules. Eur J Immunol. 1991 Sep;21(9):2069–2075. doi: 10.1002/eji.1830210915. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of protein conformation. Biochemistry. 1974 Jan 15;13(2):222–245. doi: 10.1021/bi00699a002. [DOI] [PubMed] [Google Scholar]
- Christinck E. R., Luscher M. A., Barber B. H., Williams D. B. Peptide binding to class I MHC on living cells and quantitation of complexes required for CTL lysis. Nature. 1991 Jul 4;352(6330):67–70. doi: 10.1038/352067a0. [DOI] [PubMed] [Google Scholar]
- De Magistris M. T., Alexander J., Coggeshall M., Altman A., Gaeta F. C., Grey H. M., Sette A. Antigen analog-major histocompatibility complexes act as antagonists of the T cell receptor. Cell. 1992 Feb 21;68(4):625–634. doi: 10.1016/0092-8674(92)90139-4. [DOI] [PubMed] [Google Scholar]
- Falk K., Rötzschke O., Stevanović S., Jung G., Rammensee H. G. Allele-specific motifs revealed by sequencing of self-peptides eluted from MHC molecules. Nature. 1991 May 23;351(6324):290–296. doi: 10.1038/351290a0. [DOI] [PubMed] [Google Scholar]
- Fremont D. H., Matsumura M., Stura E. A., Peterson P. A., Wilson I. A. Crystal structures of two viral peptides in complex with murine MHC class I H-2Kb. Science. 1992 Aug 14;257(5072):919–927. doi: 10.1126/science.1323877. [DOI] [PubMed] [Google Scholar]
- Gairin J. E., Oldstone M. B. Design of high-affinity major histocompatibility complex-specific antagonist peptides that inhibit cytotoxic T-lymphocyte activity: implications for control of viral disease. J Virol. 1992 Nov;66(11):6755–6762. doi: 10.1128/jvi.66.11.6755-6762.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grey H. M., Sette A., Lamont A. Biologic significance and therapeutic implications of antigen/MHC interactions. Clin Immunol Immunopathol. 1989 Nov;53(2 Pt 2):S47–S52. doi: 10.1016/0090-1229(89)90069-x. [DOI] [PubMed] [Google Scholar]
- Ljunggren H. G., Stam N. J., Ohlén C., Neefjes J. J., Höglund P., Heemels M. T., Bastin J., Schumacher T. N., Townsend A., Kärre K. Empty MHC class I molecules come out in the cold. Nature. 1990 Aug 2;346(6283):476–480. doi: 10.1038/346476a0. [DOI] [PubMed] [Google Scholar]
- Madden D. R., Gorga J. C., Strominger J. L., Wiley D. C. The structure of HLA-B27 reveals nonamer self-peptides bound in an extended conformation. Nature. 1991 Sep 26;353(6342):321–325. doi: 10.1038/353321a0. [DOI] [PubMed] [Google Scholar]
- Maryanski J. L., Verdini A. S., Weber P. C., Salemme F. R., Corradin G. Competitor analogs for defined T cell antigens: peptides incorporating a putative binding motif and polyproline or polyglycine spacers. Cell. 1990 Jan 12;60(1):63–72. doi: 10.1016/0092-8674(90)90716-r. [DOI] [PubMed] [Google Scholar]
- Matsumura M., Fremont D. H., Peterson P. A., Wilson I. A. Emerging principles for the recognition of peptide antigens by MHC class I molecules. Science. 1992 Aug 14;257(5072):927–934. doi: 10.1126/science.1323878. [DOI] [PubMed] [Google Scholar]
- Némethy G., Scheraga H. A. Protein folding. Q Rev Biophys. 1977 Aug;10(3):239–252. doi: 10.1017/s0033583500002936. [DOI] [PubMed] [Google Scholar]
- Oldstone M. B., Whitton J. L., Lewicki H., Tishon A. Fine dissection of a nine amino acid glycoprotein epitope, a major determinant recognized by lymphocytic choriomeningitis virus-specific class I-restricted H-2Db cytotoxic T lymphocytes. J Exp Med. 1988 Aug 1;168(2):559–570. doi: 10.1084/jem.168.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozato K., Hansen T. H., Sachs D. H. Monoclonal antibodies to mouse MHC antigens. II. Antibodies to the H-2Ld antigen, the products of a third polymorphic locus of the mouse major histocompatibility complex. J Immunol. 1980 Dec;125(6):2473–2477. [PubMed] [Google Scholar]
- Richards N. G., Hinds M. G., Brennand D. M., Glennie M. J., Welsh J. M., Robinson J. A. Probing the role of proline as a recognition element in peptide antigens. Biochem Pharmacol. 1990 Jul 1;40(1):119–123. doi: 10.1016/0006-2952(90)90186-o. [DOI] [PubMed] [Google Scholar]
- Rothbard J. B., Gefter M. L. Interactions between immunogenic peptides and MHC proteins. Annu Rev Immunol. 1991;9:527–565. doi: 10.1146/annurev.iy.09.040191.002523. [DOI] [PubMed] [Google Scholar]
- Rötzschke O., Falk K., Deres K., Schild H., Norda M., Metzger J., Jung G., Rammensee H. G. Isolation and analysis of naturally processed viral peptides as recognized by cytotoxic T cells. Nature. 1990 Nov 15;348(6298):252–254. doi: 10.1038/348252a0. [DOI] [PubMed] [Google Scholar]
- Rötzschke O., Falk K. Naturally-occurring peptide antigens derived from the MHC class-I-restricted processing pathway. Immunol Today. 1991 Dec;12(12):447–455. doi: 10.1016/0167-5699(91)90018-O. [DOI] [PubMed] [Google Scholar]
- Sette A., Lamont A., Buus S., Colon S. M., Miles C., Grey H. M. Effect of conformational propensity of peptide antigens in their interaction with MHC class II molecules. Failure to document the importance of regular secondary structures. J Immunol. 1989 Aug 15;143(4):1268–1273. [PubMed] [Google Scholar]
- Townsend A., Bodmer H. Antigen recognition by class I-restricted T lymphocytes. Annu Rev Immunol. 1989;7:601–624. doi: 10.1146/annurev.iy.07.040189.003125. [DOI] [PubMed] [Google Scholar]
- Townsend A., Elliott T., Cerundolo V., Foster L., Barber B., Tse A. Assembly of MHC class I molecules analyzed in vitro. Cell. 1990 Jul 27;62(2):285–295. doi: 10.1016/0092-8674(90)90366-m. [DOI] [PubMed] [Google Scholar]
- Van Bleek G. M., Nathenson S. G. Isolation of an endogenously processed immunodominant viral peptide from the class I H-2Kb molecule. Nature. 1990 Nov 15;348(6298):213–216. doi: 10.1038/348213a0. [DOI] [PubMed] [Google Scholar]
- Zinkernagel R. M., Doherty P. C. Restriction of in vitro T cell-mediated cytotoxicity in lymphocytic choriomeningitis within a syngeneic or semiallogeneic system. Nature. 1974 Apr 19;248(5450):701–702. doi: 10.1038/248701a0. [DOI] [PubMed] [Google Scholar]



