Abstract
Leishmaniases are diseases caused by protozoa of the genus Leishmania that affect more than 20 million people in the world. The initial phase of the infection is fundamental for either the progression or control of the disease. The Leishmania parasites are injected in the skin as promastigotes and then, after been phagocytized by the host macrophages, rapidly transform into amastigotes. In this phase different nonspecific cellular and humoral elements participate. We have shown previously that insulin-like growth factor (IGF)-I that is constitutively present in the skin induces growth of Leishmania promastigotes. In the present paper we show further evidence for the importance of this factor: (i) IGF-I also can induce a growth response in Leishmania (Leishmania) mexicana amastigotes; (ii) IGF-I binds specifically to a putative single-site receptor on both promastigotes and amastigotes; (iii) IGF-I induces a rapid tyrosine phosphorylation of parasite proteins with different molecular mass in promastigotes and amastigotes of L. (L.) mexicana; and, finally, (iv) the cutaneous lesion in the mice when challenged by IGF-I-preactivated Leishmania (Viannia) panamensis is increased significantly because of inflammatory process and growth of parasites. We thus suggest that IGF-I is another important host factor participating in the Leishmania–host interplay in the early stage during the establishment of the infection and presumably also in the later stages.
Keywords: tyrosine phosphorylation/protozoa/binding/leishmaniasis
Leishmaniases are diseases caused by protozoa of the genus Leishmania that affect more than 20 million people in the world (1). The infectious cycle in the vertebrate host is initiated when Leishmania promastigotes are injected into the skin by the insect vector. In the host the promastigotes transform into amastigotes inside the macrophages where they continue proliferating. Immunity in leishmaniasis is considered mainly to be T cell-mediated (2, 3), but more and more nonspecific factors acting in the early stage of the infection have been considered as important for the outcome of the infection (3, 4). Besides complement, polymorphonuclear neutrophils, and eosinophils (2, 5), other growth factors or cytokines such as granulocyte–macrophage colony-stimulating factor, transforming growth factor β, and tumor necrosis factor (6–9) have been reported to participate in this phase. Macrophages and natural killer cells also have been considered important in this phase as source of interleukin-12 and interferon γ, respectively, and thus guiding the preferential activation of T helper 1 or T helper 2 subpopulation of CD4+ T cells (10, 11).
Insulin-like growth factors (IGFs) are evolutionary well conserved polypeptides with an approximate molecular mass of 7.5 kDa. The most abundant forms are IGF-I and -II. They are detectable both in circulation and in tissues, mainly associated to IGF-binding proteins. Most cell types have the ability to produce IGFs although the main site of production is the liver. Upon stimulation several cell types, including monocytes and mitogen-stimulated T cells, display an enhanced production of IGF-I (for excellent reviews see refs. 12–14). Functionally, IGFs have been shown to affect cell metabolism (e.g., glucose) and to be an important endocrine growth and differentiation factor in inflammation, immune activation, and wound healing (14–16). In the skin it is possible to detect a constitutive production of IGF-I, and an increase in the mRNA level of IGF-I at the site of the lesion has been reported when a wound is inflicted (17). In the macrophages, habitat of Leishmania amastigotes, immunoreactive IGF-I has been detected (18). Therefore, it is likely that IGF-I is one of the first growth/growth-inducing factors that the Leishmania promastigotes encounter in the skin soon after their transmission and that amastigotes interact with within the macrophages. We have shown previously that IGF-I but not IGF-II induces a growth response of promastigotes of several species of Leishmania (19). In the present report we have extended these studies and show that IGF-I also induces a growth response in Leishmania amastigote-like forms. IGF-I has a relatively high specific binding to both forms and induces a stage-specific phosphorylation of tyrosine residues of respective parasite proteins. Finally, we show that Leishmania promastigotes preactivated with IGF-I greatly enhance the size of the lesion and the number of intact parasites when injected in vivo.
MATERIALS AND METHODS
Mice.
Six- to 10-week-old inbred BALB/c strain mice of both sexes were obtained from our own breeding facility at the Medical School at the University of Sao Paulo, Brazil.
Parasites.
Leishmania (Viannia) panamensis (HSJD-1 strain) (20) and Leishmania (Leishmania) amazonensis (IOC-L l85 strain, WHOM/BR/OO/LTBOOl6) were used. The established axenic culture of amastigote-like forms of Leishmania (Leishmania) mexicana (MNYC/BZ/62/M379), originally from D. G. Russel’s laboratory (Washington University School of Medicine), was kindly provided by S. C. Alfieri (University of Sao Paulo) (21). Leishmania (Leishmania) mexicana promastigotes were derived from this strain. The promastigotes were maintained in NNN medium overlayered by RPMI 1640 medium (GIBCO) with 10% heat-inactivated fetal calf serum (FCS) (WL. Imunoquímica, Rio de Janeiro, Brazil) at 25°C, with periodical passage in BALB/c mice. The parasite culture was expanded in RPMI 1640 medium with 10% FCS and grown until stationary phase for experiments. After washing, for injection in mice the concentration was adjusted to 2 × 108 parasites per ml in RPMI 1640 medium. The amastigote-like forms were maintained at 33°C in M-54 medium, pH 6.0, consisting in medium 199 with Hanks’ salts (GIBCO/BRL) supplemented with 2.5 mg/ml glucose/5 mg/ml trypticase/750 μg/ml l-glutamine/20 μg/ml hemin/5 μg/ml gentamicin/20% of heat-inactivated FCS and 25 mM Hepes (21).
Reagents.
Recombinant human IGF-I and -II, produced in Escherichia coli, was a kind gift from M. Lake, Kabi-Pharmacia, Sweden (for details on purification see ref. 15) or purchased from R & D Systems. Monoclonal anti-phosphotyrosine antibody (22) was kindly provided by R. I. Schumacker (University of Sao Paulo, Brazil). 125I (100 mCi/ml), 125I -IGF (6.6 μCi/μg), 125I-protein A (35 μCi/μg), and [3H]thymidine (2 Ci/mmol), and 14C-Rainbow molecular weight markers were purchased from Amersham. Phenylmethylsulfonyl fluoride, aprotinin, leupeptin, sodium pyrophosphate, sodium fluoride, sodium orthovanadate, tris(hydroxymethyl)aminomethane (Tris), Triton X-100, and BSA RIA grade (with no detectable amounts of IGF contamination as determined by a radioreceptor assay; Daniel Gianella Neto, personal communication) were from Sigma. Enzymobeads for lactoperoxidase labeling were purchased from Bio-Rad. Mouse monoclonal anti-phosphotyrosine IgG3 antibody secreted by the hybridoma cell line FB2 and purified by affinity chromatography (22, 23) was kindly provided by R. I. Schumacher (University of Sao Paulo, Brazil).
Measurement of Proliferative Responses to IGF-I and -II.
Stationary-phase L. (L.) amazonensis and L. (L.) mexicana promastigotes and L. (L.) mexicana amastigote-like forms after washing in 0.01 M PBS, pH 7.2, had the concentration adjusted to 107 parasites/ml in RPMI 1640 medium without FCS. Parasites (2 × 105/well) in 96-well flat-bottomed microculture plates (Costar) were cultured in the presence of recombinant IGF-I or IGF-II (1–50 ng/ml) and in the presence of FCS (4–8%) as indicated in the figures. The cultures were pulsed with [3H]thymidine (1 μCi/well) either at time 0 (for 24-hr culture) or after 24 hr (for 48-hr culture) and incubated either at 25°C (promastigotes) or 33°C (amastigote-like forms) for an additional 24 hr. The parasite cultures were then harvested onto glass-fiber filter paper (Flow Laboratories) by using a cell harvester (Titertek, Huntsville, AL). The incorporated radioactivity was assessed by liquid scintillation in a beta counter (Beckman). Besides [3H]thymidine incorporation, the proliferation of L. (L.) mexicana amastigote-like forms grown in the presence of 0, 10, or 50 ng/ml of IGF-I in RPMI medium with 4% FCS also was evaluated after 24 hr, counting the actual number of amastigotes under light microscope. The results are expressed either in cpm or as parasite index = number of parasites in cultures with IGF-I/number of parasites in cultures without IGF-I.
Radioiodination.
When commercial 125I-IGF-I was not available, 0.5 μg of recombinant IGF-I was labeled with 0.5 mCi 125I by lactoperoxidase using Enzymobeads according to the protocol supplied by the manufacturer (Bio-Rad). The labeling resulted in a specific activity of 30–40 μCi/μg, and the free 125I represented less than 1% as determined by trichloroacetic acid precipitation.
Determination of IGF-I Binding to Parasites.
Homologous displacement was performed essentially as described in ref. 25. To each tube containing 1 ml of 6 × 106 L.(L.) mexicana promastigotes or amastigote-like forms in ice-cold buffer (100 mM Hepes/120 mM NaCl/5 mM KCl/1.2 mM MgCl2/1 mM EDTA/0.2% BSA, pH 7.4) 25 μl of 0.6 nM 125I-IGF-I was added in the absence or in the presence of increasing amounts of unlabeled IGF-I ligand and left overnight at 4°C. After incubation the samples were immediately centrifuged at 10,000 × g for 3 min, the supernatants were collected, and the pellet was washed twice with ice-cold PBS, resuspended in 0.2 M NaOH, and incubated for 1 hr at 37°C with mild agitation. Both the supernatants and the lysates were measured for radioactivity in a gamma counter (Beckman). The data were analyzed by the ligand computer program (24).
Analysis of Tyrosine Phosphorylation.
Detection of tyrosine phosphorylation was done essentially as described in refs. 22 and 24. L. (L.) mexicana promastigotes or amastigote-like forms (2 × 108 parasites/ml) were stimulated with IGF-I (50 ng/ml) for 1, 5, and 25 min in RPMI 1640 medium in the presence of 4% FCS. The parasites then were lysed in 1 ml of lysis buffer (20 mM Tris⋅HCl, pH 7.4/10 mM sodium pyrophosphate/50 mM sodium fluoride/2 mM sodium ortho-vanadate/1 μg/ml leupeptin/1 μg/ml aprotinin/1 μM phenylmethylsulfonyl fluoride/1% Triton X-100) for 30 min on ice. Thereafter, the solubilisates were centrifuged at 12,000 × g for 10 min, and an aliquot of the supernatants was adjusted to the same protein concentration and separated on a 7% SDS/PAGE gel under reducing conditions. Then the proteins were electrotransferred by using a semidry blotter (Bio-Rad) onto nitrocellulose membranes (GIBCO), blocked for 2 hr with 5% BSA in 20 mM Tris-buffered saline (TBS), pH 7.4, and thereafter incubated with 5 μg/ml of a monoclonal anti-phosphotyrosine antibody for 18 hr. After extensive washing with TBS/0.05% Triton X-100, 4 μCi of 125I-protein A was added and allowed to react for 1 hr. The membranes again were washed extensively, and the bands representing tyrosine-phosphorylated proteins were detected by autoradiography by using X-Omat-AR film (Kodak) and cassette with enhancing screen (Amersham). Laser densitometric analysis was done by using an Ultrascan XL (LKB).
Analysis of the in Vivo Effect of IGF-I.
L. (V.) panamensis promastigotes were preincubated with or without 50 ng/ml of IGF-I in PBS for 5 min. Thereafter, 107 stationary phase promastigotes in 50 μl were injected in the right hind-footpad of age- and sex-matched BALB/c mice (five in each group). The growth of the lesion was monitored at indicated time points by measuring the thickness of the footpad using a dial caliper (Mitsutoyo, Tokyo, Japan). The contralateral footpad of each animal injected with PBS represented the control value and the swelling calculated as: thickness of the right footpad − thickness of the left footpad. The data were analyzed by Mann–Whitney test.
Quantitative Morphometric Analysis.
At 24 and 48 hr and 7 and 30 days postinfection (PI), five animals from each group were killed and the specimens from the inoculation site were taken for quantitative morphometric analysis. The skin specimens were fixed in 0.01 M phosphate-buffered 4% paraformaldehyde and embedded in plastic resin (Technovit 7100). Semithin sections (1 μm) were obtained in 11800 Pyramitone (LKB) and stained with hematoxylin-eosin. The morphometric analysis was made on three different levels of semithin section per animal by using a graticule eyepiece in an area of 0.01 mm2 with an Olympus planapochromatic immersion objective lens (×100). In these levels 900 cells were counted, where each cell type constituted at least 10% of the total to keep the relative SE below 0.1% as detailed in ref. 25. Samples from five experimental animals were analyzed in each of these points, and the data were submitted to the Mann–Whitney test.
RESULTS
In Vitro Growth Response of Leishmania Amastigotes to IGFs.
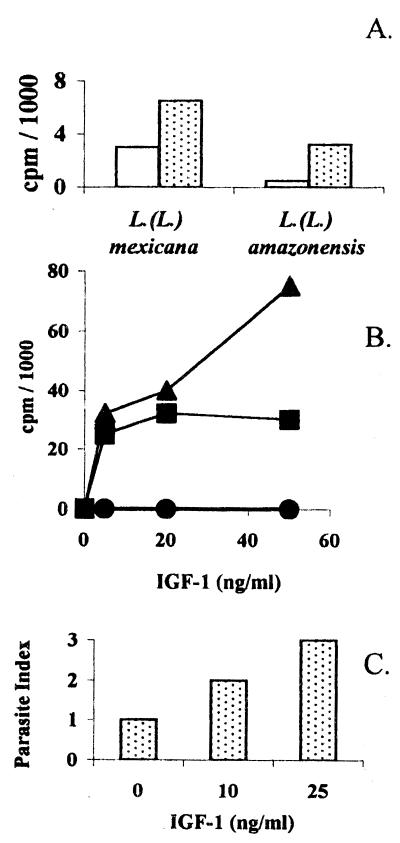
We have shown previously the growth-inducing effect of recombinant IGF-I (but not IGF-II) on various Leishmania promastigotes (19). To analyze whether amastigotes could respond to IGF-I or -II we used cell-free amastigotes or so-called amastigote-like forms derived from the promastigotes of L. (L.) mexicana and adapted to axenic culture conditions (21). These amastigotes showed a growth response similar to the promastigotes as shown both by [3H]thymidine incorporation and actual increase in the parasite number (Fig. 1 A– C). As for promastigotes, this growth-inducing effect was observed under suboptimal concentration of fetal calf serum, i.e., concentration where no spontaneous proliferative response occurs. The growth response occurred during the first 24 hr in both promastigotes and amastigote-like forms, and no further growth was detected in the following 24 hr (data not shown).
Figure 1.
(A) In vitro proliferative response of Leishmania (L.) amazonensis and Leishmania (L.) mexicana promastigotes to IGF-I. Stationary-phase Leishmania (L) amazonensis and Leishmania (L.) mexicana promastigotes were tested for proliferative response ([3H]thymidine incorporation) with IGF-I in the presence of 4% FCS: control (open bar); with 50 ng/ml IGF-I (hatched bar). Each point represents data from triplicate determinations with less than 5% SD of the mean. (B) In vitro proliferative response of Leishmania (L.) mexicana amastigote-like forms to IGF-I ([3H]thymidine incorporation). Stationary-phase Leishmania (L) mexicana amastigote-like forms were tested for proliferative response with IGF-I in the presence of 0% FCS (circle), 4% FCS (square), or 8% FCS (triangle). (C) In vitro proliferative response of Leishmania (L.) mexicana amastigote-like forms to IGF-I (parasite counting). Stationary-phase Leishmania (L) mexicana amastigote-like forms were tested for proliferative response to IGF-I in the presence of 4% FCS. Parasite index = number of parasites in cultures with IGF-I/number of parasites in cultures without IGF-I. For further details see Materials and Methods.
Analysis of Specific Binding of IGF-I to Leishmania Promastigotes and Amastigote-Like Forms.
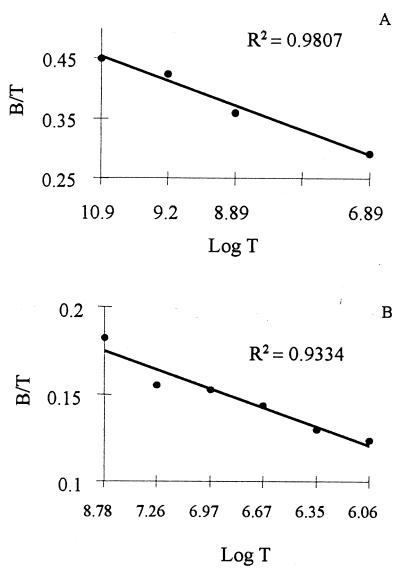
To analyze the specific binding and the characteristics of interaction between IGF-I and Leishmania, we performed homologous displacement analysis by using 125I-labeled and unlabeled IGF-I (cold). Fig. 2 shows one of two experiments that yielded similar curves for the analysis of the binding of IGF-I to L. (L.) mexicana promastigotes and amastigote-like forms. IGF-I binding to amastigote-like forms consistently was higher than to promastigotes, although both forms presented a high to medium affinity binding. Other calculated values are shown in Table 1 and show a remarkably high number of specific receptors for IGF-I. The analysis showed a single-site binding for IGF-I on both promastigotes and amastigote-like forms.
Figure 2.
Determination of the specific binding of IGF-I to Leishmania (L) mexicana promastigotes and amastigote-like forms. The binding of IGF-I to Leishmania (L) mexicana promastigotes (A) and amastigote-like forms (B) were analyzed by homologous displacement as described in Materials and Methods.
Table 1.
Physical constants and the number of receptors for the binding of IGF-I to Leishmania (L) mexicana promastigotes and amastigote-like forms
| Parameter | Promastigotes | Amastigotes |
|---|---|---|
| Ka, liter/mol | 3 × 107 | 1 × 107 |
| Kd, mol/liter | 4 × 10−8 | 10 × 10−8 |
| Receptor number/parasite | 2 × 106 | 6 × 106 |
The values were obtained from the computer program ligand as described in Materials and Methods.
IGF-I-Mediated Tyrosine Phosphorylation in Leishmania Promastigotes and Amastigote-Like Forms.
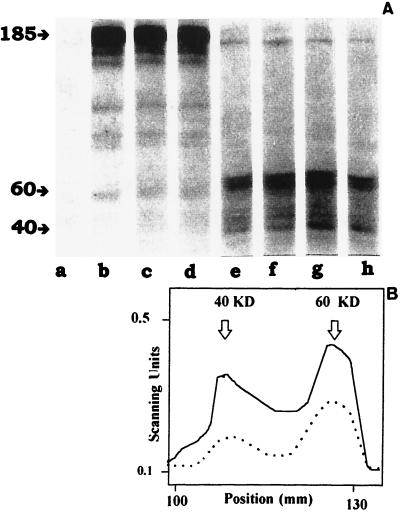
To further strengthen the link between IGF-I and the induced proliferative response in Leishmania parasites we studied the time-dependent induction of protein phosphorylation occurring at the tyrosine residues upon stimulation with IGF-I for 1–25 min. This was done by immunoblotting parasite proteins with a mAb recognizing phosphotyrosine. IGF-I induced tyrosine phosphorylation of proteins both in promastigotes and amastigote-like forms of L. (L.) mexicana from 1-min stimulation. Promastigotes and amastigote-like forms showed different phosphorylation patterns. Promastigotes showed strong tyrosine phosphorylation of a 185-kDa protein and some less-pronounced lower-molecular-mass bands while amastigote-like forms showed tyrosine phosphorylation of 60- and 40-kDa proteins (Fig. 3 A and B).
Figure 3.
(A) IGF-I-induced tyrosine phosphorylation. Analysis of IGF-I-induced tyrosine phosphorylation in Leishmania (L.) mexicana promastigotes (lanes a–d) and amastigote-like forms (lanes e–h) was done by immunoblotting with a monoclonal antiphosphotyrosine antibody. Stimulation time: 0, lanes a and e; 1 min, lanes b and f; 5 min, lanes c and g; and 25 min, lanes d and h. (B) Quantitative analysis of the IGF-I-induced tyrosine phosphorylation in Leishmania (L.) mexicana amastigote-like forms. Evaluation of the phosphorylation in Leishmania (L) mexicana promastigotes at 0 (broken line) and 5 min (solid line) of IGF-I induction was performed by using laser densitometry.
In Vivo Effect of IGF-I on Leishmania Infection.
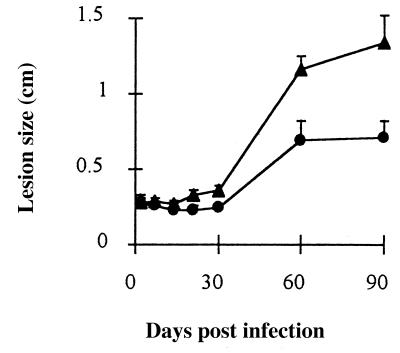
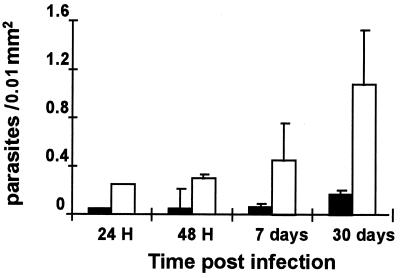
Finally, to evaluate the importance of IGF-I for the establishment of the Leishmania infection in vivo we used a L. (V.) panamensis-induced leishmaniasis model that we characterized recently (21, 26). We infected susceptible BALB/c mice with L. (V.) panamensis with or without IGF-I. This was done by preincubating and preactivating the parasite with IGF-I (50 ng/ml) for 5 min before injection in the hind footpad. This procedure resulted in significantly increased foot swelling from 21 days and onward postinfection. One of two independent experiments showing a significant difference between the IGF-I-activated Leishmania-injected and control groups is shown in Fig. 4. We also made morphometric analysis in the skin lesions in the acute phase at 24 and 48 hr PI and later at 7 and 30 days PI. Counting the number of intact parasites/0.01 mm2, the density of parasites was higher in mice injected with IGF-I-activated parasites (Fig. 5).
Figure 4.
In vivo development of the cutaneous lesions after challenge with Leishmania (V.) panamensis promastigotes preincubated with IGF-1. Leishmania (V) panamensis promastigotes preincubated with 50 ng/ml of IGF-1 (triangle) or without IGF-I (circle) were injected in the hind footpad, and the lesion size (mean ± SEM) was determined as stated in Materials and Methods.
Figure 5.
Density of intact parasites (mean ± SEM) in the skin lesion of BALB/c mice infected with Leishmania (V.) panamensis promastigotes with and without preincubation with IGF-I. Leishmania (V.) panamensis promastigotes preincubated with 50 ng/ml of IGF-I (open column) or without IGF-I (solid column) were injected in the hind footpad, and the skin samples taken from five animals from each group at different time points were submitted to morphometric analysis as stated in Materials and Methods.
DISCUSSION
Directly after transmission into the vertebrate host, the Leishmania promastigotes encounter drastic changes of physiological nature, such as changes in temperature and pH, and a highly oxidative milieu (ref. 27; R. Chebabo, unpublished data). The parasite also will encounter host-derived humoral factors, including growth factors (5, 7), that can be beneficial for the host leading to the elimination of the parasites, but it is also possible that they can directly or indirectly favor the parasite survival in the initially hostile host environment. IGFs, particularly IGF-I, could belong to this category of factors since IGF-I is an evolutionary well conserved polypeptide, constitutively present in the skin, and known to be associated with inflammation and wound healing (12–17). Moreover, the effect of IGFs has been reported on the protozoan Giardia lamblia (28) and on cells from primitive vertebrates such as Cottus scorpius (sea scorpion), Raja clavata (ray), and Myxine glutinosa (Atlantic hagfish) (29). Furthermore, we previously have analyzed and shown the growth-inducing effect of IGF-I on promastigotes of different species of Leishmania (19).
In this study we extended our previous observation analyzing the effect of IGF-I on amastigotes and also how parasites already stimulated by IGF-I would behave during an experimental infection in vivo.
In the presence of suboptimal concentrations of fetal calf serum, IGF-I but not IGF-II could induce a rapid growth response of Leishmania amastigote-like forms in vitro as it could of promastigotes. The amastigote-like forms used here are very similar to amastigotes directly taken from the lesion, and it has been shown that they induce cutaneous lesions in mice (30). In G. lamblia it was shown that IGF-II induced a growth response while IGF-I was inert; however, both showed the capacity to bind to the parasite (28). We cannot completely rule out the effect of IGF-II on Leishmania under circumstances not tested here, but the difference may reflect species or phylogenetic differences.
Both amastigotes and promastigotes showed a strong specific binding of IGF-I through a single-site putative receptor of IGF-I. The strength of their binding is comparable to that of IGF-I to erythrocytes (31), fibroblasts, and smooth muscle cells (D. Gianella Neto, unpublished data).
IGF-I could induce a rapid tyrosine phosphorylation of a number of proteins in L. (L.) mexicana promastigotes and amastigotes. Reports on phosphorylation-dependent signaling in protozoas and particularly in Leishmania are still sparse and mainly show differences in the total phosphorylation pattern in various species of Leishmania promastigotes depending on whether the growth phase is stationary or exponential (32). In the study presented here and also by us in ref. 23, a single specific ligand (IGF-I) is used to study the tyrosine phosphorylation in Leishmania. One of the more prominent phosphorylation occurred at the protein of 185 kDa in promastigotes, and it represents a hitherto nonreported tyrosine-phosphorylated molecule in Leishmania. This is interesting because it corresponds in molecular mass to the molecule described in mammalian cells as an endogenous substrate for the IGF-I and insulin receptor, the insulin receptor substrate-1 (33, 34). The tyrosine phosphorylation of proteins in the amastigote-like forms upon IGF-I stimulation was shown to be different and occurred at 60- and 40-kDa proteins. The 60- and 40-kDa proteins also were present in the control sample from nonstimulated amastigotes and could represent autophosphorylating molecules or could reflect the experimental difficulties in achieving completely synchronized and quiescent parasites. However, stimulation with the IGF-I led to a significantly increased phosphorylation as shown by laser densitometry. Interestingly, an autophosphorylating protein kinase of 60 kDa has been described in Trypanosoma brucei (35). The difference in phosphorylation pattern found between promastigotes and amastigote-like forms could reflect evolutionary adaptation to different environments of the parasites, i.e., promastigotes for the extracellular and the amastigotes for the intracellular milieu. The use of the same strain of Leishmania for the study on promastigote and amastigote-like forms strengthens the significance of this difference in pattern. Similar cell cycle-dependent differences in the tyrosine-phosphorylation pattern in protozoa has been described in T. brucei (36, 37). A more detailed study and discussion on the IGF-1-mediated phosphorylation of Leishmania parasites is published in ref. 23.
The exact mode of action of IGF-I to induce proliferation of Leishmania promastigotes and amastigotes in vitro has to be elucidated. Here we present evidence for an IGF-I-induced sequential event starting with an initial binding, induction of intracellular signaling through tyrosine phosphorylation, and increase in both DNA synthesis rate and number of parasites. The requirement for suboptimal levels of FCS points to the previously suggested endocrine role of IGF-I, in which IGF-I induces expression of receptors for other growth or activation factors (12–15).
To test the hypothesis that IGF-I could activate the parasites directly when entering in the host at the site of infection and thus influence the outcome of the infection, the in vivo experiment was designed to make the challenge with parasites already activated with a physiological dose of IGF-I. Two independent experiments showed that Leishmania promastigotes preactivated with IGF-I induced a significantly bigger lesion. The actual increase in the number of intact parasites also observed suggests that IGF-I may have induced a rapid proliferative response of the parasites and thus would have increased the number of available and infectious parasites. An alternative mechanism could be an increase of the virulence of the parasites by altering or inducing surface molecules (e.g., lectin-like) or the induction of mechanisms that prevent parasite death, as in the case of granulocyte–macrophage colony-stimulating factor (6).
The in vitro data showing a specific response of the Leishmania amastigotes indicate that IGF-I also may affect later stages of the infection. The operational mechanisms for such an intracellular effect remain unclear. Intracellular IGF-I in macrophages detected by immunohistochemistry have been reported (18). Whether extracellular IGF-I also can reach the parasitophorous vacuole, however, remains speculative. A similar in vitro effect on facultative intracellular Mycobacteria by epidermal growth factor has been described (38).
Thus, we conclude that IGF-I with a growth-inducing effect on Leishmania parasites in vitro and effect on the course of infection in vivo may constitute another important pathogenic factor in leishmaniasis.
Acknowledgments
We acknowledge Drs. Silvia C. Alfieri, Daniel Gianella Neto, Marcia Dalastra Laurenti, Vania L. R. da Matta, Rosa Fukui, and, especially, Drs. M. Lake and H. Wigzell for helpful discussions, comments, technical advice, and reagents. This work was supported by Cancerfonden (M.G.), Conselho Nacional de Pesquisas, Fundação de Amparo à Pesquisa do Estado de São Paulo, LIM 50-Hospital das Clinicas da Facudade de Medicina da Universidade de São Paulo.
ABBREVIATION
- IGF
insulin-like growth factor
References
- 1.World Health Organization. Control of Leishmaniasis. Technical- Report Series: report of a WHO Expert Committee; 1990. p. 793. [Google Scholar]
- 2.Sacks D L, Louis J A, Wirth D F. In: Immunology and Molecular Biology of Parasitic Infection. Warren K S, editor. Oxford: Blackwell Scientific; 1993. pp. 237–268. [Google Scholar]
- 3.Reiner L S, Locksley R M. Annu Rev Immunol. 1995;13:151–177. doi: 10.1146/annurev.iy.13.040195.001055. [DOI] [PubMed] [Google Scholar]
- 4.Shankar A, Titus R G. J Exp Med. 1995;181:845–855. doi: 10.1084/jem.181.3.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laurenti M D, Corbett C E P, Sotto M N, Sinhorini I L, Goto H. Int J Exp Pathol. 1996;77:15–24. doi: 10.1046/j.1365-2613.1996.958096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barcinski M A, Schehtman D, Quintão L G, Costa D A, Soares R B, Moreira M E C, Charlab R. Infect Immunol. 1992;60:3523–3527. doi: 10.1128/iai.60.9.3523-3527.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barcinski M A, Moreira M E C. Parasitol Today. 1994;10:352–355. doi: 10.1016/0169-4758(94)90246-1. [DOI] [PubMed] [Google Scholar]
- 8.Barral-Neto M, Barral A, Brownell C E, Sheiky Y A W, Ellingsworth L R, Twardzik D R, Reed S G. Science. 1992;257:545–548. doi: 10.1126/science.1636092. [DOI] [PubMed] [Google Scholar]
- 9.Clark I A, Cowden W B. Immunol Ser. 1992;56:365–407. [PubMed] [Google Scholar]
- 10.Scharton-Kersten T, Afonso L C C, Wysocka M, Trincheri G, Scott P. J Immunol. 1995;154:5320–5330. [PubMed] [Google Scholar]
- 11.Scott P. J Immunol. 1991;147:3149–3155. [PubMed] [Google Scholar]
- 12.Humbel R E. Eur J Biochem. 1990;190:445–462. doi: 10.1111/j.1432-1033.1990.tb15595.x. [DOI] [PubMed] [Google Scholar]
- 13.Rechler M M, Nissley S P. In: Insulin-Like Growth Factors in Peptide Growth Factors and Their Receptors, Handbook of Pharmacology. Sporn B, Roberts A B, editors. Heidelberg: Springer; 1990. pp. 263–282. [Google Scholar]
- 14.Jones J I, Clemons D R. Endocrine Rev. 1995;16:3–34. doi: 10.1210/edrv-16-1-3. [DOI] [PubMed] [Google Scholar]
- 15.Kratz G, Haegerstrand A, Lake M, Forsberg G, Gidlund M. Exp Cell Res. 1992;202:381–385. doi: 10.1016/0014-4827(92)90089-q. [DOI] [PubMed] [Google Scholar]
- 16.Kratz G, Gidlund M. Scand J Plast Reconstr Surg Hand Surg. 1994;28:107–112. doi: 10.3109/02844319409071187. [DOI] [PubMed] [Google Scholar]
- 17.Werner H, LeRoit D. Diabetes Rev. 1995;3:28–37. [Google Scholar]
- 18.Kooijman R, Hooghe-Peters E L, Hooghe R. Adv Immunol. 1996;63:377–454. doi: 10.1016/s0065-2776(08)60860-3. [DOI] [PubMed] [Google Scholar]
- 19.Gomes C M C, Goto H, Corbett C E P, Gidlund M. Acta Trop. 1997;64:225–228. doi: 10.1016/s0001-706x(96)00633-x. [DOI] [PubMed] [Google Scholar]
- 20.Rojas J I, Tani E, Örn A, Sánchez C, Goto H. Int J Exp Pathol. 1993;74:481–491. [PMC free article] [PubMed] [Google Scholar]
- 21.Pral E M F, Buovsky T, Balanco J M F, Alfiere S C. Exp Parasitol. 1993;77:62–73. doi: 10.1006/expr.1993.1061. [DOI] [PubMed] [Google Scholar]
- 22.Peranovich T M S, Silva A M, Fries D M, Stern A, Monteiro H P. Biochem J. 1995;306:613–619. doi: 10.1042/bj3050613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gomes C M C, Monteiro H P, Gidlund M, Corbett C E P, Goto H. J Eukaryotic Microbiol. 1998;45:352–355. doi: 10.1111/j.1550-7408.1998.tb04548.x. [DOI] [PubMed] [Google Scholar]
- 24.Munson J. Methods Enzymol. 1983;92:543–576. doi: 10.1016/0076-6879(83)92044-x. [DOI] [PubMed] [Google Scholar]
- 25.Weibel E R, Gomez D M. J Appl Physiol. 1962;17:343–48. doi: 10.1152/jappl.1962.17.2.343. [DOI] [PubMed] [Google Scholar]
- 26.Goto H, Rojas J I, Sporrong L, de Carreira P, Sanchéz C, Örn A. Rev Inst Med Trop São Paulo. 1995;37:475–481. doi: 10.1590/s0036-46651995000600001. [DOI] [PubMed] [Google Scholar]
- 27.Antoine J C. Pathol Biol. 1995;43:215–223. [PubMed] [Google Scholar]
- 28.Luján H D, Mowatt M R, Helman L J, Nash T E. J Biol Chem. 1994;269:13069–13072. [PubMed] [Google Scholar]
- 29.Drakenberg K, Sara V R, Falkmer S, Gammeltoft S, Maake C, Reinecke M. Regul Pept. 1993;43:73–81. doi: 10.1016/0167-0115(93)90409-2. [DOI] [PubMed] [Google Scholar]
- 30.Bates P A, Robertson C D, Tetley L, Coombs G H. Parasitology. 1992;105:193–202. doi: 10.1017/s0031182000074102. [DOI] [PubMed] [Google Scholar]
- 31.El-Andere W, Lerário A C, Giannella-Neto D, Wajcenberg B L. Metabolism. 1995;44:923–928. doi: 10.1016/0026-0495(95)90246-5. [DOI] [PubMed] [Google Scholar]
- 32.Mukhopadhyay N K, Asish K S, Lovelace J K, Silva R, Sacks D L, Glew R H. J Protozool. 1988;35:601–607. doi: 10.1111/j.1550-7408.1988.tb04158.x. [DOI] [PubMed] [Google Scholar]
- 33.Sun X J, Rothenberg P, Kanh C R, Backer J M, Araki E, Winden P A, Cahil D A, Goldstein B J, White M, Kahn C R. Nature (London) 1991;352:73–77. doi: 10.1038/352073a0. [DOI] [PubMed] [Google Scholar]
- 34.Rothenberg P L, Lane W S, Karasik A, Backer J, White M, Kahn C R. J Biol Chem. 1991;266:8302–8311. [PubMed] [Google Scholar]
- 35.Hide G, Graham T, Chanan A, Tait A, Keith K. Parasitology. 1994;108:161–166. doi: 10.1017/s0031182000068256. [DOI] [PubMed] [Google Scholar]
- 36.Parsons M, Valentine M, Deans J. Parasitology. 1990;44:241–249. doi: 10.1016/0166-6851(91)90091-j. [DOI] [PubMed] [Google Scholar]
- 37.Parsons M, Valentine M, Carter V. Proc Natl Acad Sci USA. 1993;90:2656–2660. doi: 10.1073/pnas.90.7.2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bermudez L E, Petrofsky M, Shelton K. Infect Immunol. 1996;64:2917–2922. doi: 10.1128/iai.64.8.2917-2922.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]