Abstract
Acetate kinase, a member of the ASKHA (Acetate and Sugar Kinases, Hsp70, Actin) phosphotransferase superfamily is a central enzyme in prokaryotic carbon and energy metabolism. Recently extensive structural and biochemical studies of acetate kinase and related carboxylate kinases have been conducted. Analysis of the kinetic properties of wild-type and mutant enzymes has been impeded by the nature of the current assays for acetate kinase activity. These assays have the disadvantages of being either discontinuous or insensitive or of utilizing compounds that interfere with activity measurements. We have developed a novel continuous assay that depends on the purine nucleoside phosphorylase-based spectroscopic measurement of the inorganic phosphate generated by hydroxylaminolysis of one of the products of the acetate kinase reaction, acetyl phosphate. This assay has enabled a determination of the kinetic parameters of the Thermotoga maritima acetate kinase that indicates a lower Km for acetate than previously published.
Keywords: Acetyl phosphate, Acetate kinase, ASKHA phosphotransferase, purine nucleoside phosphorylase, MESG
1. Introduction
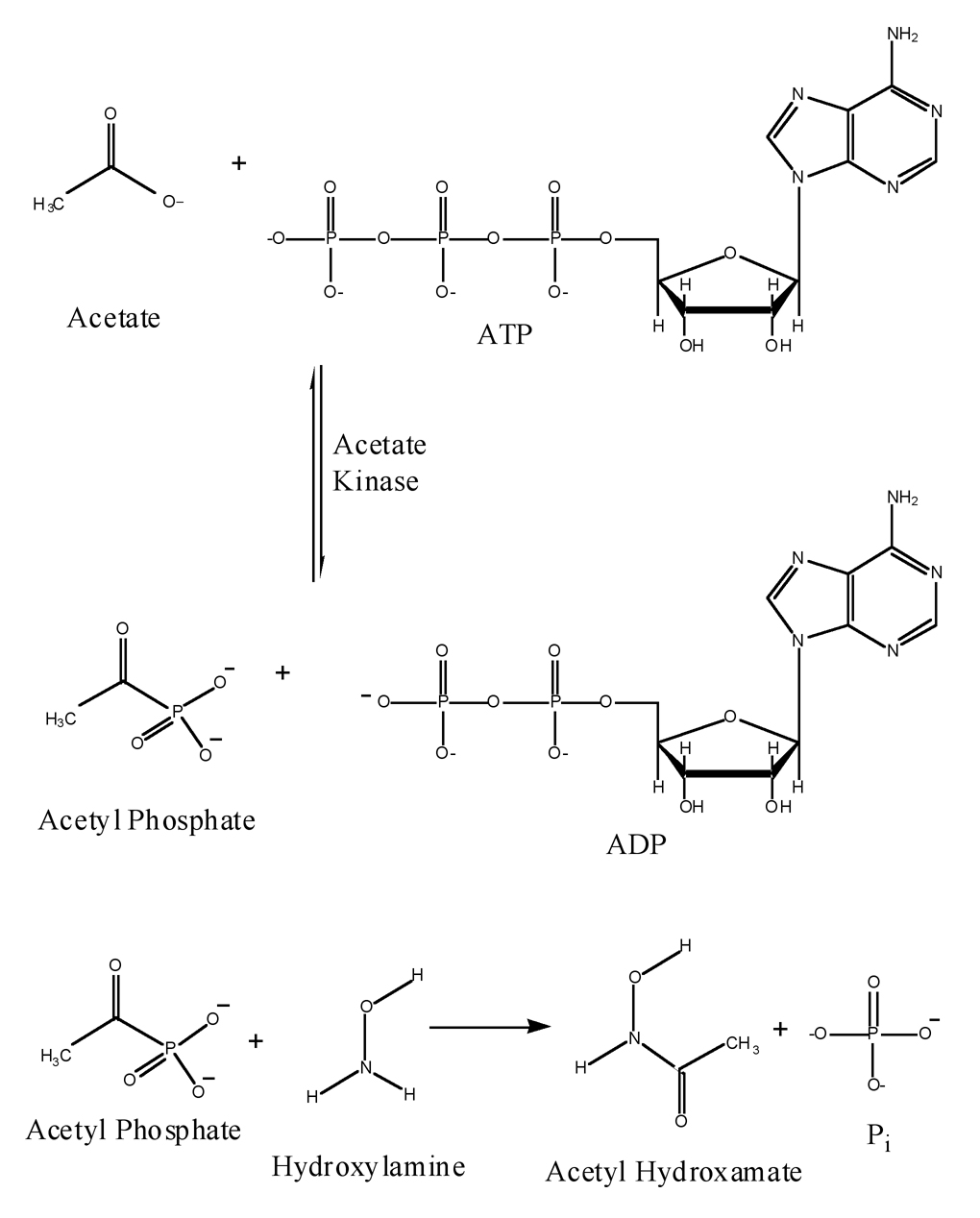
Acetate kinase, is central to carbon and energy metabolism in bacteria and plays a central role in archaeal methanogenesis [1,2]. The enzyme catalyzes the reversible transfer of the γ-phosphoryl group from ATP to the carboxylate group in acetate to form acetyl phosphate (Fig 1). Acetate kinase is homologous to the propionate and butyrate/isobutyrate kinases [3]. The 3-dimensional structure of the Methanosarcina thermophila acetate kinase has been determined, and the enzyme has been demonstrated to be a member of the ASKHA (Acetate and Sugar Kinases, Hsp70, Actin) phosphotransferase superfamily [4]. A combination of structural and biochemical approaches involving mutant enzymes have been employed to investigate the mechanism of acetate kinase. Progress has been hampered by the challenges posed by the current assays for acetate-kinase activity. The most commonly used assays for the catalysis of the reaction of ATP and acetate by acetate kinase are the hydroxamate assay, which measures acetyl-phosphate formation and an enzyme-linked assay, which detects ADP formation. The hydroxamate assay, an adaptation of the method of Lipmann and Tuttle [5] and Rose [6], utilizes the reaction of acetyl phosphate with neutral hydroxylamine to form acetyl hydroxamate (Fig 1), which forms a colored complex with ferric ions. Inorganic phosphate (Pi) is released as a result of the hydroxylaminolysis. The assay is discontinuous and insensitive, because spectrophotometric detection at 540nm of the ferric-hydroxamate complex requires termination of the reaction with acidic ferric chloride. In addition, the high concentration of hydroxylamine (704mM) used in the reaction was reported to produce nonlinear and noncompetitive inhibition of acetate kinase [7].
Fig. 1.
Acetate kinase catalyzes the reaction of acetate and ATP producing acetyl phosphate and ADP. High-energy acetyl phosphate reacts with hydroxylamine forming acetyl hydroxamate and inorganic phosphate (Pi).
The enzyme-linked assay couples ADP formation to the oxidation of NADH (ε340 = 6.22mM−1 cm−1) through pyruvate kinase (PyK) and lactic dehydrogenase (LDH) [8]. Pyruvate kinase utilizes phosphoenolpyruvate to phosphorylate the ADP thereby forming ATP and pyruvate. Lactate dehydrogenase couples the reduction of pyruvate to lactate with the oxidation of NADH to NAD+. This method has been the preferred continuous assay to measure acetate-kinase kinetic parameters [7]. However, this assay has disadvantages deriving from the contamination of nucleoside triphosphate preparations with nucleoside diphosphates and issues concerning sensitivity and the nucleotide specificity of the pyruvate kinase [9].
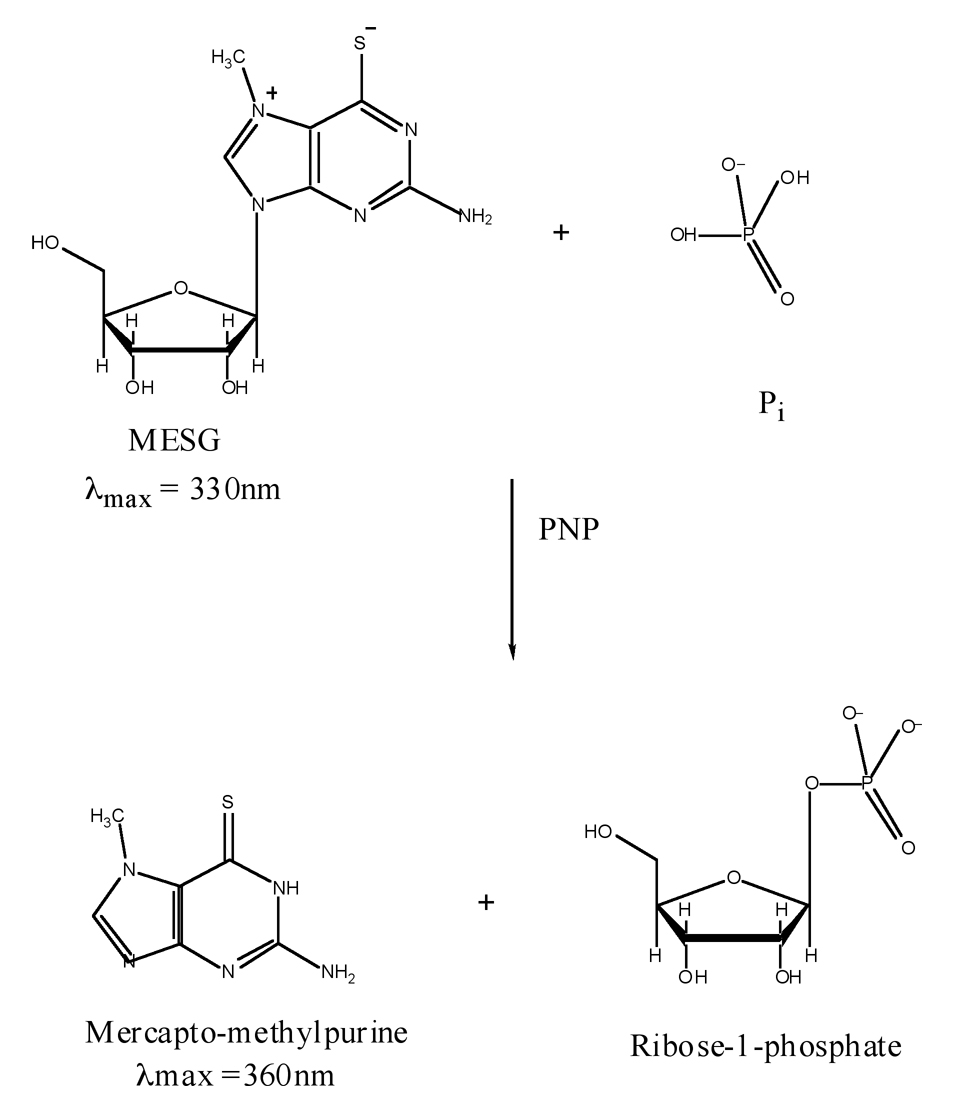
To circumvent these limitations we have developed an assay that can measure the released Pi at a nanomolar level. This assay depends upon a commercially available kit that can measure the inorganic phosphate released upon hydroxylaminolysis of acetyl phosphate using hydroxylamine concentrations that are not inhibitory to the acetate-kinase reaction. The kit is based on the method described by Webb [10] wherein purine nucleoside phosphorylase (PNP) utilizes inorganic phosphate (Pi) to convert the substrate 2-amino-6-mercapto-7-methylpurine ribonucleoside (MESG) to ribose-1-phosphate and 2-amino-6-mercapto-7-methylpurine (MES) (Fig 2). The Pi-dependent enzymatic removal of the ribose moiety from MESG results in a spectrophotometric shift in the wavelength of maximum absorbance (λmax) from 330nm for the substrate to 360nm for the product [10]. The increase in absorbance at 360nm can therefore be used to measure Pi concentration.
Fig. 2.
Enzymatic conversion of 2-amino-6-mercapto-7-methyl-purine riboside (MESG) to ribose-1-phosphate and 2-amino-6-mercapto-7-methyl-purine (MES) by purine nucleoside phosphorylase (PNP). The accompanying change in absorption at 360nm allows quantification of the inorganic phosphate (Pi) consumed in the reaction.
2. Materials and Methods
2.1 Materials
ATP, hydroxylamine-hydrochloride, lithium potassium acetyl phosphate, trisodium phosphoenolpyruvate, rabbit muscle pyruvate kinase and lactate dehydrogenase were purchased from SIGMA. Analytical grade acetate was obtained from Mallinckrodt. The EnzChek Phosphate Assay kit purchased from Molecular Probes contained 2-amino-6-mercapto-7-methylpurine riboside (MESG) and Purine nucleoside phosphorylase (PNP).
2.2 Purification of Acetate Kinase from Thermotoga maritima
T. maritima AK was expressed from a pET-17b-based vector (gift of P. Schoenheit) in BL21-CodonPlus-RIL cells (Stratagene). A single colony was inoculated into 4 liters of Luria Broth medium containing 50µg/ml of carbenicillin and grown to an O.D.600 of 0.8 at 37°C. Protein expression was induced by adding 1mM isopropyl-β-D-thiogalactopyranoside. Cells were harvested after 4h of growth by centrifugation at 5855×g at 4°C, frozen in liquid nitrogen and stored at −80°C. The cell pellet was suspended in lysis buffer (50mM Tris-HCl (pH 8.4), 5mM MgCl2, 0.07mM Thesit) and lysed in a French Press cell at 18000 psi. The inclusion of 0.07mM Thesit was found to increase enzyme solubility, and therefore the detergent was included in all purification buffers. The lysate was incubated at 70°C for 20 minutes, and the cell debris was removed by ultracentrifugation at 184692×g for 1hr. The soluble fraction was loaded onto a Q-Sepharose column pre-equilibrated with buffer A (50mM Tris-HCl (pH 8.0), 5mM MgCl2). Protein was eluted with a gradient of 0–2M NaCl in buffer B (50mM Piperazine (pH 6.5), 5mM MgCl2). The fractions containing AK protein were pooled and dialyzed against buffer C (50mM Tris-HCl (pH 7.5), 5mM MgCl2) overnight. The protein was loaded onto a heparin column equilibrated with buffer C and eluted with buffer C containing 1M NaCl using a linear gradient. Fractions of protein were collected and diluted 1:2 with buffer D (50mM Tris-HCl (pH 7.5), 5mM MgCl2, 3M (NH4)2SO4). The protein solution was loaded onto a Phenyl Sepharose column with equilibration buffer E (50mM Tris-HCl (pH 7.5), 5mM MgCl2, 2M (NH4)2SO4), and the purified protein was eluted with buffer A. The protein containing fractions were dialyzed in buffer A, concentrated to 40–50 mg/ml, frozen in liquid nitrogen and stored at −80°C.
2.3. Assay of purine nucleoside phosphorylase activity
Purine nucleoside phosphorylase activity assays were conducted in the presence and absence of various concentrations of phosphate, acetyl phosphate and hydroxylamine. The reaction mixture contained 10µl of 1M Tris-HCl (pH 7.5), 20mM MgCl2, 40µl 1mM MESG substrate solution, a varying volume of 500µM KH2P04 or of 500µM lithium potassium acetyl phosphate and a varying volume of neutralized 2M hydroxylamine. A final volume of 198µl was achieved by the addition of the appropriate amount of water. The reactions were started by adding 2µl of PNP (1U) followed by incubation for 30 minutes at 37°C. The release of Pi was quantified by measuring the absorbance at 360nm with a Cary 100-Bio UV-Visible Spectrophotometer. In the examination of the question of whether hydroxylaminolysis was rate-limiting a variation of the assay was conducted in which the concentration of hydroxylamine was kept constant (250mM) and the 1U of PNP was added prior to the addition of varying volumes of 500µM lithium potassium acetyl phosphate and incubated for 1 minute at 37°C.
2.4 Assay of coupled acetate kinase and purine nucleoside phosphorylase activities
The acetate kinase/purine nucleoside phosphorylase reaction mixture contained 50mM Tris-HCl (pH 7.5), 1mM MgCl2, 0.2mM MESG, varying concentrations of neutralized hydroxylamine (0–704 mM), 5mM ATP, 5mM MgCl2, 200mM sodium acetate (pH 7.0) and 1U of PNP. The reaction was initiated by addition of 0.2µg of T. maritima acetate kinase and monitored at 37°C. The Km and Vmax values were determined through a nonlinear regression data analysis fit to the Michaelis-Menten equation utilizing the program Kaleidagraph (Synergy Software, Reading, PA).
2.5 Assay of coupled acetate kinase, pyruvate kinase, and lactate dehydrogenase activities
For comparative purposes acetate kinase activity was assayed through a coupled reaction in the presence of pyruvate kinase and lactate dehydrogenase. Assay solutions contained 60mM HEPES (pH 7.0), 5mM MgCl2, 3mM trisodium phosphoenolpyruvate, 12 units of rabbit muscle pyruvate kinase and 24 units of lactate dehydrogenase, 0.4mM NADH, 5mM ATP, and 5mM MgCl2, and varying concentrations of sodium acetate (pH 7.0). The reaction was initiated with the addition of 0.03µg of T. maritima acetate kinase and followed at 37°C. Absorbance changes were monitored at 340nm with a Cary 100-Bio UV-Visible Spectrophotometer.
3. Results and discussion
3.1 Measurement of phosphate generated by hydroxylaminolysis of acetyl phosphate
In the reaction catalyzed by acetate kinase acetyl phosphate and ADP are produced from ATP and acetate. Assays of acetate kinase activity have depended upon assaying ADP generation or measurement of acetyl hydroxamate resulting from the reaction of acetyl phosphate with hydroxylamine. In order to develop a sensitive, versatile and continuous assay, we decided to attempt to measure the other product of hydroxylaminolysis of acetyl phosphate, inorganic phosphate. The spectrophotometric quantification of Pi can be achieved through measurement of the phosphorylysis of 2-amino-6-mercapto-7-methylpurine ribonucleoside (MESG) to ribose-1-phosphate and 2-amino-6-mercapto-7-methylpurine (MES) by purine nucleoside phosphorylase. Sensitivity of the assay is in the range of 2–150µM, and the reaction can be performed over a pH range of 6.5 to 8.5.
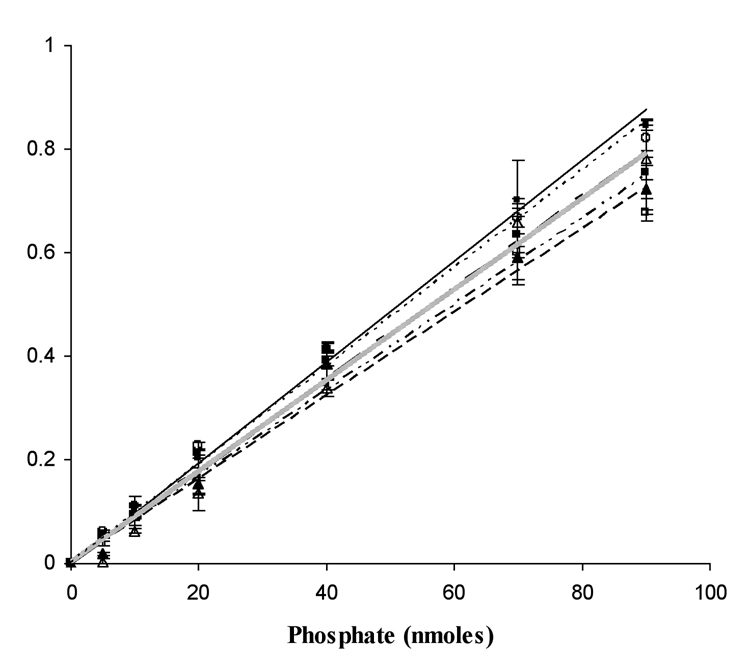
In order to assess the suitability of the PNP reaction for quantification of Pi generated by hydroxylaminolysis of acetyl phosphate it was necessary to demonstrate that hydroxylamine does not interfere with the PNP reaction. The PNP assay to measure phosphate concentration was conducted in the presence of varying concentrations of hydroxylamine. It can be seen that there was minimal inhibition of the reaction at the various hydroxylamine concentrations (Fig. 3).
Fig. 3.
Purine nucleoside phosphorylase reaction dependence on phosphate and hydroxylamine concentrations. (●——) 0mM, (□ — — —) 704mM, (△—— - ——) 250mM, (■·········) 200mM, (○-------) 150mM, (▲—— --- ——-) 100mM hydroxylamine. KH2PO4 was used as the source for inorganic phosphate.
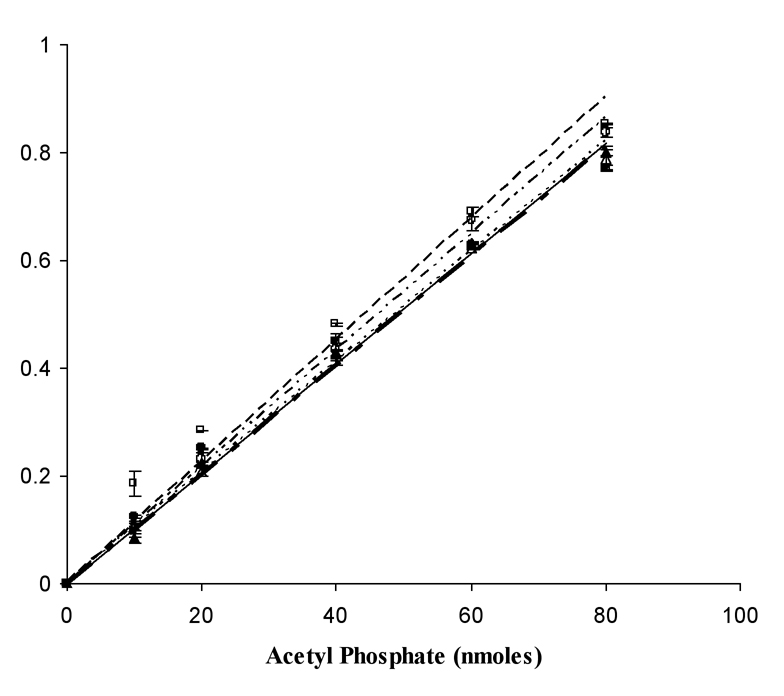
It was also necessary to examine the hydroxylamine-concentration dependence of the release of Pi by the hydroxylaminolysis of acetyl phosphate. At concentrations as low as 100mM hydroxylamine, the acetyl phosphate is completely hydrolyzed as measured by Pi release (Fig. 4). In order to determine whether the hydroxylaminolysis of acetyl phosphate was rate-limiting, a comparison of the rate of the measured reaction when acetyl phosphate or PNP was the last component added to the mixture was conducted. The results indicated that hydroxylaminolysis was very rapid and not rate-limiting for the assay under the reaction conditions (data not shown).
Fig. 4.
Purine nucleoside phosphorylase reaction dependence on acetyl phosphate and hydroxylamine concentrations. (●——) 704mM, (□ — — —) 250mM, (△—— - ——) 200mM, (▲------- ) 150mM, (○——---——-) 100mM hydroxylamine. Lithium potassium acetyl phosphate was used as the source for acetyl phosphate.
3.2 Assay of acetate kinase and determination of its kinetic parameters
We were able to determine that the assay as described in the Materials and Methods is linear for quantities of T. maritima acetate kinase (TMAK) up to 0.3 µg (data not shown). A comparison of the kinetic parameters of TMAK as measured by the pyruvate kinase/lactate dehydrogenase (PK/LD) assay and the PNP assay was conducted. There was some dependence of the Km of TMAK for acetate on the concentration of hydroyxlamine in the reaction (Table 1). It had been previously demonstrated that hydroxylamine inhibits the Methanosarcina thermophila acetate kinase reaction in a nonlinear and noncompetitive fashion [7]. At 704mM hydroxylamine there was substantial inhibition of the acetate-kinase reaction, whereas at 250mM hydroxylamine, inhibition was minimal. There is an elevated Km of TMAK for acetate in the presence of 704mM hydroxylamine. The measured kinetic parameters appear to be optimal at 250mM hydroxylamine.
Table 1.
Km of TMAK for acetate in presence of different amounts of hydroxylamine.
| Amounts of Hydroxylamine (mM) | Km(mM) | Vmax × 10−5 (moles/min) |
|---|---|---|
| 704 | 22.3 ± 2.8 | 4.0 ± 0.1 |
| 250 | 9.8 ± 1.4 | 4.9 ± 0.2 |
| 200 | 15.7 ± 2.4 | 3.9 ± 0.2 |
| 150 | 13.0 ± 1.8 | 3.9 ± 0.1 |
| 100 | 11.5 ± 1.2 | 3.9 ± 0.1 |
Analysis of the TMAK kinetic parameters derived from the PK/LD and PNP assays indicates that the Km of TMAK for acetate as measured in the PNP assay is much lower than that determined in the PK/LD assay (Table 2). There is a 2.6-fold increase in the kcat/Km value as measured in the PNP assay. It appears plausible that differences in the parameters arise from the fact that the affinity of acetyl phosphate for acetate kinases is much greater than that of acetate, and it therefore may compete for binding to the enzyme. In the PNP assay the acetyl phosphate is continuously consumed.
Table 2.
The comparison of kinetic parameters for two coupled assays
| Assay Type | Km(mM) | kcat(s−1) | kcat/Km (M−1s−1) |
|---|---|---|---|
| PNP-linked assay(new) | 9.8 ± 1.4 | 72.5 | 7370 |
| PK/LD assay (old) | 80.6 ± 7.6 | 225 | 2800 |
4. Conclusion
A new, sensitive, and continuous assay of acetate kinase activity has been developed. It measures the generation of acetyl phosphate by acetate kinase through hydroxylaminolysis of the product and detection of the inorganic phosphate produced. A lower Km of the Thermotoga maritima acetate kinase for acetate was measured than that determined by earlier assay methods. The Km of Thermotoga maritima acetate kinase was previously determined to be 40mM when measured at 55° C (11). The lower Km value measured here is more consistent with the concentrations of acetate to which cells are exposed and the internal acetyl phosphate concentrations that have been measured experimentally (12). A future improvement to the assay will be necessary to investigate the kinetic properties of the enzyme at its optimal temperature of 90°C (11).
Acknowledgement
This research was supported by NIH grant RO1-GM57056 and by David and Lucille Packard Foundation Fellowship 99-1463 to MSH and DAS, NSF CAREER award 99-84919-MCB to DAS and NIH Cancer Center support at Purdue University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Thauer RK, Zinkhan DM, Spormann AM. Annu. Rev. Microbiol. 1989;43:43–67. doi: 10.1146/annurev.mi.43.100189.000355. [DOI] [PubMed] [Google Scholar]
- 2.Ferry JG. J. Bacteriol. 1992;174:5489–5495. doi: 10.1128/jb.174.17.5489-5495.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ingram-Smith C, Gorrell A, Lawrence SH, Iyer P, Smith K, Ferry JG. J. Bacteriol. 2005;187:2386–2394. doi: 10.1128/JB.187.7.2386-2394.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buss KA, Cooper DR, Ingram-Smith C, Ferry JG, Sanders DA, Hasson MS. J.Bacteriol. 2001;183:680–686. doi: 10.1128/JB.183.2.680-686.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lipmann F, Tuttle LC. J. Biol. Chem. 1945;159:21–28. [PubMed] [Google Scholar]
- 6.Rose IA. Methods in Enz. 1955;1:591–595. [Google Scholar]
- 7.Gorrell A, Lawrence SH, Ferry JG. J. Biol. Chem. 2005;280:10731–10742. doi: 10.1074/jbc.M412118200. [DOI] [PubMed] [Google Scholar]
- 8.Aceti DJ, Ferry JG. J. Biol. Chem. 1988;263:15444–15448. [PubMed] [Google Scholar]
- 9.Smith CR, Knowles VL, Plaxton WC. Eur. J. Biochem. 2000;267:4477–4485. doi: 10.1046/j.1432-1327.2000.01494.x. [DOI] [PubMed] [Google Scholar]
- 10.Webb MR. Proc. Natl. Acad. Sci. USA. 1992;89:4884–4887. doi: 10.1073/pnas.89.11.4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bock AK, Glasemacher J, Schimdt R, Schoenheit P. J. Bacteriol. 1999;181:1861–1867. doi: 10.1128/jb.181.6.1861-1867.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolfe AJ. Microbiol. Mol. Biol. Rev. 2005;69:12–50. doi: 10.1128/MMBR.69.1.12-50.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]