Abstract
Purpose:
To quantify radiation-induced changes in normal appearing white matter (NAWM) before, during and after radiation therapy (RT) in cerebral tumor patients.
Methods and Materials:
Twenty-five patients with low-grade glioma, high-grade glioma or benign tumor treated with RT were studied using diffusion tensor MRI. The biologically corrected doses ranged from 50 to 81 Gy. Temporal changes were assessed before, during, and till 45 weeks after start of RT. The mean diffusivity of water <D>, fractional anisotropy (FA) of diffusion, diffusivity perpendicular (λ⊥) and parallel (λ∥) to white matter fibers were calculated in normal-appearing genu and splenium of the corpus callosum.
Results:
In the genu and splenium, FA decreased and <D>, λ∥, and λ⊥ increased linearly and significantly over time (p< 0.01). At 45 weeks after start of RT, λ⊥ increased ∼30% in the genu and splenium, while λ∥ increased 5% in the genu and 9% in the splenium, suggesting demyelination is predominant. The increases in λ⊥ and λ∥ were dose-dependent starting at 3 weeks and continuing to 32 weeks from the start of RT. The dose-dependent increase in λ⊥ and λ∥ were not sustained after 32 weeks indicating the transition from the focal to diffuse effects.
Conclusions:
The acute and sub-acute changes in normal appearing white matter fibers indicate radiation-induced demyelination and mild structural degradation of axonal fibers. The structural changes after RT are progressive, with early dose-dependent demyelination and subsequent diffuse dose-independent demyelination and mild axonal degradation. DT-MR imaging is potentially a marker for assessment of radiation-induced white matter injury.
Keywords: Diffusion tensor magnetic resonance imaging; normal appearing white matter; radiation-induced demyelination; cerebral tumors, cranial radiation therapy
INTRODUCTION
Radiation therapy (RT) is a common treatment and prophylactic modality for brain tumors, however, RT can have detrimental effects on the central nervous system resulting in neurological complications. The response of cerebral tissue to radiation is dynamic, 1, 2 and extending beyond the targeted tumor volume and influencing neural structures directly or indirectly. Clinical and neurological symptoms can occur within minutes after irradiation and up to 30 years after completion of treatment. 3, 4 Acute side effects that occur within two weeks from start of RT include headache, nausea and drowsiness. Radiation-induced demyelination5 may be the cause of early-delayed neurological symptoms that occur between two week and 3 to 4 months of RT. 6, 7 Mechanisms of the late effects of radiation necrosis include vascular damage 8, white matter injury 1 5, and coagulation necrosis 9. Demyelination, inflammation and breakdown of the blood-brain barrier are among the main neurotoxic effects 10 observed in primates, dogs, rats and mice brain exposed to radiation 5, 7, 11, 12. There are limited data from clinical studies on radiation-induced changes of neuroanatomical structures. In a postmortem study of 25 glioma patients, a host of responses were reported, ranging from late delayed radiation necrosis, to injury of cerebral tissue in the vicinity of the tumor, as well as demyelination suggestive of early delayed necrosis 13. White matter injury can be either focal or diffuse, but hard to identify acutely or subacutely with either conventional MRI or CT 14. Periventricular white matter lesions after radiation are not distinguished on conventional MRI or CT until 12 to 18 months after RT or even later 14, 15. Dysfunction in white matter structures including the corpus callosum can lead to deficits in sensory and neurocognitive functions 16-18. Therefore early detection of neuroanatomical structural changes may aid in replanning therapy to avoid regions at risk for damage.
Diffusion tensor imaging (DTI) is the most sensitive technique to identify white matter pathologies, and can detect well-before structural changes are visible with any other imaging method. DTI can provide information on the density and orientation of white matter fiber tracts. In addition, the quantitative measures obtained from DTI can aid in distinguishing between myelin loss and axonal injury, which has been confirmed with pathology in myelin-deficient rats 19. In a recent study of pediatric medulloblastoma treated with craniospinal irradiation, decreased anisotropy of water diffusion in white matter was found to be correlated with radiation dose 20, but myelin and axon injury were not distinguished. Furthermore, this cross-sectional study investigated only a single snapshot of the dose-dependent neurotoxic effects. An investigation of the longitudinal effects and changes in white matter structures is important to make the association with delayed neurological deficits.
In the present study we used DTI to non-invasively identify radiation-induced changes in normal appearing white matter, and quantitatively characterized the changes during and up to 45 weeks after start of RT. We hypothesized that in brain tumor patients treated with RT, radiation causes progressive loss of structural integrity that is initially predominated by demyelination of white matter. Further, the degree of radiation-induced demyelination would be dependent on the radiation dose delivered. We investigated the anisotropic diffusion characteristics of water in large fibers of normal appearing white matter tissue to understand the sequence of events that manifest in structural degradation. Specifically, we studied the genu and splenium of the corpus callosum, the large white matter tracts that connect the two cerebral hemispheres, because their anatomy is well-defined, their fiber orientation is uniform, and they are involved in a host of neurological functions, including cognitive and motor functions.
METHODS
Patients
Twenty-five patients, eight females and seventeen males, with cerebral tumors participated in institutional review board (IRB) approved clinical MRI studies (Table I). The median age was 60 years (range 23-75). Histopathological diagnosis was based on surgically resected or biopsied tissue. Nineteen patients had grade IV gliomas, three had grade-II gliomas, and three had other benign tumors (Table I). All patients underwent conformal RT of approximately six weeks duration, with fraction sizes ranging from 1.8 to 2.7 Gy. The effect of fraction size on late effects in normal tissue was taken into account by calculating a biologically-corrected dose using an α/β = 2.5 21. Hereafter, the biologically-corrected doses will be referenced and abbreviated as dose. Median RT dose to the planning target volume (PTV) was 60 Gy (range: 50 to 81 Gy). Fifteen patients had concurrent temozolomide with RT. MRI studies were performed just prior to RT and 3, 10, 19, 32 and 45 weeks after start of RT.
Table I.
Gender, age, tumor pathology, anatomical location of the tumor and the total biologically corrected dose (α/β = 2.5) of the 25 patients studied.
| Patient # | Age | Gender | Tumor Pathology |
Anatomical Location of Tumor |
Total Tumor Dose (Gy) |
Concurrent RT + Temozolomide |
|---|---|---|---|---|---|---|
| 1 | 39 | M | GBM | Left Temporal | 72 | Yes |
| 2 | 33 | M | Diffuse Gemistocytic Astrocytoma |
Right Temporal | 70 | No |
| 3 | 62 | M | GBM | Right Fronto- Temporal |
75 | Yes |
| 4 | 40 | M | GBM | Right Occipito- Parietal |
81 | Yes |
| 5 | 31 | F | GBM | Left Temporal | 72 | Yes |
| 6 | 64 | M | GBM | Right Temporal | 78 | Yes |
| 7 | 66 | M | GBM | Right Parietal | 70 | No |
| 8 | 54 | F | GBM | Right Parietal | 70 | No |
| 9 | 60 | M | GBM | Right Parietal | 75 | Yes |
| 10 | 55 | M | Secretory Meningioma |
Left Medial Sphenoid |
54 | No |
| 11 | 68 | F | GBM | Left Frontal | 60 | No |
| 12 | 70 | M | GBM | Right Frontal | 60 | No |
| 13 | 29 | M | Craniopharyngioma | Suprasellar | 56 | No |
| 14 | 66 | M | GBM | Right Posterior Temporal |
75 | Yes |
| 15 | 23 | F | Gliosarcoma | Right Temporal | 66 | Yes |
| 16 | 71 | M | Null cell pituitary Adenoma |
Suprasellar | 50 | No |
| 17 | 30 | M | Oligoastrocytoma | Left Fronto- Parietal |
58 | No |
| 18 | 73 | M | GBM | Right Temporal | 75 | Yes |
| 19 | 56 | F | GBM | Right Frontal | 81 | Yes |
| 20 | 44 | M | GBM | Right Fronto- Parietal |
78 | Yes |
| 21 | 62 | F | GBM | Right Parietal | 75 | Yes |
| 22 | 73 | F | GBM | Left Occipit- Parietal |
75 | Yes |
| 23 | 69 | M | GBM | Right Temporal | 81 | Yes |
| 24 | 75 | F | GBM | Right Parietal | 75 | Yes |
| 25 | 39 | M | Mixed Oligoastrocytoma |
Right Fronto- Temporal |
60 | No |
MR Imaging
All MRI and DTI scans were acquired on a 1.5T GE clinical scanner (General Electric Medical Systems, Milwaukee, WI). The imaging protocol included T1- and T2-weighted imaging, fluid-attenuated inversion recovery imaging (FLAIR), post-contrast T1-weighted, and diffusion tensor imaging. DTI were acquired using a spin-echo echo-planar imaging sequence with repetition time (TR) of 10,000 ms, echo time (TE) of 70 ms, 360 mm2 field of view, 128×128 matrix, and 4 mm slice thickness with a 0 mm gap. Diffusion-sensitizing gradient encoding with diffusion weighting factor of b=1000 s/mm2 was applied in nine directions, and one set of null images with b=0 s/mm2 were acquired. Ten images were obtained for each axial slice.
Image Registration & Processing
Diffusion tensor images acquired prior to, during and after completion of RT were co-registered to the post-contrast T1-weighted image set acquired before initiation of RT. The diffusion weighted images were registered using mutual information and affine transformation which included 12 parameters (9 for 3×3 rotation and shearing matrix and 3 for translation) to optimize and compensate for head motion, slice misalignment, and geometric distortion due to magnetic field susceptibility. Similarly, the treatment planning CT and the spatially-distributed radiation dose maps were co-registered to the post-contrast T1-weighted image set acquired prior to RT.
Diffusion Tensor Imaging (DTI)
DTI that measures the restricted motion of water molecules in tissue was used to assess structural changes in white matter fiber tracts. Several DTI indices can be calculated from the diffusion tensor. The elements of the diffusion tensor consist of isotropic and anisotropic components of water diffusion. The two commonly reported DTI indices are the mean diffusivity (<D>) and fractional anisotropy of diffusion (FA). The mean diffusivity, the average of the three diffusivities in the three orthogonal directions, and is the orientation-independent measure of the displacement of water molecules in tissue. FA is a quantitative DTI index that measures the degree of anistropic diffusion of water in the fiber-like structure. The FA values range between zero and one, with a higher value denoting a highly-ordered white matter structure in which water diffusion is restricted in the directions perpendicular to the fiber long axis. The mean diffusivities in gray and white matter are similar, but FA, the orientation-dependent diffusion index of water molecules is inherently different in white and gray matter. However, additional information on white matter structures and changes in these structures can be provided by eigen-diffusivities (λ1, λ2, and λ3) along the three principal directions. The eigen-diffusivities are obtained by diagonalizing the diffusion tensor matrix 22-24. In the brain, the largest eigen-diffusivity λ1 represents water diffusion along the direction parallel to the axonal fibers, hereafter labeled λ∥, which is sensitive to axonal injury but not demyelination 19, 25. The λ2 and λ3 components denote diffusivities in the two orthogonal directions perpendicular to the axonal fibers. The mean of λ2 and λ3 is termed as λ⊥, which is sensitive to demyelination 19, 26. Maps of <D>, FA, λ∥ and λ⊥, were calculated using an integrated functional imaging analysis software package, FIAT 27, developed in-house. FIAT is equipped with a range of tools and features that allow co-registration, mathematical manipulations, evaluation of quantitative information and data display of images. All image processing and analysis were performed using FIAT.
Volumes of Interest
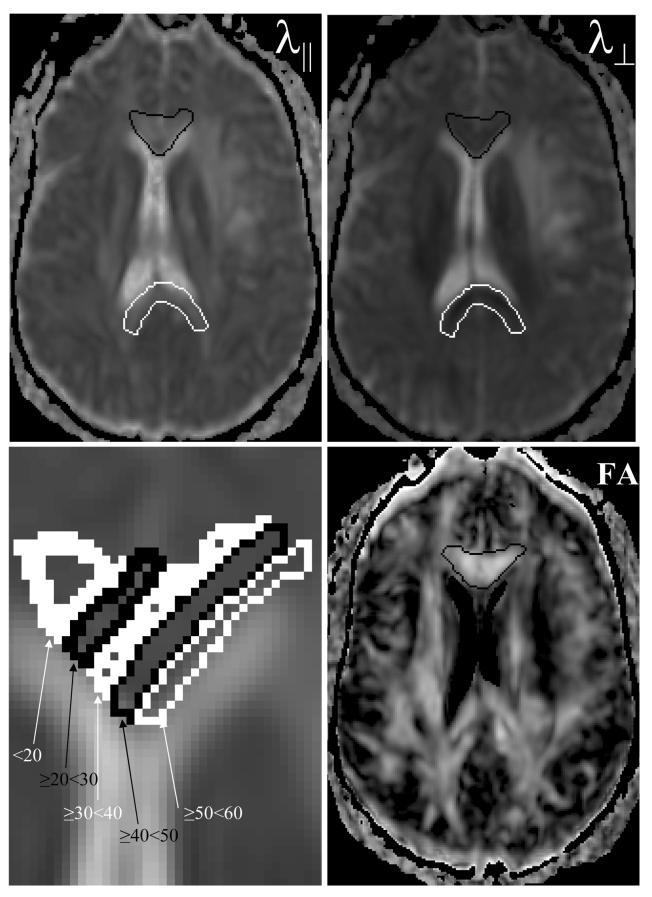
Normal appearing white matter (NAWM), that is, tissue which appeared normal on conventional T1-weighted, T2-weighted and FLAIR MR images were analyzed to study the genu and splenium of the corpus callosum. The corpus callosum is the largest white matter fiber bundle in the brain connecting the two cerebral hemispheres, anteriorly at the genu and posteriorly at the splenium (Figure 1). The commissural fibers passing through the genu that connect the prefrontal cortices and frontal lobes, and the fibers passing through the splenium that connect the temporal, parietal and occipital lobes were identified and manually contoured on the FA image that was color-coded with the direction of fibers. For each patient, the volumes of interest (VOI) in NAWM of genu and splenium were defined on the axial slice at the level of the third ventricle, and in the adjacent superior and inferior slices. The VOIs were then transferred onto FLAIR images, which accentuated the differences between gray and white matters, and edited to exclude any tumor or peritumoral tissue. The volumes of normal-appearing genu and splenium were further divided into subvolumes which were binned at 10-Gy intervals (Figure 1). These volumes were obtained from RT dose map and used to analyze for dose-dependent changes.
Figure 1.
Images of the parallel (λ∥, left top) and perpendicular (λ⊥, right top) diffusivities, and fractional anisotropy (FA, right bottom) maps from patient #5 before RT. The genu outlined in black and the splenium in white, appear bright onλ∥ and dark on theλ⊥ images, indicating that diffusion is greater in the direction parallel to the fiber long axis than in the perpendicular direction, representative of anisotropic diffusion. In contrast, cerebral spinal fluid (CSF) appears bright with similar intensities on both λ∥ and λ⊥. The fractional anisotropy allows better visualization of white matter (WM) fiber tracts. The bright regions on the FA image correspond to WM; while, CSF which lacks anisotropic diffusion appears dark. The genu and splenium volumes of interest were further divided into subvolumes as 10 Gy intervals of biologically-corrected doses. An enlarged view of the genu with dose-dependent subvolumes overlaid on the mean diffusivity image is shown in the lower left panel.
Statistical Analysis
Statistical analysis was performed with the commercially available Statistical Software Package for Social Sciences program (SPSS Software Products, Chicago, IL). Descriptive statistics of <D>, FA,λ∥, and λ⊥ were obtained for the genu and splenium.
Two-tailed Student's t-test was used to assess statistical differences between the genu versus the splenium and between time-points (pre, during and post RT). Associations between the DTI indices, and time of MRI scan, and radiation dose were evaluated using Pearson's product-moment correlation. If there was a statistically significant correlation, a linear least squares fit was used to obtain the regression equation. Results were considered statistically significant at the level of p < 0.05.
RESULTS
Pre-RT DTI Characteristics
Representative images of the DTI indices, <D>, FA, λ∥ and λ⊥ from patient #5 (Table I) are illustrated in Figure 1. The mean ± SE of <D>, FA, λ∥ and λ⊥ for the 25 patients, evaluated before RT in NAWM of the genu and splenium are given in Table II. Before RT, the <D>, FA and λ⊥ values in the genu were significantly different from those in the splenium (p<0.05), suggesting there are structural differences between the genu and splenium, but there was no difference in λ∥. We evaluated the correlation between FA in the genu or splenium and age prior to RT, for the purpose of quality assurance, and found there were significant correlations (p<0.01, data not shown). Furthermore, we correlated the changes in DTI indices after the start of RT with age, and did not find any significant correlations (p>0.05), indicating that the changes in DTI indices after the start of RT were not related to patient age.
Table II.
Mean ± SE (n=25 patients) of the mean diffusivity (<D>), fractional anisotropy (FA), the parallel (λ∥) and perpendicular (λ⊥) diffusivities before RT in the genu and splenium of normal-appearing white matter.
| DTI Index/Structure |
<D> x10−6 mm2/s |
FA | λ∥ x10−6 mm2/s |
λ⊥ x10−6 mm2/s |
|---|---|---|---|---|
| Genu | 985 ± 24 | 0.465 ± 0.016 | 1538 ± 24 | 709 ± 29 |
| Splenium | 957 ± 25 | 0.493 ± 0.016 | 1534 ± 33 | 668 ± 26 |
Temporal Changes in DTI Characteristics
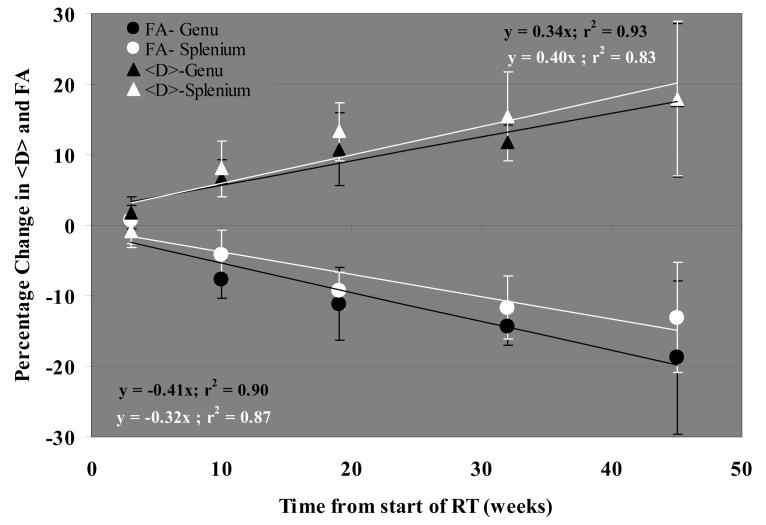
The effects of radiation in normal-appearing genu and splenium of the corpus callosum were assessed by changes in FA and <D> up to 45 weeks after the start of RT. We performed quantitative analysis of the temporal changes in FA and <D> data pooled from 25 patients (Figure 2). Overall, in both the genu and splenium, FA decreased and <D> increased linearly and significantly over time (p< 0.01, linear regression analysis), suggesting degradation of white matter after irradiation. The increases in <D> were similar for the genu and splenium, with an 18% increase at 45 weeks after the start of RT. The magnitude of decline in FA was greater in the genu than in the splenium, but this difference was not significant (Figure 2). FA decreased by 19% in the genu 45 weeks after the start of RT, about one-third more than the 13% decrease in the splenium. The increases in <D> and the decreases in FA became significant 10 weeks after the start of RT in the genu (p< 0.03) and 19 weeks in the splenium (p<0.01). Figure 3 shows an example of changes in FA of the genu; in this case, FA decreased from 0.52 before RT to 0.37 at 19 weeks after the start of RT, a 29% decrease.
Figure 2.
Temporal profile of the percentage changes in the mean diffusivity, <D>, and fractional anisotropy, FA, in the genu and splenium during and after RT relative to the respective pre RT values. The mean diffusivity, <D>, increased and FA decreased linearly over time both in the genu and splenium. The data is average from all 25 patients in the study, and the error bars represent the standard error of the mean.
Figure 3.
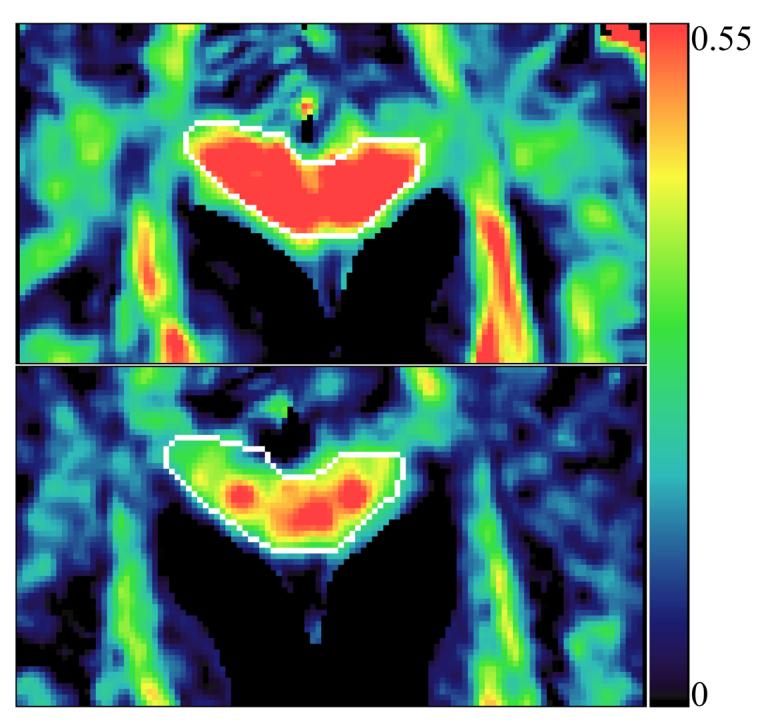
The fractional anisotropy (FA) maps from patient # 7 before RT (top panel) and 10 weeks after the start of RT (lower panel). FA is color-coded, with blue color corresponding to low anisotropy and red denoting higher anisotropy. The pre and post RT maps are windowed to the same scale. FA in the genu (outlined in white) decreased from 0.52 before RT to 0.37 at 10 weeks from start of RT (1 month after the completion of RT), suggesting radiation-induced injury.
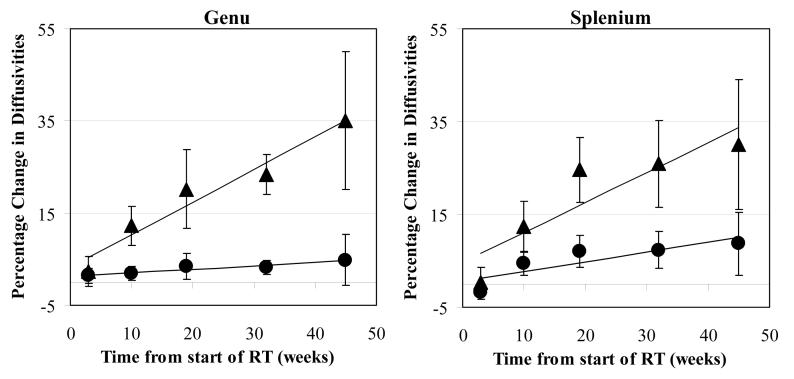
The effects of radiation on normal-appearing genu and splenium were further analyzed with λ∥ and λ⊥, which could provide information about white matter injury in addition to that obtained from FA and <D>. Linear regression analysis revealed that both λ∥ and λ⊥ in the genu increased approximately linearly with time (p<0.04 and 0.002 respectively, Figure 4). The λ⊥ increases were similar in the genu and splenium, approximately 0.7% per week, resulting in a ∼30% increase 45 weeks after the start of RT. However, the increase in λ∥ were significantly less than those in λ⊥ in both the genu and splenium (p<0.016 and p<0.010, respectively). In the genu, λ∥ increased approximately 0.08% per week, resulting in a ∼ 3.6% increase 45 weeks after the start of RT, while in the splenium, λ∥ increased approximately 0.21% per week, resulting in a 9% increase 45 weeks after the start of RT. The predominance of change in λ⊥ compared to λ∥ suggests that radiation-induced injury in these fibers is predominantly by demyelination as opposed to axonal injury. In the genu, the increase in λ⊥ became significant 10 weeks after the start of RT (p<0.0013), but, the λ∥ increase was not significant at this timepoint. In the splenium, the increases in both λ⊥ and λ∥ became significant 19 weeks from start of RT (p<0.005). These differences in radiation-induced changes between the genu and splenium indicate the heterogeneous effects of RT in the corpus callosum.
Figure 4.
Scatter plot of the percentage changes over time in the parallel (λ∥ circles) and perpendicular (λ⊥ triangles) diffusivities in normal appearing white matter tissue in the genu (left panel) and splenium (right panel) of the corpus callosum. The data are the average of all patients in the study and the bars denote the standard error of the mean; the solid lines are the best fit curves. The increases in λ⊥ (triangles) were greater than in λ∥ (circles by seven-fold in the genu and three-fold in the splenium, indicating radiation-induced injury is predominantly due to demyelination, with a possible contribution of mild axonal degradation .
Dose-Dependent Changes in DTI
Dose-dependent changes in λ∥ and λ⊥ of the genu and splenium were evaluated during and after RT. The data from FA and <D> are not presented since they provide results similar to λ∥ and λ⊥.
(A) Genu
We found that λ⊥ of the genu was significantly correlated with the dose to the genu as early as 3 weeks after the start of RT (p<0.004), and the significant correlation persisted to 19 weeks after the start of RT (Table III). By 32 weeks, however, the correlation with dose diminished, while the increase in λ⊥ continued long after the full dose had been delivered. In contrast, the effect of radiation on λ∥ was smaller compared with the effect on λ⊥. Three weeks after the start of RT, when the increase in λ∥ was minimum (see previous section), λ∥ was not correlated with the dose. By 10 weeks, correlation with dose became significant (p<0.02), and this relationship remained significant to 19 weeks after the start of RT (Table III). By 32 weeks, a correlation was longer found.
Table III.
Dose-dependent changes in the perpendicular and (λ⊥) parallel (λ∥) diffusivities during and post RT in the genu and splenium of normal-appearing white matter. The slope (units are in 10−6mm2/s per Gy) of the linear regression fit of λ⊥ vs. dose and λ∥ vs. dose, and p value of the linear fit are listed for weeks 3, 10 and 19 from start of RT. The underlined values denotes there was no significant correlation between diffusivity and dose.
| DTI Index | Genu | Splenium | ||||
|---|---|---|---|---|---|---|
| Dose-dependency of DTI Indices |
3 weeks | 10 weeks | 19 weeks | 3 weeks | 10 weeks | 19 weeks |
| λ⊥ slope p |
0.058 0.004 |
0.049 0.002 |
0.071 0.0001 |
0.031 0.03 |
0.0007 >0.1 |
0.038 0.03 |
| λ∥ slope p |
ns |
0.047 0.014 |
0.062 0.002 |
−0.038 0.07 |
−0.008 >0.1 |
0.087 0.001 |
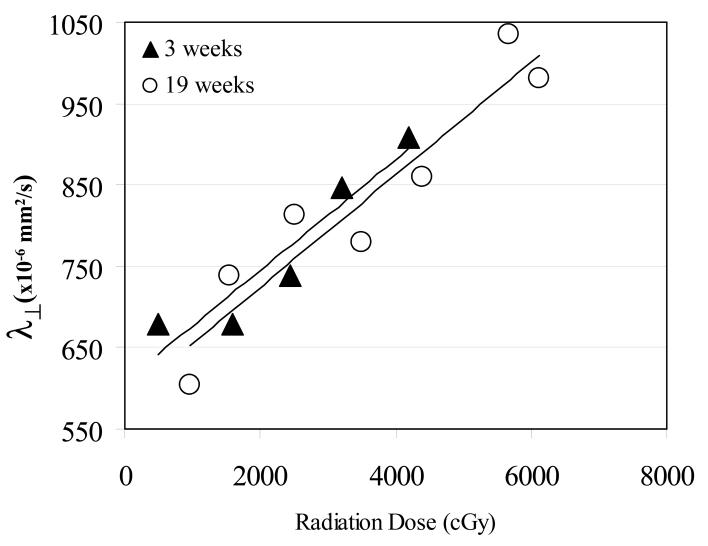
Further analysis showed that there were no significant differences between the slopes of the linear regression fits of λ⊥ vs. the dose at 3, 10 and 19 weeks after the start of RT (Figure 5, Table III). Note that the accumulated dose at 10- and 19-weeks were the same, and approximately double the dose at 3 weeks after the start of RT. The similar slopes of linear regression fits at 3, 10, and 19 weeks imply that doubling the dose also doubled the increase in λ⊥.
Figure 5.
Dose-dependency of the perpendicular diffusivity (λ⊥) in the genu 3 weeks (solid triangles) and 19 weeks after the start of RT (open circles) with the solid lines representing the linear regression fits. The shorter solid line is the linear regression fit at 3 weeks and the longer line is the regression fit at 19 weeks from start of RT. Note that the regression line at 3 weeks was similar to the one at 19 weeks with respective slopes of 0.06 and 0.07 ×10−6mm2/s per Gy, suggesting that doubling the dose also doubled the effect of radiation on the genu.
(B) Splenium
The dose-dependent changes in λ⊥ and λ∥ of the splenium were similar to the genu (Table III). Again, significant correlation between the diffusivity and dose was observed in the direction perpendicular to the fiber axis (λ⊥) as early as 3 weeks after the start of RT (p<0.03) while the significant correlation in λ∥ was delayed until 19 weeks after the start of RT (p<0.01). The correlation between λ⊥ and the dose was also significant at 19 weeks (p<0.03) but not however at 10 weeks after the start of RT (Table III). Similar to the genu, by 32 weeks after the start of RT, the increases in λ⊥ and λ∥ of the splenium were not correlated with the dose any more, suggesting that the delayed effect of radiation on white matter is diffuse. Finally, there was no significant difference in the slopes of the linear regression fits of λ⊥ vs. the dose, at 3 weeks and 19 weeks after the start of RT (Table III).
DISCUSSION
In this study, we observed that white matter fiber integrity is compromised ensuing a course of RT in normal appearing tissue of cerebral tumor patients. DTI results indicated a substantial increase in λ⊥ but only a mild increase in λ∥ following RT, suggesting demyelination is the predominant effect on white matter fibers 28. The increases in λ⊥ and λ∥ diffusivities occurred gradually and persisted until shortly after RT, albeit the magnitudes of increase were different. Furthermore, the increases in diffusivities were dose-dependent starting as early as 3 weeks from the start of RT. The dose-dependent increase in λ⊥ and λ∥ were not sustained later than 32 weeks after the start of RT, indicating that demyelination is not limited to regions receiving high radiation doses but becomes diffuse over time. The comprehensive analysis of our longitudinal DT imaging studies elucidates white matter sequelae that include dose-dependent demyelination during RT and acutely up to three months after RT with subsequent mild axonal degradation and diffuse demyelination subacutely, four to six months post RT. These findings suggest a window of opportunity in which treatment could be administered to arrest or retard degradation of white matter.
Demyelination and axonal damage are the hallmarks of white matter injury, each playing a different role in neurological function; thus a distinction between the two pathologies is important. White matter in the brain is known to be highly vulnerable to radiation 1, 2, 29. In primates, post-radiation responses included perivascular and diffuse demyelination manifest in degeneration of myelin accompanied by degeneration of axonal fibers, in the later stages 11, 30. There is no noninvasive biological marker that can differentiate between the white matter pathologies of demyelination and axonal damage. Studies suggest that among the indices that can be measured with DTI, comparison of the parallel (λ∥) and perpendicular (λ⊥) components of diffusivity is useful to distinguish between myelin dysfunction and axonal degradation. A DTI study of myelin-deficient/absent Shiverer mice validated that an elevation in λ⊥ with unchanged λ∥ was caused by demyelination 26. Conversely in an animal model of axonal loss from the optic nerve after retinal injury, an unaltered λ⊥ accompanied with a change in λ∥ was confirmed histologically to be due to axonal degeneration without demyelination19. Our observation of the substantial increase in λ⊥ with a smaller increase in λ∥ over a 45-weeks period suggests that demyelination is the predominant early response of NAWM to RT. Also, neither remyelination, more severe demyelination, or radiation necrosis were evident up to 45 weeks after the start of RT in the normal-appearing genu and splenium. Remyelination would be indicated by renormalization or decrease in λ⊥ 31, 32. More severe demyelination would be evident as hyperintense signal on T2 images 33, while radiation necrosis could be indicated by alterations in both T1 and T2 33. However, these developments did not present in our data.
Radiation response of cerebral white matter tissue appears to be progressive with dose-dependent demyelination early in regions receiving high radiation doses, followed by dose-independent demyelination not limited to high dose regions and mild axonal degradation, four to six months after completion of RT. Both early and diffuse pathological changes have been reported in animal and post-mortem human studies 13, 34, 35, with myelin breakdown detected within weeks of irradiation 11. In monkeys treated with neutron irradiation progressive demyelination was documented, but the demyelination was not accompanied by axonal, glial, neuronal or inflammatory responses 30. In a post-mortem study of glioma patients, radiation was shown to produce selective demyelination in tissue adjacent to the neoplasm 13. Our observations concur with the above reports; radiation-induced abnormalities in NAWM were continuous, progressing from early (during and up to three months after RT) dose-dependent demyelination, to ongoing demyelination that became dose-independent subacutely (four to six months after completion of RT), indicative of diffuse demyelination. To avoid radiation injury to critical white matter fibers that connect the large neuronal network diffuse demyelination poses a major challenge in the treatment planning paradigm. The current finding of the progressive but mild demyelination initially and latency in degradation of axonal structures up to several months opens a window of opportunity during which interventional therapies can be implemented to salvage, arrest or retard further deterioration of white matter structures and thereby minimize neurocognitive dysfunction.
Cerebral white matter plays a vital role in carrying action potentials from one gray matter region to another. The primary effect of demyelination is to slow transmission from one region to another, while injury to axons causes more severe disruption of transmission. Clinical evidence suggests that limited RT-induced demyelination is associated with minimal or mild neurocognitive deficits, while more extensive demyelination, termed RT encephalopathy or RT leukoencephalopathy is associated with more severe cognitive dysfunction as well as motor and possibly visual deficits 14 36 37 38 39. The patterns of neurocognitive impairments include decline or loss of memory, attention, learning, and executive functions 40 41, sometimes severe enough to meet the definition of dementia. The most common motor manifestation is gait impairment, sometimes termed gait apraxia 41. In a rodent model, neurological deficits included paralysis 4 to 7 months post irradiation 42. A study of the relationship between radiation-induced white matter injury and compromised neurocognitive functions demonstrated a significant correlation between the DTI fractional anisotropy index and cognitive decline months to years after whole brain radiation in children with medulloblastoma and acute lymphoblastic leukemia pediatric patients 43. Whether the early changes we demonstrated in white matter after cranial irradiation are predictive of delayed neurological dysfunction will be tested in a future study.
Our study aimed to characterize the temporal changes before, during and after RT, thus providing information on the time-sequence of structural changes in normal-appearing white matter, and allowed us to use patients' as their own control and eliminated the need to use a control population. Therefore, factors such as patient age, disease grade and tumor pathology were not relevant. By using quantitative DTI indices, absolute values were calculated to evaluate changes over time.
CONCLUSIONS
The acute and sub-acute changes in normal appearing white matter fibers of the corpus callosum observed using quantitative diffusion tensor MR imaging indicate radiation-induced demyelination and mild structural degradation of axonal fibers. The structural changes in white matter after cranial irradiation are progressive, with early dose-dependent demyelination and subsequent mild axonal degradation and diffuse demyelination. DT-MR imaging therefore is potentially a marker for assessment of radiation-induced white matter injury. Latency in axonal degradation and further progression in demyelination may allow remedial therapy to be administered.
Acknowledgments
This work was supported by National Institute of Health Grants 3PO1 CA59827, PO2 CA85878 and R21 CA11369901. The authors thank Kristin Brierley for research study coordination, Dan Tatro for dosimetry assistance and Zhou Shen for computer software support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Meeting Presentation:
Part of this work was presented at the 48th ASTRO Annual Meeting, November 5 -9, 2006, Philadelphia.
Conflicts Of Interest:
None of the authors have any actual or potential conflicts of interest.
REFERENCES
- 1.Schultheiss TE, Kun LE, Ang KK, et al. Radiation response of the central nervous system. Int J Radiat Oncol Biol Phys. 1995;31:1093–1112. doi: 10.1016/0360-3016(94)00655-5. [DOI] [PubMed] [Google Scholar]
- 2.Tofilon PJ, Fike JR. The radioresponse of the central nervous system: a dynamic process. Radiat Res. 2000;153:357–370. doi: 10.1667/0033-7587(2000)153[0357:trotcn]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 3.Zeman W. Radiosensitivities of nervous tissues. Brookhaven Symp Biol. 1961;14:176–199. [PubMed] [Google Scholar]
- 4.Keime-Guibert F, Napolitano M, Delattre JY. Neurological complications of radiotherapy and chemotherapy. J Neurol. 1998;245:695–708. doi: 10.1007/s004150050271. [DOI] [PubMed] [Google Scholar]
- 5.Calvo W, Hopewell JW, Reinhold HS, et al. Time- and dose-related changes in the white matter of the rat brain after single doses of X rays. Br J Radiol. 1988;61:1043–1052. doi: 10.1259/0007-1285-61-731-1043. [DOI] [PubMed] [Google Scholar]
- 6.Monro P, Mair WG. Radiation effects on the human central nervous system 14 weeks after x-radiation. Acta Neuropathol (Berl) 1968;11:267–274. doi: 10.1007/BF00686723. [DOI] [PubMed] [Google Scholar]
- 7.Chiang CS, McBride WH, Withers HR. Radiation-induced astrocytic and microglial responses in mouse brain. Radiother Oncol. 1993;29:60–68. doi: 10.1016/0167-8140(93)90174-7. [DOI] [PubMed] [Google Scholar]
- 8.Reinhold HS, Calvo W, Hopewell JW, et al. Development of blood vessel-related radiation damage in the fimbria of the central nervous system. Int J Radiat Oncol Biol Phys. 1990;18:37–42. doi: 10.1016/0360-3016(90)90264-k. [DOI] [PubMed] [Google Scholar]
- 9.Glass JP, Hwang TL, Leavens ME, et al. Cerebral radiation necrosis following treatment of extracranial malignancies. Cancer. 1984;54:1966–1972. doi: 10.1002/1097-0142(19841101)54:9<1966::aid-cncr2820540930>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 10.Sheline GE, Wara WM, Smith V. Therapeutic irradiation and brain injury. Int J Radiat Oncol Biol Phys. 1980;6:1215–1228. doi: 10.1016/0360-3016(80)90175-3. [DOI] [PubMed] [Google Scholar]
- 11.Price RE, Langford LA, Jackson EF, et al. Radiation-induced morphologic changes in the rhesus monkey (Macaca mulatta) brain. J Med Primatol. 2001;30:81–87. doi: 10.1034/j.1600-0684.2001.300202.x. [DOI] [PubMed] [Google Scholar]
- 12.Benczik J, Tenhunen M, Snellman M, et al. Late radiation effects in the dog brain: correlation of MRI and histological changes. Radiother Oncol. 2002;63:107–120. doi: 10.1016/s0167-8140(02)00028-2. [DOI] [PubMed] [Google Scholar]
- 13.Burger PC, Mahley MS, Jr., Dudka L, et al. The morphologic effects of radiation administered therapeutically for intracranial gliomas: a postmortem study of 25 cases. Cancer. 1979;44:1256–1272. doi: 10.1002/1097-0142(197910)44:4<1256::aid-cncr2820440415>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 14.Constine LS, Konski A, Ekholm S, et al. Adverse effects of brain irradiation correlated with MR and CT imaging. Int J Radiat Oncol Biol Phys. 1988;15:319–330. doi: 10.1016/s0360-3016(98)90011-6. [DOI] [PubMed] [Google Scholar]
- 15.Packer RJ, Zimmerman RA, Bilaniuk LT. Magnetic resonance imaging in the evaluation of treatment-related central nervous system damage. Cancer. 1986;58:635–640. doi: 10.1002/1097-0142(19860801)58:3<635::aid-cncr2820580307>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 16.Smith DH, Meaney DF, Shull WH. Diffuse axonal injury in head trauma. J Head Trauma Rehabil. 2003;18:307–316. doi: 10.1097/00001199-200307000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Meythaler JM, Peduzzi JD, Eleftheriou E, et al. Current concepts: diffuse axonal injury-associated traumatic brain injury. Arch Phys Med Rehabil. 2001;82:1461–1471. doi: 10.1053/apmr.2001.25137. [DOI] [PubMed] [Google Scholar]
- 18.Okamoto T, Hashimoto K, Aoki S, et al. Cerebral blood flow in patients with diffuse axonal injury--examination of the easy Z-score imaging system utility. Eur J Neurol. 2007;14:540–547. doi: 10.1111/j.1468-1331.2007.01742.x. [DOI] [PubMed] [Google Scholar]
- 19.Song SK, Sun SW, Ju WK, et al. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage. 2003;20:1714–1722. doi: 10.1016/j.neuroimage.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 20.Khong PL, Leung LH, Chan GC, et al. White matter anisotropy in childhood medulloblastoma survivors: association with neurotoxicity risk factors. Radiology. 2005;236:647–652. doi: 10.1148/radiol.2362041066. [DOI] [PubMed] [Google Scholar]
- 21.Steel GG. Basic Clinical Radiobiology. 3rd Edition ed. Arnold Publishers; London, U.K.: 2002. [Google Scholar]
- 22.Pierpaoli C, Basser PJ. Toward a quantitative assessment of diffusion anisotropy. Magn Reson Med. 1996;36:893–906. doi: 10.1002/mrm.1910360612. [DOI] [PubMed] [Google Scholar]
- 23.Pierpaoli C, Jezzard P, Basser PJ, et al. Diffusion tensor MR imaging of the human brain. Radiology. 1996;201:637–648. doi: 10.1148/radiology.201.3.8939209. [DOI] [PubMed] [Google Scholar]
- 24.Basser PJ, Pierpaoli C. A simplified method to measure the diffusion tensor from seven MR images. Magn Reson Med. 1998;39:928–934. doi: 10.1002/mrm.1910390610. [DOI] [PubMed] [Google Scholar]
- 25.Ono J, Harada K, Takahashi M, et al. Differentiation between dysmyelination and demyelination using magnetic resonance diffusional anisotropy. Brain Res. 1995;671:141–148. doi: 10.1016/0006-8993(94)01335-f. [DOI] [PubMed] [Google Scholar]
- 26.Song SK, Sun SW, Ramsbottom MJ, et al. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage. 2002;17:1429–1436. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- 27.Cao Y. Development of Image Software Tools for Radiation Therapy Assessment. Medical Physics. 2005;32:2136. [Google Scholar]
- 28.Song SK, Yoshino J, Le TQ, et al. Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage. 2005;26:132–140. doi: 10.1016/j.neuroimage.2005.01.028. [DOI] [PubMed] [Google Scholar]
- 29.Schultheiss TE, Stephens LC. The pathogenesis of radiation myelopathy: widening the circle. Int J Radiat Oncol Biol Phys. 1992;23:1089–1091. doi: 10.1016/0360-3016(92)90920-d. discussion 1093-1084. [DOI] [PubMed] [Google Scholar]
- 30.Pickering JE, Vogel FS. Demyelinization induced in the brains of monkeys by means of fast neutrons; pathogenesis of the lesion and comparison with the lesions of multiple sclerosis and Schilder's disease. J Exp Med. 1956;104:435–442. doi: 10.1084/jem.104.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harsan LA, Poulet P, Guignard B, et al. Brain dysmyelination and recovery assessment by noninvasive in vivo diffusion tensor magnetic resonance imaging. J Neurosci Res. 2006;83:392–402. doi: 10.1002/jnr.20742. [DOI] [PubMed] [Google Scholar]
- 32.Sun SW, Liang HF, Trinkaus K, et al. Noninvasive detection of cuprizone induced axonal damage and demyelination in the mouse corpus callosum. Magn Reson Med. 2006;55:302–308. doi: 10.1002/mrm.20774. [DOI] [PubMed] [Google Scholar]
- 33.Valk PE, Dillon WP. Radiation injury of the brain. AJNR Am J Neuroradiol. 1991;12:45–62. [PMC free article] [PubMed] [Google Scholar]
- 34.Lo EH, DeLaPaz RL, Frankel KA, et al. MRI and PET of delayed heavy-ion radiation injury in the rabbit brain. Int J Radiat Oncol Biol Phys. 1991;20:689–696. doi: 10.1016/0360-3016(91)90010-2. [DOI] [PubMed] [Google Scholar]
- 35.Corn BW, Yousem DM, Scott CB, et al. White matter changes are correlated significantly with radiation dose. Observations from a randomized dose-escalation trial for malignant glioma (Radiation Therapy Oncology Group 83-02) Cancer. 1994;74:2828–2835. doi: 10.1002/1097-0142(19941115)74:10<2828::aid-cncr2820741014>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 36.van der Putten JJ, Hobart JC, Freeman JA, et al. Measuring change in disability after inpatient rehabilitation: comparison of the responsiveness of the Barthel index and the Functional Independence Measure. J Neurol Neurosurg Psychiatry. 1999;66:480–484. doi: 10.1136/jnnp.66.4.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Armstrong CL, Corn BW, Ruffer JE, et al. Radiotherapeutic effects on brain function: double dissociation of memory systems. Neuropsychiatry Neuropsychol Behav Neurol. 2000;13:101–111. [PubMed] [Google Scholar]
- 38.Cole PD, Kamen BA. Delayed neurotoxicity associated with therapy for children with acute lymphoblastic leukemia. Ment Retard Dev Disabil Res Rev. 2006;12:174–183. doi: 10.1002/mrdd.20113. [DOI] [PubMed] [Google Scholar]
- 39.Chang EL, Akyurek S, Avalos T, et al. Evaluation of peritumoral edema in the delineation of radiotherapy clinical target volumes for glioblastoma. Int J Radiat Oncol Biol Phys. 2007 doi: 10.1016/j.ijrobp.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 40.Meyers CA, Geara F, Wong PF, et al. Neurocognitive effects of therapeutic irradiation for base of skull tumors. Int J Radiat Oncol Biol Phys. 2000;46:51–55. doi: 10.1016/s0360-3016(99)00376-4. [DOI] [PubMed] [Google Scholar]
- 41.Moretti R, Torre P, Antonello RM, et al. Neuropsychological evaluation of late-onset post-radiotherapy encephalopathy: a comparison with vascular dementia. J Neurol Sci. 2005;229-230:195–200. doi: 10.1016/j.jns.2004.11.027. [DOI] [PubMed] [Google Scholar]
- 42.Rubin P, Whitaker JN, Ceckler TL, et al. Myelin basic protein and magnetic resonance imaging for diagnosing radiation myelopathy. Int J Radiat Oncol Biol Phys. 1988;15:1371–1381. doi: 10.1016/0360-3016(88)90233-7. [DOI] [PubMed] [Google Scholar]
- 43.Khong PL, Leung LH, Fung AS, et al. White matter anisotropy in post-treatment childhood cancer survivors: preliminary evidence of association with neurocognitive function. J Clin Oncol. 2006;24:884–890. doi: 10.1200/JCO.2005.02.4505. [DOI] [PubMed] [Google Scholar]
- 44.Pfefferbaum A, Sullivan EV, Hedehus M, et al. Age-related decline in brain white matter anisotropy measured with spatially corrected echo-planar diffusion tensor imaging. Magn Reson Med. 2000;44:259–268. doi: 10.1002/1522-2594(200008)44:2<259::aid-mrm13>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 45.Mukherjee P, Miller JH, Shimony JS, et al. Normal brain maturation during childhood: developmental trends characterized with diffusion-tensor MR imaging. Radiology. 2001;221:349–358. doi: 10.1148/radiol.2212001702. [DOI] [PubMed] [Google Scholar]