Abstract
Cell migration is critical for animal development and physiological as well as pathological responses. One important step during cell migration is the formation of lamellipodia at the leading edge of migrating cells. Here we report that the second messenger cAMP inhibits the migration of mouse embryonic fibroblast cells and mouse breast tumor cells. cAMP acts downstream of the small GTPase Rac and interferes with the formation of lamellipodia. Moreover, cAMP decreases the phosphorylation of the myosin light chain at the leading edge of cells and increases the phosphorylation of the vasodilator-stimulated phosphoprotein. Together with our previous report of a positive role of another second messenger, cGMP, in lamellipodium formation, our data indicate that cAMP and cGMP play opposite roles in modulating lamellipodium formation.
Cell migration is a cellular event that is critical for various physiological processes and pathological responses such as embryonic development, angiogenesis, immune function and inflammation, axonal guidance and neural development, tissue repair, and tumor metastasis (1, 2). Cell migration is a sequential and interrelated multistep process. It involves the formation of lamellipodia/membrane protrusions at the front edge, cycles of adhesion and detachment to the extracellular matrix, cell body contraction and translocation, and tail retraction. For efficient migration to occur, these activities need to be spatially and temporally coordinated through complex signaling events. A better understanding of the regulation of cell migration will lead to the development of novel therapeutics for human disease conditions such as tumor metastasis.
Lamellipodium formation is an important step during cell migration (3, 4). The lamellipodium is a specialized subcellular structure at the front of a migrating cell. It is mainly a cytoskeletal actin projection. The tips of lamellipodia localize and harness actin polymerization for cell migration. Lamellipodia display characteristic highly active behavior. They spread forwards quickly with sometimes retracting, ruffling, or bubbling (3).
Cells migrate in response to specific external signals. This orchestrated movement is subjected to modulation. cAMP is a ubiquitous cellular second messenger and could regulate a wide range of cellular processes, including cell migration (5–10). In Xenopus spinal neurons and rat sensory neurons, the ratio of cAMP to cGMP is important in axonal guidance (11, 12). Although cAMP could modulate cell migration, the mechanism by which cAMP plays its role in regulating fibroblast and tumor cell migration is not clear.
Here we use both mouse embryonic fibroblasts (MEFs)2 and mouse 4T1 breast tumor cells to study the modulation of cell migration by cAMP. We found that cAMP inhibits the migration of MEFs and 4T1 breast tumor cells by interfering with the formation of lamellipodia at the leading edge during cell migration.
EXPERIMENTAL PROCEDURES
Cells and Reagents—MEF cells, Gαs-/- cells, and 4T1 breast tumor cells were described previously (13–16). MEF cells were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum and 2 mm glutamine. 4T1 cells were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum. PDGF-BB was from Sigma. LPA was from Cayman Chemical Co. Forskolin, 6-benzoyl-cAMP, and H-89 were from Calbiochem.
In Vitro Wound-healing Cell Migration Assay—Cell migration assays were performed as described previously (13–15). Cells were allowed to form a confluent monolayer in a 24-well plate coated with gelatin before wounding. The wound was made by scraping a conventional pipette tip across the monolayer. Cell migration was induced by adding medium supplemented with 10% fetal bovine serum and 20 ng/ml PDGF (for MEF cells) or 10 μm LPA (for 4T1 cells). For MEF cells, it typically took 8–10 h for the wound to close. For 4T1 cells, it typically took 12–14 h for the wound to close. When the wound for the positive control closed, cells were fixed with 3.7% formaldehyde and stained with crystal violet staining solution.
Boyden Chamber Cell Migration Assay—MEF and 4T1 cells (5 × 104) suspended in starvation medium were added to the upper chamber of an insert (6.5-mm diameter, 8-μm pore size; BD Biosciences), and the insert was placed in a 24-well plate containing starvation medium with or without 10% fetal bovine serum abd 20 ng/ml PDGF (for MEF cells) or 10 μm LPA (for 4T1 cells). When used, inhibitors were added to both chambers. Migration assays were carried out for 4 h, and cells were fixed with 3.7% formaldehyde. Cells were stained with crystal violet staining solution, and cells on the upper side of the insert were removed with a cotton swab. Three randomly selected fields (×10 objective) were photographed, and the migrated cells were counted.
Immunofluorescence Microscopy— Preparation of samples for fluorescence microscopy was preformed as described previously (15, 17–19). Cells cultured on gelatin-coated glass coverslips were fixed with 3.7% formaldehyde in phosphate-buffered saline for 10 min at room temperature, permeabilized with 0.1% Triton X-100 for 5 min, and then washed three times with phosphate-buffered saline. To block nonspecific binding, the cells were incubated with a solution of phosphate-buffered saline containing 1% bovine serum albumin for 30 min and then incubated with primary antibody at appropriate dilutions (1:100 for rabbit anti-phospho-Ser-19 MLC antibody (Cell Signaling Technology), 1:2000 for anti-tubulin antibody, and 1:1000 for anti-VASP antibody (Cell Signaling Technology)) for 1 h. Alexa Fluor 488-conjugated phalloidin (Molecular Probes) was used to visualize F-actin. After incubation with primary antibody, cells were washed three times with phosphate-buffered saline and incubated with rhodamine-conjugated secondary antibody (Molecular Probes). The coverslips were then fixed on slides and imaged using a Zeiss fluorescence microscope.
Microtubule Organizing Center (MTOC) Reorientation—MTOC reorientation was analyzed as described previously (20) with modification. Cells were allowed to grow to confluence on a glass coverslip coated with gelatin. Serum was added immediately after wounding. MTOC reorientation was assessed 2 h after wounding by immunolabeling using anti-pericentrin antibody. Cells in which the MTOC was within the quadrant facing the wound were scored positive, and for each condition, at least 100 wound-edge cells were examined.
Statistical Analysis—Data are expressed as the means ± S.D. from three experiments and analyzed by one-way analysis of variance followed by Dunnett's multiple comparison test with significance defined as p < 0.05.
RESULTS AND DISCUSSION
Increase of cAMP Plays an Inhibitory Role in the Migration of Fibroblast and Breast Tumor Cells—During our investigation of the role of the second messenger cGMP in cell migration (20), we noticed that IBMX, a broad-spectrum phosphodiesterase inhibitor preventing the breakdown of cAMP and cGMP, inhibited serum-induced migration of MEF cells (Fig. 1A). Because we had shown that cGMP plays a positive role in serum-induced MEF cell migration (20), here we investigated a possible inhibitory role of cAMP in MEF cell migration. We used two complementary approaches to study serum-induced MEF cell migration in the presence or absence of forskolin, a direct activator of adenylyl cyclase leading to the increase of cellular cAMP (16). In the qualitative in vitro wound-healing assay, MEF cells were grown to confluence. A wound was made in the middle of the culture plate with a pipette tip. After ∼10 h in the presence of serum, whereas control MEF cells migrated and covered the “wound,” the addition of 50 μm forskolin significantly inhibited serum-induced MEF cell migration (Fig. 1B). These results were confirmed with the quantitative Boyden chamber assays with 4 h of forskolin treatment (Fig. 1C). Treatment of MEF cells with forskolin resulted in a dosage-dependent inhibition of cell migration with an IC50 of ∼30 μm (Fig. 1D). Furthermore, treatment of MEF cells with isoproterenol, which activates the endogenous Gs-coupled β-adrenergic receptor and results in the elevation of cellular cAMP, also inhibited PDGF-induced cell migration (Fig. 1E). This inhibitory effect was abolished in Gαs-/- cells. Forskolin was still able to inhibit PDGF-induced MEF cell migration in the absence of Gαs (Fig. 1E). These data demonstrate that an increase of cellular cAMP could inhibit serum-induced MEF cell migration.
FIGURE 1.
Increase of cAMP plays an inhibitory role in the migration of MEF cells. A, IBMX (0.5 mm) inhibited serum-induced migration of MEF cells in the wound-healing assay. B, the addition of 50 μm forskolin inhibited serum- or PDGF-induced MEF cell migration (wound-healing assay). C, Boyden chamber assays show that forskolin treatment decreased MEF cell migration induced by serum or PDGF. D, dosage-dependent inhibition of serum-induced MEF cell migration by forskolin is shown. E, isoproterenol (10 μm) activation of endogenous β-adrenergic receptors inhibited PDGF-induced MEF cell migration. This inhibitory effect was abolished in Gαs-/- cells. Forskolin was still able to inhibit PDGF-induced MEF cell migration in the absence of Gαs. Data are representative of three experiments. Error bars show the means ± S.D. of three experiments. *, p < 0.05.
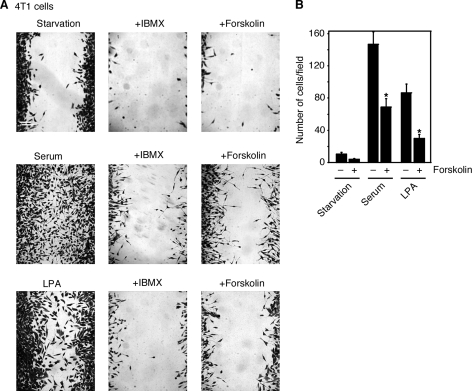
To investigate whether this inhibitory role of cAMP in cell migration is unique to MEF cells, we studied the effect of forskolin on the migration of highly invasive mouse 4T1 breast tumor cells. As shown by both the wound-healing assay (Fig. 2A) and the Boyden chamber assay (Fig. 2B), the addition of 50 μm forskolin inhibited serum-induced 4T1 cell migration. Similarly, treatment with IBMX decreased serum-induced 4T1 cell migration (Fig. 2A). These results show that cAMP has an inhibitory role in the migration of breast tumor cells in addition to MEF cells.
FIGURE 2.
IBMX and forskolin inhibit the migration of breast tumor cells. A, wound-healing assay shows that IBMX (0.5 mm) and forskolin (50 μm) inhibited serum- or LPA-induced 4T1 cell migration. B, Boyden chamber assay shows that forskolin treatment decreased serum- or LPA-induced 4T1 cell migration. Data are representative of three experiments. Error bars show the means ± S.D. of three experiments. *, p < 0.05.
Because serum contains various growth factors, we next studied whether cAMP inhibits cell migration induced by several growth factors known to have chemotactic function, including PDGF and LPA. PDGF efficiently induced the migration of MEF cells, and forskolin treatment inhibited PDGF-induced MEF cell migration (Fig. 1, B and C). Similarly, LPA increased the migration of the 4T1 breast tumor cells (Fig. 2, A and B). Forskolin treatment decreased LPA-induced 4T1 cell migration (Fig. 2, A and B). Although PDGF works on its receptor tyrosine kinase, LPA acts through its G protein-coupled receptor. Collectively, our data suggest that increases of cAMP play an inhibitory role in controlling MEF and 4T1 cell migration induced by various factors.
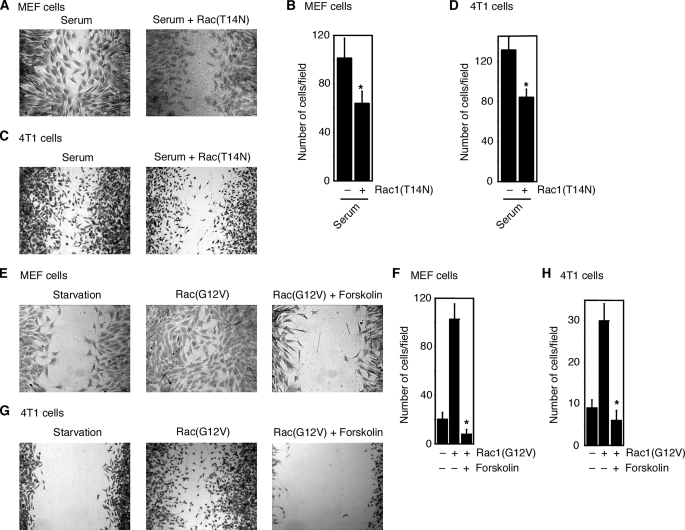
cAMP Acts Downstream of the Small GTPase Rac—To explore the molecular mechanism by which cAMP inhibits serum-induced cell migration, we first investigated at which stage cAMP acts. The Rho family small GTPase Rac plays an essential role in serum-induced cell migration (14, 15, 21). Expression (through retroviral infection) of dominant-negative Rac (Rac1(T14N)) in MEF cells or in 4T1 breast tumor cells decreased serum-induced migration of MEF cells or 4T1 cells, respectively (Fig. 3, A–D). These results indicate that Rac is required for serum-induced MEF and 4T1 cell migration. Furthermore, as shown in Fig. 3, E–H, Rac is also sufficient to induce MEF and 4T1 cell migration, as expression of constitutively active Rac (Rac1(G12V)) in MEF cells or 4T1 cells induced the migration of these cells.
FIGURE 3.
cAMP acts downstream of small GTPase Rac. A and B, expression of dominant-negative Rac (Rac1(T14N)) in MEF cells decreased serum-induced migration of MEF cells as assayed by wound-healing assay (A) or Boyden chamber assay (B). C and D, dominant-negative Rac inhibited serum-induced 4T1 cell migration as assayed by wound-healing assay (C) or Boyden chamber assay (D). E and F, forskolin (50 μm) inhibited MEF cell migration induced by constitutively active Rac (Rac1(G12V)) as assayed by wound-healing assay (E) or Boyden chamber assay (F). G and H, forskolin inhibited 4T1 cell migration induced by constitutively active Rac as assayed by wound-healing assay (G) or Boyden chamber assay (H). Data are representative of three experiments. Error bars show the means ± S.D. of three experiments. *, p < 0.05.
To study whether cAMP acts upstream or downstream of Rac, we examined the effect of forskolin on the migration induced by constitutively active Rac. MEF cells were infected with retroviruses carrying constitutively active Rac1(G12V) or control retroviral vector. Cells were then treated with forskolin. As shown in Fig. 3 (E–H), forskolin decreased the migration of MEF cells or 4T1 cells induced by constitutively active Rac. These data are consistent with a model that cAMP plays its inhibitory role by acting downstream of (or on) Rac in the pathway of serum-induced cell migration.
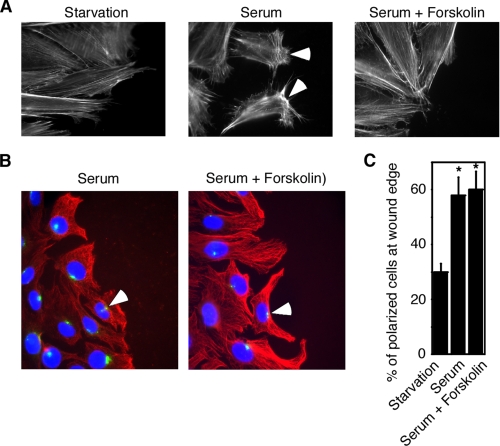
cAMP Inhibits Lamellipodium Formation—To further investigate the molecular mechanism of the cAMP action, we studied the effect of cAMP on cellular events during cell migration. Rac is known to mediate serum-induced lamellipodium formation and focal adhesion formation and turnover during cell migration (1). We first examined the lamellipodium formation. Lamellipodia are membrane extensions at the front of migrating cells (3). This structure could be visualized by the staining of actin filaments at the leading edge. Two h after making the wound in the in vitro wound-healing assay, serum effectively induced the formation of lamellipodia with a distinct polarized actin cytoskeleton with strong membrane protrusions toward the leading edge (Fig. 4A). In contrast, forskolin treatment disrupted the polarized distribution of F-actin (Fig. 4A). Instead, forskolin-treated cells adopted an elongated morphology with a non-polarized F-actin meshwork. These data demonstrate that cAMP interferes with the lamellipodium formation.
FIGURE 4.
cAMP inhibits lamellipodium formation. A, forskolin (50 μm) treatment interfered with the lamellipodium formation. B and C, forskolin had no effect on MTOC reorientation, as shown by tubulin staining (B) or quantification of ∼100 cells (C). Red, tubulin staining; blue,4′,6-diamidino-2-phenylindole staining; green, pericentrin staining. Data are representative of three experiments. Error bars show the means ± S.D. of three experiments. *, p < 0.05.
We also examined the effect of cAMP on focal adhesion turnover and on the microtubule dynamics. We found that forskolin treatment had no effect on microtubule dynamics (Fig. 4, B and C) and no effect on focal adhesion turnover (data not shown). The MTOC reorientation and the formation of microtubule protrusions in the leading edge contribute to directional cell migration (1, 22). As shown in Fig. 4 (B and C), in the presence of serum, the MTOC and the microtubule cytoskeleton reorganized to face the wound. Forskolin treatment did not affect the polarization of the MTOC or the protrusion of microtubules. These data suggest that cAMP inhibits cell migration by disrupting F-actin rather than microtubule dynamics within the leading edge of migrating cells.
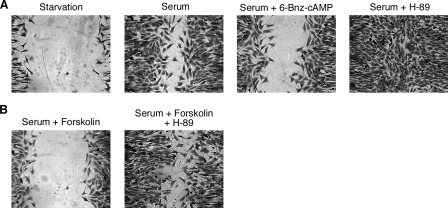
PKA and pMLC Contribute to cAMP Inhibitory Function— To gain a biochemical understanding of the inhibitory function of cAMP in MEF cell migration, we tested the participation of several signaling components in this regulatory pathway. The best characterized direct effector of cAMP is PKA. We first tested whether PKA contributes to the cAMP inhibitory function in MEF cell migration. We examined the MEF cell migration in the presence or absence of 6-benzoyl-cAMP, a selective cAMP analogue that directly activates PKA (5). As shown in Fig. 5A, the addition of 6-benzoyl-cAMP inhibited the serum-induced migration of MEF cells, implying that activation of PKA is capable of inhibiting MEF cell migration. The involvement of PKA was further investigated using H-89, a specific PKA inhibitor. In MEF cells, the addition of H-89 stimulated serum-induced cell migration, consistent with the inhibitory role of PKA in controlling cell migration (Fig. 5A). Furthermore, H-89 reduced the inhibitory effect of forskolin on serum-induced MEF cell migration (Fig. 5B). Hence, the data from both the activation of PKA and the inhibition of PKA are consistent with a negative role of PKA in MEF cell migration.
FIGURE 5.
PKA contributes to cAMP inhibitory function. A, 6-benzoyl (Bnz)-cAMP inhibited serum-induced MEF cell migration, whereas H-89 facilitated serum-induced MEF cell migration. B, H-89 reduced the inhibitory effect of forskolin on serum-induced MEF cell migration. Data are representative of three experiments.
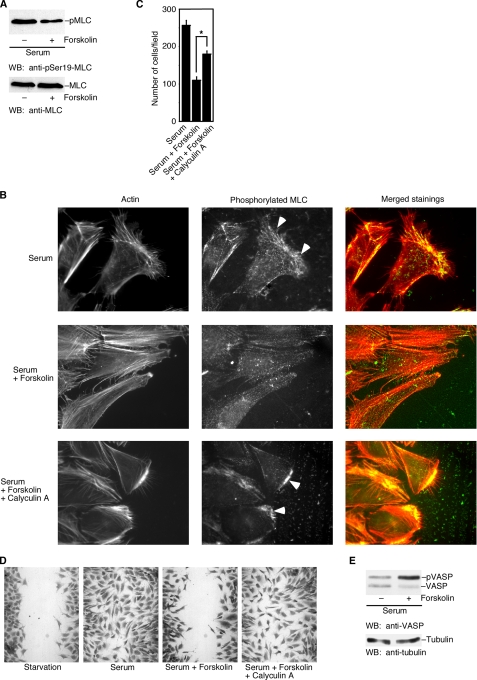
Of the reported physiological substrates for PKA, one is MLC kinase (23). PKA can phosphorylate and decrease the activity of MLC kinase (23–25). MLC kinase phosphorylates the regulatory light chain of myosin II and activates myosin II (26). The essential role of myosin II in actin cytoskeletal rearrangement and cell migration has long been appreciated (27). Myosin II regulates the retrograde flow of F-actin in the lamella and plays an essential role in F-actin-driven cell migration (28). Phosphorylated MLC (at Ser-19) appears to be strong in both the anterior and posterior regions of motile cells (15, 29, 30). Furthermore, phosphorylation of the myosin light chain is required for the anchorage of lamellipodia (3). To investigate the effect of cAMP/PKA activation on MLC phosphorylation in cell migration, we first examined whether forskolin could reduce the phosphorylation of MLC in MEF cells. As shown in Fig. 6A, forskolin treatment significantly reduced the level of phosphorylated MLC, whereas the amount of total MLC was not affected. Next, we examined the distribution of phosphorylated MLC in cells at the leading edge of a wound with an antibody recognizing phosphorylated MLC. As shown in Fig. 6B, in the absence of forskolin, strong staining of phosphorylated MLC was observed in the lamella of migrating cells (indicated by arrowheads). In contrast, activation of cAMP/PKA by forskolin disrupted the anterior staining of phosphorylated MLC. To further confirm that the cAMP effect is on the phosphorylation of MLC, we tested whether raising the phosphorylation of MLC (by inhibiting the MLC phosphatase) could attenuate the cAMP inhibitory effect. As shown in Fig. 6B, treatment of cells with low concentrations of calyculin A (0.5 nm), a specific inhibitor for MLC phosphatase (31), restored the accumulation of phosphorylated MLC at the leading edge of the cells (indicated by arrowheads). Moreover, calyculin treatment also attenuated the inhibitory effect of forskolin on serum-induced MEF cell migration as measured by the Boyden chamber assay (Fig. 6C) and the wound-healing assay (Fig. 6D). Taken together, our data indicate that forskolin decreases the phosphorylation of MLC at the leading edge and provide a possible biochemical mechanism for the inhibitory role of cAMP in cell migration.
FIGURE 6.
Forskolin decreases the phosphorylation of MLC and increases the phosphorylation of VASP. A, a Western blot (WB) shows the decreased phosphorylation of MLC after forskolin treatment. B, distribution of phosphorylated MLC in cells at the leading edge of a wound was shown by staining with an antibody recognizing phosphorylated MLC. Strong staining of phosphorylated MLC was observed in the lamella of migrating cells (indicated by arrowheads). Forskolin (50 μm) reduced the staining of phosphorylated MLC. Calyculin A (an inhibitor for MLC phosphatase) restored the accumulation of phosphorylated MLC at the leading edge of the cells (indicated by arrowheads). C and D, calyculin treatment attenuated the inhibitory effect of forskolin on serum-induced MEF cell migration as measured by Boyden chamber assay (C) and by wound-healing assay (D). E, a Western blot shows the increased phosphorylation of VASP by forskolin. Data are representative of three experiments. Error bars show the means ± S.D. of three experiments.
Another PKA substrate that is involved in lamellipodial dynamics is VASP (32, 33). VASP proteins could be targeted to the leading edge by activated Rac in migrating fibroblasts (34). VASP phosphorylation by PKA diminishes VASP binding to F-actin and suppresses the actin-nucleating activity of VASP, leading to decreased actin polymerization (33). To investigate whether the cAMP inhibitory effect on cell migration is accompanied by increased phosphorylation of VASP, we examined the phosphorylation state of VASP with or without forskolin treatment. Phosphorylation of VASP causes an electrophoretic mobility shift in SDS-PAGE (35). As shown in Fig. 6E, forskolin treatment markedly increased the phosphorylation of VASP. Together with the effect of cAMP/PKA on the phosphorylation of MLC, cAMP/PKA could have multiple targets in their effects on cell migration and lamellipodium formation.
Conclusion—In summary, cAMP inhibits the migration of MEFs and mouse 4T1 breast tumor cells. cAMP acts downstream of Rac. Furthermore, cAMP interferes with the formation of lamellipodia. cAMP decreases the phosphorylation of MLC and increases the phosphorylation of VASP. Myosin-based contractility is important for cell migration at the leading edge as well as at the trailing edge (15, 29, 30). In polarized migrating MEFs, there are two areas of phosphorylated MLC staining: leading edge staining and trailing tail staining (15). In growth factor-induced migration of MEFs, we have shown previously that Ca2+ influx, through calmodulin and MLC kinase, increases the phosphorylation of MLC at the training tail and contributes to the trailing tail contraction; Ca2+ influx has no effect on the phosphorylation of MLC at the leading edge (15). Here cAMP appears to decrease the phosphorylation of MLC at the front as well as the tail of migrating cells.
Although we examined the effect of cAMP/PKA on the phosphorylation of MLC and VASP, we did not intend to state that phosphorylations of MLC and VASP are the only or major mechanisms by which cAMP inhibits lamellipodium formation. cAMP/PKA can regulate the activity of other proteins involved in actin cytoskeletons. For example, another means by which cAMP/PKA could modulate the actin cytoskeletal reorganization is through the AKAPs (A kinase anchoring proteins) such as WAVE-1 and AKAP-Lbc because these proteins are known to regulate actin polymerization or Rho activity (36, 37). PKA phosphorylation of integrin (such as α4) within the protrusion is critical for integrin-dependent cell migration (38). With the reported positive and negative effects of cAMP/PKA on the migration of different cell types, it is likely that the spatial-temporal distribution and activation of cAMP/PKA are crucial (39).
Acknowledgments
We thank members of our laboratory for discussions and critically reading the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grant AG23202. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: MEF, mouse embryonic fibroblast; VASP, vasodilator-stimulated phosphoprotein; PDGF, platelet-derived growth factor; LPA, lysophosphatidic acid; MLC, myosin light chain; MTOC, microtubule organizing center; PKA, cAMP-dependent protein kinase; IBMX, isobutylmethylxanthine.
References
- 1.Ridley, A. J., Schwartz, M. A., Burridge, K., Firtel, R. A., Ginsberg, M. H., Borisy, G., Parsons, J. T., and Horwitz, A. R. (2003) Science 302 1704-1709 [DOI] [PubMed] [Google Scholar]
- 2.Lauffenburger, D. A., and Horwitz, A. F. (1996) Cell 84 359-369 [DOI] [PubMed] [Google Scholar]
- 3.Small, J. V., Stradal, T., Vignal, E., and Rottner, K. (2002) Trends Cell Biol. 12 112-120 [DOI] [PubMed] [Google Scholar]
- 4.Hall, A. (1998) Science 279 509-514 [DOI] [PubMed] [Google Scholar]
- 5.Beavo, J. A., and Brunton, L. L. (2002) Nat. Rev. Mol. Cell Biol. 3 710-718 [DOI] [PubMed] [Google Scholar]
- 6.Howe, A. K. (2004) Biochim. Biophys. Acta 1692 159-174 [DOI] [PubMed] [Google Scholar]
- 7.Bakre, M. M., Zhu, Y., Yin, H., Burton, D. W., Terkeltaub, R., Deftos, L. J., and Varner, J. A. (2002) Nat. Med. 8 995-1003 [DOI] [PubMed] [Google Scholar]
- 8.Hirakawa, M., Karashima, Y., Watanabe, M., Kimura, C., Ito, Y., and Oike, M. (2007) J. Pharmacol. Exp. Ther. 321 1102-1108 [DOI] [PubMed] [Google Scholar]
- 9.Lorenowicz, M. J., Fernandez-Borja, M., and Hordijk, P. L. (2007) Arterioscler. Thromb. Vasc. Biol. 27 1014-1022 [DOI] [PubMed] [Google Scholar]
- 10.Sandulache, V. C., Parekh, A., Li-Korotky, H., Dohar, J. E., and Hebda, P. A. (2007) Wound Repair Regen. 15 122-133 [DOI] [PubMed] [Google Scholar]
- 11.Nishiyama, M., Hoshino, A., Tsai, L., Henley, J. R., Goshima, Y., Tessier-Lavigne, M., Poo, M. M., and Hong, K. (2003) Nature 424 990-995 [DOI] [PubMed] [Google Scholar]
- 12.Song, H., Ming, G., He, Z., Lehmann, M., McKerracher, L., Tessier-Lavigne, M., and Poo, M. (1998) Science 281 1515-1518 [DOI] [PubMed] [Google Scholar]
- 13.Shan, D., Chen, L., Njardarson, J. T., Gaul, C., Ma, X., Danishefsky, S. J., and Huang, X.-Y. (2005) Proc. Natl. Acad. Sci. U. S. A. 102 3772-3776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shan, D., Chen, L., Wang, D., Tan, Y. C., Gu, J. L., and Huang, X.-Y. (2006) Dev. Cell 10 707-718 [DOI] [PubMed] [Google Scholar]
- 15.Yang, S., and Huang, X.-Y. (2005) J. Biol. Chem. 280 27130-27137 [DOI] [PubMed] [Google Scholar]
- 16.Sun, Y., Huang, J., Xiang, Y., Bastepe, M., Juppner, H., Kobilka, B. K., Zhang, J. J., and Huang, X.-Y. (2007) EMBO J. 26 53-64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lowry, W. E., Huang, J., Ma, Y. C., Ali, S., Wang, D., Williams, D. M., Okada, M., Cole, P. A., and Huang, X.-Y. (2002) Dev. Cell 2 733-744 [DOI] [PubMed] [Google Scholar]
- 18.McGarrigle, D., Shan, D., Yang, S., and Huang, X.-Y. (2006) J. Biol. Chem. 281 10583-10588 [DOI] [PubMed] [Google Scholar]
- 19.Wang, D., Tan, Y. C., Kreitzer, G. E., Nakai, Y., Shan, D., Zheng, Y., and Huang, X.-Y. (2006) J. Biol. Chem. 281 32660-32667 [DOI] [PubMed] [Google Scholar]
- 20.Guo, D., Tan, Y. C., Wang, D., Madhusoodanan, K. S., Zheng, Y., Maack, T., Zhang, J. J., and Huang, X.-Y. (2007) Cell 128 341-355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ridley, A. J., Paterson, H. F., Johnston, C. L., Diekmann, D., and Hall, A. (1992) Cell 70 401-410 [DOI] [PubMed] [Google Scholar]
- 22.Etienne-Manneville, S., and Hall, A. (2001) Cell 106 489-498 [DOI] [PubMed] [Google Scholar]
- 23.Nishikawa, M., de Lanerolle, P., Lincoln, T. M., and Adelstein, R. S. (1984) J. Biol. Chem. 259 8429-8436 [PubMed] [Google Scholar]
- 24.Garcia, J. G., Davis, H. W., and Patterson, C. E. (1995) J. Cell. Physiol. 163 510-522 [DOI] [PubMed] [Google Scholar]
- 25.Lamb, N. J., Fernandez, A., Conti, M. A., Adelstein, R., Glass, D. B., Welch, W. J., and Feramisco, J. R. (1988) J. Cell Biol. 106 1955-1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kamm, K. E., and Stull, J. T. (2001) J. Biol. Chem. 276 4527-4530 [DOI] [PubMed] [Google Scholar]
- 27.Spudich, J. A. (1989) Cell Regul. 1 1-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Somlyo, A. P., and Somlyo, A. V. (2003) Physiol. Rev. 83 1325-1358 [DOI] [PubMed] [Google Scholar]
- 29.Chew, T. L., Wolf, W. A., Gallagher, P. J., Matsumura, F., and Chisholm, R. L. (2002) J. Cell Biol. 156 543-553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Worthylake, R. A., Lemoine, S., Watson, J. M., and Burridge, K. (2001) J. Cell Biol. 154 147-160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gupton, S. L., and Waterman-Storer, C. M. (2006) Cell 125 1361-1374 [DOI] [PubMed] [Google Scholar]
- 32.Gomez, T. M., and Robles, E. (2004) Neuron 42 1-3 [DOI] [PubMed] [Google Scholar]
- 33.Butt, E., Abel, K., Krieger, M., Palm, D., Hoppe, V., Hoppe, J., and Walter, U. (1994) J. Biol. Chem. 269 14509-14517 [PubMed] [Google Scholar]
- 34.Reinhard, M., Jarchau, T., and Walter, U. (2001) Trends Biochem. Sci. 26 243-249 [DOI] [PubMed] [Google Scholar]
- 35.Harbeck, B., Huttelmaier, S., Schluter, K., Jockusch, B. M., and Illenberger, S. (2000) J. Biol. Chem. 275 30817-30825 [DOI] [PubMed] [Google Scholar]
- 36.Westphal, R. S., Soderling, S. H., Alto, N. M., Langeberg, L. K., and Scott, J. D. (2000) EMBO J. 19 4589-4600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Diviani, D., Soderling, J., and Scott, J. D. (2001) J. Biol. Chem. 276 44247-44257 [DOI] [PubMed] [Google Scholar]
- 38.Goldfinger, L. E., Han, J., Kiosses, W. B., Howe, A. K., and Ginsberg, M. H. (2003) J. Cell Biol. 162 731-741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Howe, A. K., Baldor, L. C., and Hogan, B. P. (2005) Proc. Natl. Acad. Sci. U. S. A. 102 14320-14325 [DOI] [PMC free article] [PubMed] [Google Scholar]