Abstract
Tumor cells strategically down-regulate Fas receptor expression to evade immune attack and up-regulate expression of Fas ligand to promote apoptosis of infiltrating T lymphocytes. Many pathways leading to apoptotic cell death require calcium release from inositol 1,4,5-trisphosphate receptors (IP3Rs). Here, we show that Fas-dependent killing of Jurkat T lymphoma cells by SW620 colon cancer cells requires calcium release from IP3R. General suppression of IP3R signaling significantly reduced SW620-mediated Jurkat cell apoptosis. Significantly, a specific inhibitor of apoptotic calcium release from IP3R strongly blocked lymphocyte apoptosis. Thus, selective pharmacological targeting of apoptotic calcium release from IP3R may enhance tumor cell immunogenicity.
Fas receptor is a member of the tumor necrosis factor-α superfamily of death receptors. Fas receptor binds to Fas ligand presented on another cell, initiating a series of events ultimately leading to the activation of proapoptotic proteases known as caspases. Fas ligand expression is restricted primarily to activated T cells and natural killer cells, where it is essential for activation-induced cell death and immune-mediated cytotoxicity. In addition, Fas ligand is constitutively expressed in select immunoprivileged tissues such as the eye and testis. Cancer cells can harness the Fas pathway to evade immune attack (1–3). Loss of Fas receptor function is frequently observed in human cancers, reducing the ability of infiltrating T cells and natural killer cells to kill cancerous cells (4–8). Modulation of Fas signaling for selective survival advantage has been documented in multiple tumor types, including colon, hepatocellular, ovarian and esophageal carcinomas, melanoma, and astrocytoma (1). Loss of Fas signaling by cancer cells is accomplished at the molecular level by transcriptional down-regulation of Fas receptor or the adaptor protein Fas-associated protein with death domain and up-regulation of negative regulatory components of the Fas pathway such as FLICE-like inhibitory protein, Bcl-XL, and Bcl-2 (2). Moreover, many cancer cells also up-regulate Fas ligand expression, which consequently results in increased T cell apoptosis and decreased infiltration (9–11). Thus, the identification of the molecular pathways modulating Fas signaling is an important goal in rational drug development that targets this adaptive strategy of cancer cells.
We have recently shown that intracellular calcium homeostasis is critical to the progression of apoptotic cell death. Inositol 1,4,5-trisphosphate receptor (IP3R)2 calcium channels regulate intracellular calcium concentration during apoptosis induced by death receptor ligation (12) and cellular damage (13). Apoptosis-specific signaling via IP3R is mediated by cytochrome c, which binds to the channel after being released from mitochondria, resulting in augmented channel function (13). This ultimately leads to cytosolic and mitochondrial calcium overload and cell death. Fas receptor signaling utilizes cytochrome c release from mitochondria to amplify the apoptotic signal (14). Therefore, we hypothesized that inhibiting IP3R-dependent apoptotic signaling in lymphocytes would protect them from apoptotic cell death induced by tumor cells expressing high levels of Fas ligand.
In this report, we show that inhibiting IP3R function in Jurkat T-lymphoma cells is cytoprotective against apoptosis induced by co-culture with Fas ligand-expressing SW620 colon cancer cells. The IP3R antagonist xestospongin C (XestC) and calcium buffering with BAPTA dose-dependently inhibited Jurkat cell apoptosis. Inhibiting exclusively IP3R activation due to proapoptotic signaling in lymphocytes by blocking cytochrome c binding to IP3R inhibited lymphocyte death with nanomolar affinity. Thus, specifically targeting proapopotic signaling through the IP3R in lymphocytes appears to be a promising therapeutic approach for enhancing tumor cell immunogenicity.
EXPERIMENTAL PROCEDURES
Materials—Peptide synthesis of the IP3R fragment encoding the cytochrome c binding domain of the type I IP3R isoform and conjugation to BODIPY-577/618 has been described elsewhere (15). This cell-permeant peptide is termed B-IP3RCYT. The aminomethoxy ester form of BAPTA (1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid; BAPTA-AM), propidium iodide, and fura-2-AM were purchased from Molecular Probes (Eugene, OR). XestC and all other chemicals were purchased from Sigma-Aldrich.
Cell Culture—Jurkat T-lymphoma (clone E6-1) and SW620 colon adenocarcinoma cells were purchased and cultured according to the guidelines of American Type Culture Collection (ATCC, Manassas, VA). SW620 cells grow as adherent monolayers, whereas Jurkat cells grow in suspension. Jurkat cells were co-cultured with SW620 cells by exchanging the culture medium of SW620 cells (80% confluent in a 10-cm dish) with 10 ml of Jurkat medium containing 1 × 106 Jurkat cells. Co-cultures were incubated for 24 h, after which cell death or caspase activity was determined in Jurkat cells. Preloading BAPTA or B-IP3RCYT into Jurkat cells was performed for 30 min at room temperature. Free BAPTA-AM or B-IP3RCYT were removed by medium exchange prior to addition to SW620 cells. XestC was similarly preincubated with Jurkat cells prior to co-culturing and remained in the culture medium for the duration of the co-culture experiment.
RNAi—Knockdown of the human IP3R-1 in Jurkat cells and rescue with the rat IP3R-1 isoform was done exactly as described elsewhere (12). The rat itpr1 gene has several base substitutions within the targeted region of the human ITPR1 gene allowing expression in the presence of this RNAi. Control experiments utilized an RNAi with several deletions and insertions (12). Co-transfection with yellow fluorescent protein (YFP) was utilized to identify cells containing the RNAi for calcium imaging and cell death assays (described below).
Calcium Imaging—SW620 cells grown on coverslips were loaded with fura-2-AM for 30 min at room temperature. Jurkat cells were loaded in suspension as described (12). Coverslips with fura-2-loaded SW620 cells were placed in an imaging chamber (Attofluor® cell chamber, Molecular Probes) and imaged at ×40 magnification as described (12). Fura-2-loaded Jurkat cells (∼1 × 104) were added by solution exchange. In ∼20% of trials (6 of 28), a Jurkat cell came into direct contact with one or more SW620 cells in the field of view. To measure responses in Jurkat cells co-transfected with RNAi and YFP, images were acquired with a ×20 objective, and ∼5 × 104 Jurkat cells were added to the imaging chamber containing SW620 cells. This methodology permitted imaging of the low abundance transfected Jurkat cells contacting SW620 cells with greater frequency at the expense of resolution. RNAi data are expressed as a histogram of the percentage of responding YFP-positive cells from a minimum of 15 cells, which were confirmed to directly contact an SW620 cell. A response was defined as an increase in 340/380 ratio greater than 0.5.
Caspase-3 Activity and Cell Death Determination—Caspase activity and cell death were determined specifically in Jurkat cells by removing the medium containing the Jurkat cells into a fresh tube. An additional rinse with culture medium was used to fully recover the Jurkat cells. Adherent SW620 cells remained attached to the culture plate after this rinse as determined by light microscopy. Jurkat cells were pelleted by centrifugation at 1000 × g, and cell death was determined by propidium iodide staining as described (12). Fluorometric determination of DEVDase (caspase-3 like) activity was performed on lysates prepared from Jurkat cells as described (12, 15).
Statistical Analysis—Statistical significance was determined by using unpaired two-tailed t tests on data points from at least three separate experiments. Data comparisons were considered significant if p was <0.05.
RESULTS AND DISCUSSION
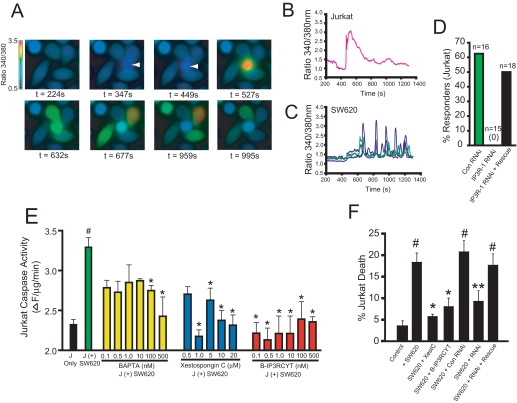
SW620 colon carcinoma cells express high levels of Fas ligand and have been shown to be potent inducers of Jurkat apoptosis in a Fas-dependent manner (16). Thus, we hypothesized that co-culture of Jurkat cells with SW620 cells would induce proapoptotic calcium release in Jurkat cells and that this calcium release would be required for lymphocyte apoptosis. To monitor intracellular calcium in co-culture experiments, SW620 cells were grown on coverslips and loaded with fura-2. Jurkat cells were also separately loaded with fura-2 to simultaneously image intracellular calcium in both cell types. After establishing baseline calcium levels in SW620 cells, Jurkat cells were added to the imaging chamber by solution exchange. In 6 of 28 trials, a Jurkat cell bound directly to one or more SW620 cells. As shown in Fig. 1, A and B, upon binding SW620 cells, a large transient rise in intracellular calcium is induced in Jurkat cells. Interestingly, after a short delay, calcium oscillations are induced in SW620 cells (Fig. 1, A and C). The functional significance of these oscillations in SW620 cells are unclear, but this finding is of significance since coupling of Fas ligand to calcium release in Fas ligand-presenting cells has not been demonstrated. Fas ligand microvesicles can induce calcium release in Jurkat cells via IP3R calcium channels (12, 15). To determine whether the calcium response in Jurkat cells was due to IP3R-mediated calcium release, we knocked down IP3R levels with a previously characterized double-stranded RNAi oligonucleotide targeting the human IP3R-1 isoform (12). Knockdown of IP3R-1 completely abrogated Jurkat calcium release in response to SW620 cell binding (0/15 cells; Fig. 1D). Importantly, calcium release could be rescued by co-transfecting the rat itpr1 cDNA (9/18 cells). As expected, Jurkat cells expressing control RNAi retained the ability to release calcium after contact with SW620 cells (10/16 cells). These results indicate that binding to SW620 cells induces IP3R-dependent calcium release in Jurkat cells.
FIGURE 1.
Calcium release from IP3R is required for SW620-mediated killing of Jurkat cells. A, Fura-2 calcium ratio imaging of SW620/Jurkat co-cultures. The first panel shows only adherent SW620 cells. At a time of 347 s, a single Jurkat cell (second panel; indicated by an arrow), comes into contact with four SW620 cells, which leads to a rise in intracellular calcium. B, calcium response in the Jurkat cell shown in A. C, calcium responses of the four SW620 cells shown in A. Qualitatively similar results were observed in all trials in which a Jurkat cell contacted at least one SW620 cell (data not shown). D, histogram of the percentage of Jurkat cells, which released calcium in response to binding SW620 cells after transfection with control RNAi (Con RNAi), IP3R-1 RNAi, or co-transfection with IP3R-1 RNAi and the cDNA encoding the rat IP3R-1. The total number of transfected cells that contacted an SW620 cell is indicated. No cells expressing IP3R-1 RNAi responded (0/15). E, caspase-3-like activity (DEVDase activity) in Jurkat cells co-cultured with SW620 cells or cultured alone (black bar). Green, no pretreatment before co-culture for 24 h with SW620 cells. Yellow, Jurkat cells were preincubated with the indicated concentrations of BAPTA-AM before co-culture. Blue, Jurkat cells were preincubated with the indicated concentrations of xestospongin C. Red, Jurkat cells were preincubated with the indicated concentrations of B-IP3RCYT. See “Experimental Procedures” for details. J = Jurkat. F, cell death in Jurkat cells prior to (Control) or 24 h after co-culture with SW620 cells (+SW620). The effects of 1 μm XestC and 10 nm B-IP3RCYT were tested by preincubation prior to co-culture. The indicated RNAis were transfected 24 h before co-culture as described elsewhere (12). All data represent the mean ± S.E. # indicates p < 0.05 relative to control (no incubation with SW620 cells). * indicates p < 0.05 relative to co-culture with SW620 cells. ** indicates p < 0.05 relative to co-culture with SW620 + control RNAi.
To examine the effects of modulators of IP3R-dependent calcium release on SW620-dependent Jurkat apoptosis, we monitored caspase-3 activation. As expected, co-culture of Jurkat cells with SW620 cells induced significant activation of caspase-3 in Jurkat cells (Fig. 1E;#, p < 0.05 relative to Jurkat cells, which were not co-cultured with SW620 cells). To determine whether intracellular calcium contributed to this increase in caspase-3 activity, we loaded Jurkat cells with various concentrations of the calcium chelator BAPTA-AM to buffer cytosolic calcium levels. Significant attenuation of caspase-3 activation was observed by incubating the cells with 100 and 500 nm BAPTA-AM (*, p < 0.05 relative to Jurkat cells co-cultured with SW620 cells without pretreatment).
To determine whether the IP3R is required for lymphocyte apoptosis, we incubated cells with various doses of the cell-permeant IP3R inhibitor XestC, a selective blocker of IP3R function when used at low micromolar concentrations (17, 18). XestC inhibited caspase-3 activation in Jurkat cells at concentrations ranging from 1 to 20 micromolar, indicating that IP3R activity was required for caspase-3 activation (Fig. 1E). Importantly, XestC potently blocked Jurkat cell death induced by coculture with SW620 cells (Fig. 1F).
During apoptosis, calcium release from IP3R is augmented by direct binding of cytochrome c to the channel (12, 13, 15). Cytochrome c binds to the cytosolic C-terminal “tail” of the IP3R, decreasing the ability of calcium to inhibit the channel and prolonging calcium release (15). Peptides derived from the cytochrome c binding domain of IP3R, when introduced into cells, inhibit calcium-dependent apoptosis without altering agonist-induced calcium release (12, 13, 15). Coupling of one of these peptides to the hydrophobic dye BODIPY 577/618 renders the peptide cell permeant, retaining antiapoptotic activity (12, 15). Thus, we tested whether this peptide, termed B-IP3RCYT, could block SW620-dependent cell death of Jurkat cells. As shown in Fig. 1E, B-IP3RCYT inhibited caspase-3 activation in Jurkat cells at nanomolar concentrations. This result is consistent with the in vitro observation that IP3RCYT has a higher affinity for cytochrome c than the cytosolic tail of IP3R and can inhibit Fas-dependent cell death induced by Fas ligand microvesicles or the cytotoxic drug staurosporine at nanomolar concentrations (15). Importantly, the B-IP3RCYT peptide also inhibited Jurkat cell death induced by SW620 co-culture (Fig. 1F). Thus, specifically blocking apoptotic calcium release via the IP3R inhibits lymphocyte apoptosis induced by Fas ligand-expressing cancer cells. Finally, RNAi-mediated knockdown of IP3R-1 also inhibited Jurkat cell death, and these effects were reversed by rescue with the rat itpr1 cDNA (Fig. 1F).
The ability of tumor cells to attain immunoprivileged status in vivo by up-regulating Fas ligand has remained relatively controversial (9, 11, 19). In this report, we demonstrate in vitro that Fas-dependent killing of a lymphocyte cell line by cancer cells requires calcium release from IP3R. Identification of the IP3Ras a mediator of lymphocyte apoptosis induced by a tumor cells highlights a novel Fas-dependent pathway that warrants further investigation in vivo (Fig. 2). Significantly, we found that lymphocyte apoptosis could be inhibited by specifically blocking apoptotic calcium release from IP3R. Lymphocyte apoptosis was inhibited by B-IP3RCYT when placed in the culture medium at low nanomolar concentrations. Since B-IP3RCYT exhibits no significant cellular toxicity and does not modify agonist-induced calcium release (12, 15), targeting IP3R/cytochrome c interactions appears to be an attractive target for enhancing the immunologic response of tumors that have acquired adaptive changes in death receptor signaling to promote survival.
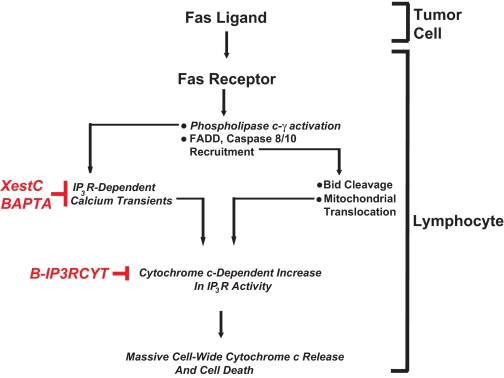
FIGURE 2.
Calcium and tumor-induced lymphocyte apoptosis. Fas ligand expressed on tumor cells activates Fas receptor on infiltrating lymphocytes. This causes activation of IP3R by coupling of Fas receptor to phospholipase C-γ1 and subsequent production of IP3 (12). The canonical components of the death-induced signaling complex are also recruited to Fas receptors such as Fas-associated death domain (FADD) and caspase 8/10. Caspase 8/10 activation induces Bid activation and translocation to mitochondria, sensitizing them to calcium-induced cytochrome c release. Cytochrome c subsequently binds to IP3R, causing further calcium release and mitochondrial calcium overload. Blocking IP3-dependent elevations in cytosolic calcium with Xestospongin C or BAPTA inhibits lymphocyte apoptosis. Specifically blocking cytochrome c binding to IP3R also inhibits lymphocyte apoptosis without modifying agonist-induced calcium release, suggesting a viable target for therapeutic intervention.
Acknowledgments
We thank Xinmin Wang for technical assistance. D. B. thanks David Boehning for useful discussions.
This work was supported, in whole or in part, by National Institutes of Health Grant GM081685 (to D. B.). This work was also supported by the University of Texas Medical Branch. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: IP3R, inositol 1,4,5-trisphosphate receptor; BAPTA, 1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid; BAPTA-AM, aminomethoxy ester form of BAPTA; XestC, Xestospongin C; YFP, yellow fluorescent protein; RNAi, RNA interference.
References
- 1.Reichmann, E. (2002) Semin. Cancer Biol. 12 309–315 [DOI] [PubMed] [Google Scholar]
- 2.Houston, A., and O'Connell, J. (2004) Curr. Opin. Pharmacol. 4 321–326 [DOI] [PubMed] [Google Scholar]
- 3.Ryan, A. E., Shanahan, F., O'Connell, J., and Houston, A. M. (2006) Cell Cycle 5 246–249 [DOI] [PubMed] [Google Scholar]
- 4.O'Connell, J., Houston, A., Bennett, M. W., O'Sullivan, G. C., and Shanahan, F. (2001) Nat. Med. 7 271–274 [DOI] [PubMed] [Google Scholar]
- 5.Houston, A., Waldron-Lynch, F. D., Bennett, M. W., Roche, D., O'Sullivan, G. C., Shanahan, F., and O'Connell, J. (2003) Int. J. Cancer 107 209–214 [DOI] [PubMed] [Google Scholar]
- 6.Bebenek, M., Dus, D., and Kozlak, J. (2006) Med. Sci. Monit. 12 CR457–CR461 [PubMed] [Google Scholar]
- 7.Yamana, K., Bilim, V., Hara, N., Kasahara, T., Itoi, T., Maruyama, R., Nishiyama, T., Takahashi, K., and Tomita, Y. (2005) Br. J. Cancer 93 544–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reimer, T., Koczan, D., Muller, H., Friese, K., Thiesen, H. J., and Gerber, B. (2002) Breast Cancer Res. 4 R9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ryan, A. E., Shanahan, F., O'Connell, J., and Houston, A. M. (2005) Cancer Res. 65 9817–9823 [DOI] [PubMed] [Google Scholar]
- 10.Bennett, M. W., O'Connell, J., Houston, A., Kelly, J., O'Sullivan, G. C., Collins, J. K., and Shanahan, F. (2001) J. Clin. Pathol. (Lond.) 54 598–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Houston, A., Bennett, M. W., O'Sullivan, G. C., Shanahan, F., and O'Connell, J. (2003) Br. J. Cancer 89 1345–1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wozniak, A. L., Wang, X., Stieren, E. S., Scarbrough, S. G., Elferink, C. J., and Boehning, D. (2006) J. Cell Biol. 175 709–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boehning, D., Patterson, R. L., Sedaghat, L., Glebova, N. O., Kurosaki, T., and Snyder, S. H. (2003) Nat. Cell Biol. 5 1051–1061 [DOI] [PubMed] [Google Scholar]
- 14.Scaffidi, C., Fulda, S., Srinivasan, A., Friesen, C., Li, F., Tomaselli, K. J., Debatin, K. M., Krammer, P. H., and Peter, M. E. (1998) EMBO J. 17 1675–1687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boehning, D., van Rossum, D. B., Patterson, R. L., and Snyder, S. H. (2005) Proc. Natl. Acad. Sci. U. S. A. 102 1466–1471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O'Connell, J., O'Sullivan, G. C., Collins, J. K., and Shanahan, F. (1996) J. Exp. Med. 184 1075–1082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gafni, J., Munsch, J. A., Lam, T. H., Catlin, M. C., Costa, L. G., Molinski, T. F., and Pessah, I. N. (1997) Neuron 19 723–733 [DOI] [PubMed] [Google Scholar]
- 18.Ta, T. A., Feng, W., Molinski, T. F., and Pessah, I. N. (2006) Mol. Pharmacol. 69 532–538 [DOI] [PubMed] [Google Scholar]
- 19.Igney, F. H., and Krammer, P. H. (2005) Cancer Immunol. Immunother. 54 1127–1136 [DOI] [PMC free article] [PubMed] [Google Scholar]