Abstract
Iron-sulfur clusters ([Fe-S] clusters) are assembled on molecular scaffolds and subsequently used for maturation of proteins that require [Fe-S] clusters for their functions. Previous studies have shown that Azotobacter vinelandii produces at least two [Fe-S] cluster assembly scaffolds: NifU, required for the maturation of nitrogenase, and IscU, required for the general maturation of other [Fe-S] proteins. A. vinelandii also encodes a protein designated NfuA, which shares amino acid sequence similarity with the C-terminal region of NifU. The activity of aconitase, a [4Fe-4S] cluster-containing enzyme, is markedly diminished in a strain containing an inactivated nfuA gene. This inactivation also results in a null-growth phenotype when the strain is cultivated under elevated oxygen concentrations. NifU has a limited ability to serve the function of NfuA, as its expression at high levels corrects the defect of the nfuA-disrupted strain. Spectroscopic and analytical studies indicate that one [4Fe-4S] cluster can be assembled in vitro within a dimeric form of NfuA. The resultant [4Fe-4S] cluster-loaded form of NfuA is competent for rapid in vitro activation of apo-aconitase. Based on these results a model is proposed where NfuA could represent a class of intermediate [Fe-S] cluster carriers involved in [Fe-S] protein maturation.
The in vivo maturation of simple [Fe-S] proteins is proposed to require preassembly of [Fe-S] species on molecular scaffolds. The first [Fe-S] cluster assembly system to be described is the NIF2 system from Azotobacter vinelandii. This system consists of a cysteine desulfurase, encoded by nifS, which supplies the S for [Fe-S] cluster formation, and a proposed scaffold protein, encoded by nifU (1). The NIF system is specialized for the maturation of [Fe-S] proteins involved in nitrogen fixation.
A. vinelandii also contains a second [Fe-S] protein maturation system designated ISC. The ISC system is required for the general maturation of cellular [Fe-S] proteins involved in intermediary metabolism, such as aconitase (2). The ISC system is more complicated than the NIF system as it includes the products of eight contiguous genes: iscR, iscS, iscU, iscA, hscB, hscA, fdx, and iscX (3). Although the NIF and ISC systems exhibit physiological target specificity, each can partially replace the function of the other, when expressed at high levels (4, 5).
Even though the NIF and ISC systems are differentiated by their apparent target specificities, they share a number of common structural and functional features. For example, NifS and IscS have similar sequences, and they both exhibit cysteine desulfurase activity (2). IscU also shares considerable sequence identity when compared with the N-terminal domain of NifU, including conservation of three cysteine residues that are likely to provide the nucleation site(s) for [Fe-S] cluster assembly (2, 6).
NifU is a modular protein that contains three distinct domains (Fig. 1). The central domain contains a stable redoxactive [2Fe-2S] cluster with an as-yet-unknown function (7). In vitro and in vivo experiments have established that labile [Fe-S] clusters can be assembled on both the N-terminal and C-terminal domains of NifU, and such cluster-loaded forms of NifU can be used for activation of the nitrogenase Fe-protein (8, 9). Thus, NifU contains two different sites upon which labile [Fe-S] clusters can be assembled in vitro, but the functional relationship between these sites is not yet known.
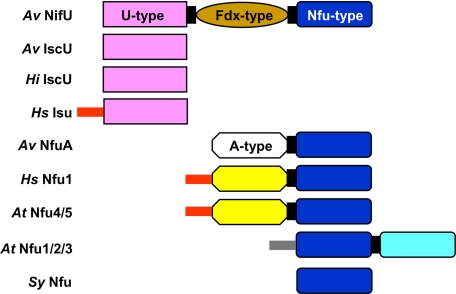
FIGURE 1.
Schematic representation of domain structures of IscU/NifU/Nfu proteins from bacteria (A. vinelandii Av IscU, Av NifU, Av NfuA, and Haemophilus influenzae Hi IscU), mitochondria (human Hs Isu, Hs Nfu1, and Arabidopsis thaliana At Nfu4/5), plant chloroplast (A. thaliana At Nfu1/2/3) and cyanobacteria (Synechocystis sp. Sy Nfu). The pink boxes represent U-type domains, and the brown box represents a Fdx-like domain. Nfu-type domains with a CXXC motif are colored deep blue and those without the CXXC motif are colored light blue. The white box represents the N-terminal A-type domain (without the conserved cysteine residues) that is present in bacterial Nfu-type proteins. The yellow boxes represent the N-terminal domain of mitochondrial Nfu-type proteins. The red and gray sticks represent mitochondrial and plastid targeting sequences, respectively.
There are no genes within the ISC transcriptional unit that encode proteins with sequence similarity to the C-terminal domain of NifU. However, located elsewhere on the A. vinelandii genome is a gene, designated nfuA, whose product encodes a protein having a C-terminal sequence similar to the C-terminal domain of NifU (Fig. 1). The sequence conservation between NifU and NfuA includes two cysteine residues that are required for the in vitro assembly of [Fe-S] clusters within the NifU C-terminal domain. Like NifU, NfuA also appears to be a modular protein because the amino acid sequence within its N-terminal region shares some sequence similarity with another protein involved in [Fe-S] protein maturation designated IscA (Fig. 1). IscA is a nonessential protein encoded within the ISC transcriptional unit, and it has been proposed to serve as an alternative [Fe-S] cluster assembly scaffold (10-12) or as an iron donor during the [Fe-S] cluster assembly process (13). IscA contains three cysteine residues that are essential for its physiological function (3). However, sequence conservation between IscA and the N-terminal domain of NfuA does not include the three cysteine residues contained within IscA.
Sequence conservation between the N-terminal region of NfuA and the IscA family of proteins, as well as the conservation between the C-terminal domains of NfuA and NifU suggest that NfuA could play a role in [Fe-S] protein maturation. This possibility is supported by evidence that [Fe-S] clusters can be assembled on NfuA-like proteins from other organisms (14-17). In some cases, loss of NfuA-like function impairs [Fe-S] protein maturation (17-20). In the present work we have performed a genetic, biochemical, and spectroscopic analysis of NfuA from A. vinelandii to gain insight into its cellular function in this organism.
EXPERIMENTAL PROCEDURES
Materials—Materials used in this work were purchased from Sigma, Fisher Scientific, New England Biolabs, Invitrogen, or from other sources as indicated throughout the text.
Bioinformatics and DNA Analysis—Sequence alignments were performed using the SDSC Biology Workbench program. The Lasergene 7 software was used to analyze DNA and protein sequences. When necessary, A. vinelandii genomic DNA was extracted, amplified, and sequenced as described previously (5).
Plasmid and Strain Construction—Construction of A. vinelandii strains via transformation with appropriate plasmids has been described previously (21). In this work, DJ1707 was obtained by transformation of our wild-type laboratory strain designated DJ (ATCC BAA-1303) with pDB1598, a plasmid that includes a kanamycin resistance cartridge inserted into the XhoI sites of nfuA. This construct results in deletion of a 290-bp fragment from the nfuA gene. DJ1759 and DJ1769, respectively, produce the NfuAC152A- and NfuAC155A-substituted forms of NfuA and were constructed using plasmids pDB1610 and pDB1614, which contain site-directed cysteine to alanine substitutions (codon change of UGC to GCC) at residue positions 152 and 155, respectively. Site-directed mutagenesis procedures were conducted using the GeneEditor™ system by Promega. The parent plasmid used for deletion, insertion, or amino acid substitutions was pDB1577, which contains the intact nfuA gene, as well as 570-bp downstream of the gene, in the pUC-7 vector.
Abundant production and controlled expression of NifUS and their variants were achieved by placing the desired genes under the control of the inducible arabinose regulatory elements as described previously (5). Plasmid pDB1598 was used to inactivate nfuA in DJ1626, a strain where nifUS is placed under the control of the araBAD promoter with concomitant in-frame deletion of the nif-regulated nifUS (5). This inactivation yielded strain DJ1772. Similarly, strains DJ1773 and DJ1791 were constructed, which contain nifU variants with cysteine to alanine substitutions at residues 35 and 275, respectively, and are otherwise isogenic to DJ1772.
Plasmid pDB1417 was designed by insertion of nfuA, flanked by NdeI and BamHI restriction sites, into the NdeI and BamHI sites of pT7-7 for recombinant expression. Similarly, plasmids pDB1582 and pDB1583 were constructed for recombinant expression of NfuA152CA and NfuA155CA, respectively.
Cell Growth at Ambient and Elevated Oxygen Levels—All strains used in this study were cultured in Burk's medium supplemented with 13 mm ammonium acetate (22). When appropriate, media were also supplemented with 20 mml-arabinose. To expose cells to elevated oxygen concentrations, Petri plates were placed in BBL GasPak jars and filled with a gas mixture containing 40% O2 as described previously (3).
Aconitase and Isocitrate Dehydrogenase Assays of A. vinelandii Crude Extracts—A. vinelandii cells were grown in liquid cultures containing Burk's medium supplemented with 13 mm ammonium acetate until they reached A600 = 0.5. Cells were subsequently harvested by centrifugation at 5000 rpm for 5 min, and stored at -20 °C, if not used immediately. To prepare crude extracts, cells were first resuspended in degassed buffer containing 25 mm Tris at pH 7.4 and were lysed using a french pressure cell at 12,000 psi. Lysed cells, which were maintained in airtight vials filled with Argon gas, were centrifuged at 35,000 rpm for 45 min. Crude extracts were separated from the cell debris pellet inside a Coy anaerobic glove box containing 4% H2 gas and 96% N2 to minimize exposure to oxygen. Aconitase assays were prepared in 1-ml quartz cuvettes (sealed airtight with rubber septa) containing 700 μl of 100 mm Tris-HCl buffer at pH 8.0 and 0.1 mg of crude extract. The reaction was initiated with the addition of 25 mm sodium citrate, and activity was monitored at 240 nm (23). Isocitrate dehydrogenase assays of crude extracts were performed in quartz cuvettes containing 900 μl of 50 mm MOPS buffer at pH 7.3, 2.5 mm MgCl2, 0.45 mm NADP+, and 0.1 mg of crude extract. The reaction was started with the addition of 80 mm DL isocitric acid (trisodium salt) in the MOPS/MgCl2 buffer (described above) and monitored at 340 nm (24).
Purification of NfuA and Its Variants—Escherichia coli BL21(DE3) cells transformed with plasmids pDB1417, pDB1582, or pDB1583 were used for the heterologous expression of NfuA, NfuA152CA, or NfuA155CA, respectively. Cells were grown in LB medium containing 0.1 mg/ml ampicillin until they reached A600 = 0.5, at which point, they were induced with 29 mm lactose and allowed to continue to grow for 3 h at 30°C. Cells were then harvested by centrifugation at 5000 rpm for 5 min and stored at -20 °C until use.
Cells expressing recombinant NfuA or its alanine-substituted variants were resuspended in degassed 50 mm Tris-HCl, pH 7.4 buffer (buffer A) and lysed either by sonication (1-min pulse/30-s pause for a total of 20 min) or by a french pressure cell. Crude extracts were prepared as described above. All purification procedures were conducted anaerobically with Schlenk lines filled with argon gas. Crude extracts were loaded at 2.5 ml/min using a peristaltic pump with a 25-ml Q-Sepharose column previously equilibrated with 5 bed volumes of buffer A. The column was subsequently washed with 50 ml of buffer A and 75 ml of buffer A containing 100 mm NaCl. To elute NfuA, 200 ml of 0.1-1.0 m NaCl gradient were applied, and the contents of each fraction were analyzed by 15% SDS-PAGE. The fractions containing NfuA, which eluted between 40 and 94 ml of the gradient, were consolidated, and (NH4)2SO4 was added to a final concentration of 1 m. This sample was then loaded onto a 25-ml phenyl-Sepharose column that was previously equilibrated with buffer A containing 1 m (NH4)2SO4. Following binding of the sample onto the column, the latter was washed with 125 ml of buffer A containing 1 m (NH4)2SO4 followed by 25 ml of 0.5 m (NH4)2SO4 in buffer A. The protein was then eluted using a 100-ml gradient of 0.5 to 0.0 m (NH4)2SO4 followed by 50 ml of buffer A. The fractions containing NfuA, collected from the last 28 ml of the gradient, were then concentrated by Amicon ultrafiltration using a YM10 membrane. The concentrated sample was loaded onto a 320-ml Superdex S75 gel filtration column equilibrated with 100 mm Tris-HCl buffer (pH 8.0) with 1 mm dithiothreitol (buffer B) and 150 mm NaCl. The purest fractions of NfuA, as judged by SDS-PAGE, were pooled and concentrated by Amicon ultrafiltration using a YM10 membrane, and frozen as pellets in liquid N2 until used. The resulting samples of NfuA were >95% pure as judged by gel densitometry.
In Vitro Reconstitution of NfuA—Purified NfuA, NfuA152CA, or NfuA155CA (0.24 mm) were incubated with NifS (12 μm), ferrous ammonium sulfate (4.8 mm), and l-cysteine (4.8 mm) in buffer B for 3 h at room temperature under argon inside a Vacuum Atmospheres glove box (<2 ppm O2). Excess reagents were removed from the sample by loading it onto a Hi-Trap Q-Sepharose column and eluting with a 0 to 1.0 m NaCl gradient using buffer B. The holoprotein eluted between 0.45 and 0.55 m NaCl and was concentrated by ultrafiltration using an Amicon YM10 membrane. 57Fe-enriched ferrous sulfate (>95% enrichment) was used in place of natural abundance ferrous ammonium sulfate in the preparation of Mössbauer samples.
Determination of the Oligomeric State of Apo- and Holo-NfuA—The oligomeric states of both apo- and holo-NfuA were determined by gel-filtration chromatography using a 25-ml Superdex S75 column. The elution buffer was buffer B with 100 mm KCl, which was applied to the column at a flow rate of 0.4 ml/min. Molecular weight standards used were blue dextran (Mr 2,000,000), β-amylase (Mr 200,000), alcohol dehydrogenase (Mr 150,000), bovine serum albumin (Mr 66,000), carbonic anhydrase (Mr 29,000), and cytochrome c (Mr 12,400).
Analytical and Spectroscopic Analyses—Protein concentrations were determined by the DC protein assay (Bio-Rad) using bovine serum albumin as the standard. Iron concentrations were determined colorimetrically using bathophenanthroline under reducing conditions, after digestion of the protein in 0.8% KMnO4/0.2 m HCl (25) or by using the commercial Quantichrom™ iron assay kit (DIFE-250) from Bioassay Systems. Samples for spectroscopic studies were prepared and handled under argon in a Vacuum Atmospheres glove box (O2 < 2 ppm). UV-visible absorption spectra were recorded at room temperature using a Shimadzu UV-3101PC or Cary 50 Bio spectrophotometer. Resonance Raman spectra were recorded as previously described, using an Instrument SA Raman or U1000 spectrometer coupled with a Coherent Sabre argon ion laser, with 20-μl frozen droplets of 2-3 mm sample mounted on the cold finger of an Air Products Displex Model CSA-202E closed cycle refrigerator (26). Signal-to-noise was improved by multiple scans and bands due to the frozen buffer solution were subtracted from all the spectra shown in this work after normalization of lattice modes of ice centered at 230 cm-1. Variable temperature magnetic circular dichroism (VTMCD) spectra were recorded using samples containing 55% (v/v) ethylene glycol in 1-mm cuvettes using an Oxford Instruments Spectromag 4000 (0-7 T) split-coil superconducting magnet (1.5-300 K) mated to a Jasco J-715 spectropolarimeter (27). X-band (∼9.6 GHz) EPR spectra were recorded using a Bruker ESP-300E EPR spectrometer equipped with a dual-mode ER-4116 cavity and an Oxford Instruments ESR-9 flow cryostat. Mössbauer spectra were recorded using the previously described instrumentation (28). Analysis of the Mössbauer data was performed with the program WMOSS (Web Research).
Activation of Apo-aconitase by Reconstituted NfuA—Apo-aconitase was prepared by incubating recombinantly expressed and purified A. vinelandii aconitase A (AcnA) (29) with EDTA and potassium ferricyanide as described previously (30). Activation mixtures contained 4 μm apo-AcnA and between 0 and 12 μm [4Fe-4S] cluster-loaded NfuA in buffer B. The concentration of [4Fe-4S] cluster-loaded NfuA corresponds to the concentration of [4Fe-4S]2+ clusters calculated by using a molar extinction coefficient of ε400 = 15.0 mm-1 cm-1 per [4Fe-4S]2+ cluster. Activation mixtures were incubated at room temperature (22 °C) under anoxic conditions, and 10-μl samples were taken at different time points and assayed for AcnA activity. AcnA activity was measured spectrophotometrically at 240 nm at 22 °C by following the formation of cis-aconitate from citrate or isocitrate, using a molar absorption coefficient ε240 of 3400 mm-1 cm-1 for cis-aconitate (23). Assays (1 ml) were carried out in sealed anoxic cuvettes containing 900 μl of 100 mm Tris/HCl (pH 8.0) and initiated by addition of 100 μl of 200 mm citrate or isocitrate. Anaerobically reconstituted samples of A. vinelandii AcnA containing one [4Fe-4S] cluster per protein monomer exhibited maximal specific activity of 25 units/mg using citrate (∼100% activity) and 79 units/mg using isocitrate (∼100% activity) (29). The time course of holo-AcnA formation at 22 °C was analyzed by fitting to second order kinetics, based on the initial concentrations of apo-AcnA and [4Fe-4S]2+ clusters on NfuA, using the Chemical Kinetics Simulator software package (IBM).
RESULTS
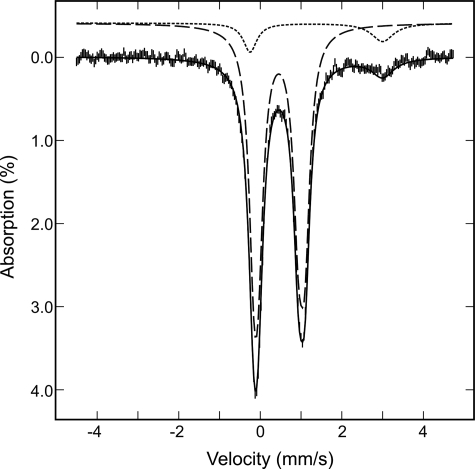
In Vitro Assembly of a [4Fe-4S] Cluster within NfuA—When A. vinelandii NfuA is heterologously produced in E. coli and purified under anoxic conditions, it does not contain a [Fe-S] cluster. Gel exclusion chromatography indicated that as-isolated, recombinant NfuA is an approximately equal mixture of dimeric and tetrameric species (data not shown). When as-isolated NfuA is incubated with NifS, l-cysteine, and Fe2+, a [Fe-S] cluster is assembled. Gel exclusion chromatography revealed that the cluster-bound form of NfuA is resolved into a single dimeric species. Analytical data, coupled with the UV-visible absorption spectrum and extinction coefficients (Fig. 2), indicate that the reconstituted sample contains approximately one [4Fe-4S]2+ cluster per NfuA dimer. Repurified samples of reconstituted NfuA contain 2.0 ± 0.3 Fe/NfuA monomer, and the visible absorption comprises a broad shoulder centered at 400 nm (A400/A280 = 0.23 ± 0.2) with a molar extinction coefficient (ε400 = 7.5 ± 0.5 mm-1 cm-1 based on the concentration of NfuA monomer) that is indicative of ∼0.5 [4Fe-4S]2+ clusters/NfuA monomer (6). More definitive assessments of the [Fe-S] cluster type and content were provided by analysis of the Mössbauer spectrum of a 57Fe-reconstituted NfuA shown in Fig. 3 (vertical bars), which revealed that 90% of the iron is associated with a [4Fe-4S]2+ cluster, and the remaining 10% of the iron with an adventitiously bound Fe2+ species. The spectrum of the [4Fe-4S]2+ cluster (dashed line) is simulated as a superposition of two equal intensity quadrupole doublets arising from the two valence delocalized pairs with δ = 0.47 mm/s, ΔEQ = 1.25 mm/s for doublet 1, and δ = 0.45/mm/s, ΔEQ = 0.99 mm/s for doublet 2. The spectrum of the Fe2+ species (dotted line) is simulated with an asymmetric quadrupole doublet with δ = 1.38 mm/s and ΔEQ = 3.25 mm/s. The presence of [2Fe-2S] clusters was not detected.
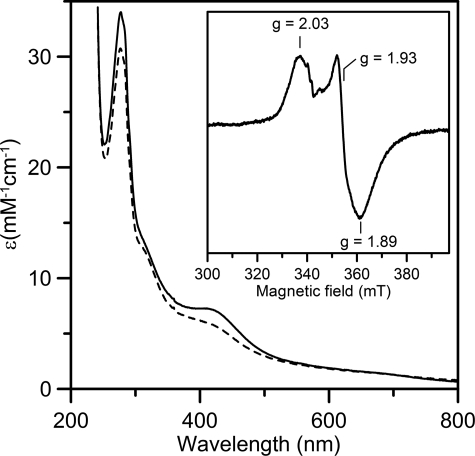
FIGURE 2.
UV-visible absorption spectra of reconstituted A. vinelandii NfuA as prepared (solid line) and after anaerobic reduction with one reducing equivalent of dithionite (dashed line). Molar extinction coefficients are expressed per NfuA monomer. The inset shows the X-band EPR spectrum of the sample reduced with one reducing equivalent of dithionite. EPR conditions: microwave frequency, 9.60 GHz; microwave power, 10 milliwatt; modulation amplitude, 0.63 mT; temperature, 10 K.
FIGURE 3.
Mössbauer spectrum of 57Fe-reconstituted A. vinelandii NfuA recorded at 4.2 K with a field of 50 mT applied parallel to the γ-radiation. The solid black line is the composite spectrum including the simulated spectrum of a [4Fe-4S]2+ cluster (dashed line), scaled to 90% of the total iron absorption, and the simulated spectrum of a Fe2+ species (dotted line), accounting for 10% of the iron absorption. The parameters used for the simulations are provided in the text.
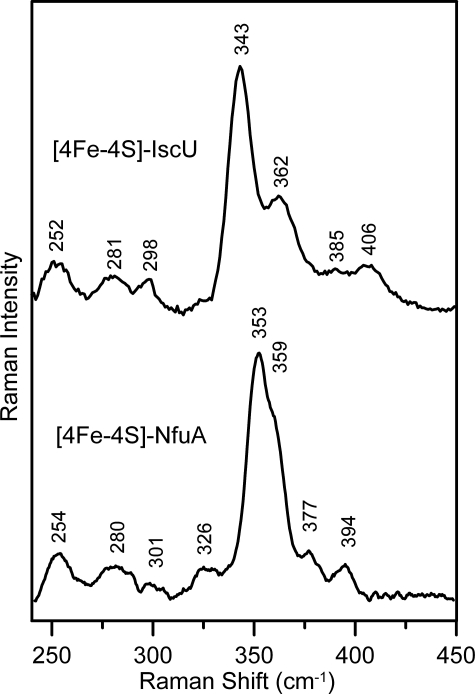
While the absorption and Mössbauer results provide unambiguous evidence for anaerobic reconstitution of a [4Fe-4S]2+ cluster on NfuA, the vibrational properties, as determined by resonance Raman spectroscopy, are somewhat atypical compared with well-characterized biological [4Fe-4S]2+ centers (31-33) and exhibit characteristics that have been observed previously only for the subunit-bridging [4Fe-4S]2+ cluster in the MgATP-bound nitrogenase iron protein (34). This feature is illustrated in Fig. 4, which shows a comparison of the resonance Raman spectra of the [4Fe-4S]2+ clusters in reconstituted forms of A. vinelandii IscU and NfuA in the Fe-S stretching region using 457-nm excitation. The major difference lies in the anomalously high frequency for the most intense band in the spectrum (353 cm-1 for the NfuA [4Fe-4S]2+ center compared with 335-343 cm-1 in other biological [4Fe-4S]2+ centers), which is generally assigned to the symmetric breathing mode of the [4Fe-4S] cubane core. Analogous spectra with similar band frequencies and relative intensities were observed for the reconstituted NfuA using 488- and 514-nm excitation, indicating that this behavior cannot be attributed to anomalous excitation profiles for discreet bands. One possible explanation is that the intense bands at 353 and 359 cm-1 for the [4Fe-4S]2+ center in NfuA correspond to asymmetric Fe-S(Cys) stretching modes and that the symmetric breathing modes primarily involving the [4Fe-4S] core and the Fe-S(Cys) bonds are only weakly enhanced as a result of distortions of the core and/or the cluster environment. This possibility is tentatively supported by the observation of similar frozen solution resonance Raman spectra for the [4Fe-4S]2+ cluster in MgATP-bound nitrogenase iron protein, which was found to be split into two [2Fe-2S] fragments separated by ∼5 Å in the crystalline state (34). Normal mode calculations coupled with 57/54Fe and 34/32S isotope shifts and measurement of depolarization ratios are planned to test this hypothesis and to provide vibrational assignments for the [4Fe-4S]2+ center in NfuA.
FIGURE 4.
Comparison of the resonance Raman spectra for [4Fe-4S] 2+ cluster-loaded forms of A. vinelandii IscU and NfuA. The samples (∼3 mm in [4Fe-4S] clusters) were in the form of frozen droplets at 17 K. The spectra were recorded using 457-nm excitation with 100 milliwatts of laser power at the sample. Each spectrum is the sum of 100 scans, with each scan involving photon counting for 1 s at 1-cm-1 increments with 7-cm-1 resolution. Bands due to lattice modes of the frozen buffer solution have been subtracted from both spectra.
The nature of the cluster ligation for the [4Fe-4S] center on NfuA was addressed by amino acid substitution of the two conserved cysteine residues located within the Cys-X-X-Cys motif. It was not possible to reconstitute a [Fe-S] cluster on variant forms of NfuA proteins that have either of these two cysteine residues substituted by alanine (data not shown). This information, together with the analytical and spectroscopic data, are reasonably interpreted in terms of cluster-loaded NfuA having a [4Fe-4S]2+ cluster that is symmetrically bridged between two identical subunits and coordinated by conserved cysteine residues from opposing subunits.
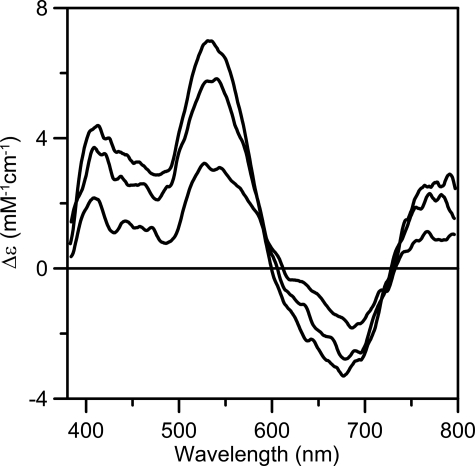
The redox properties of the [4Fe-4S]2+ cluster assembled on NfuA were assessed using a combination of UV-visible absorption, EPR, and VTMCD spectroscopy (Figs. 2 and 5). Anaerobic addition of one reducing equivalent of dithionite results in reversible bleaching of the visible absorption (Fig. 2) due to partial reduction to yield an S = 1/2 [4Fe-4S]+ cluster, as evidenced by parallel EPR and VTMCD studies. EPR revealed a near-axial resonance (g = 2.03, 1.93, 1.89) with relaxation properties (observable only below 40 K) indicative of a [4Fe-4S]+ cluster, that accounts for 0.4 spins/NfuA dimer (see inset in Fig. 2). The low spin quantification is in accord with the partial bleaching of the visible absorption and appears to reflect partial reduction. No low-field EPR signals indicative of a S = 3/2 [4Fe-4S]+ clusters were observed. In accord with the EPR results, the VTMCD spectrum is consistent with a S = 1/2 [4Fe-4S]+ cluster rather than a S = 3/2 [4Fe-4S]+ cluster (35), and the intensity indicates ∼0.4 [4Fe-4S]+ clusters/NfuA dimer. Partial reduction by one reducing equivalent of dithionite implies a redox potential for the [4Fe-4S]2+,+ couple that is close to that of dithionite at pH 8.0 (-450 mV). Such a low potential redox process is unlikely to be physiologically relevant. Moreover, the reduced cluster is unstable and is rapidly degraded within minutes in the presence of excess dithionite even under strictly anoxic conditions. Similar redox behavior has been reported for the subunit bridging [4Fe-4S] cluster on IscU (36). Hence, only the oxidized form of the subunit-bridging [4Fe-4S] cluster on NfuA is likely to be relevant in vivo.
FIGURE 5.
VTMCD spectra of dithionite-reduced reconstituted A. vinelandii NfuA. Reconstituted NfuA (0.25 mm in [4Fe-4S] clusters) was reduced with one reducing equivalent of dithionite after addition of 55% (v/v) ethylene glycol. MCD spectra recorded in a 1-mm cell with a magnetic field of 6 T at 1.7, 4.2, and 15 K. All bands increased in intensity with decreasing temperature, and Δε values are expressed per NfuA monomer.
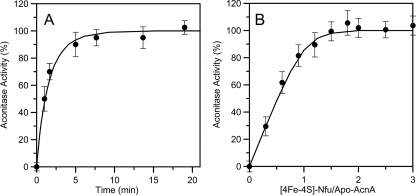
Activation of Apo-aconitase using [4Fe-4S] Cluster-loaded NfuA—Fig. 6A shows the time-dependent activation of apoaconitase that occurs when it is mixed with [4Fe-4S] cluster-loaded NfuA. Initial experiments duplicated the conditions established to achieve optimal aconitase activation using [4Fe-4S] cluster-loaded IscU (29) and involved incubation of cluster-loaded NfuA (12 μm in [4Fe-4S] clusters) with 4 μm apo-aconitase, i.e. a 3-fold stoichiometric excess of [4Fe-4S] clusters. The activation is remarkably rapid, having a second order rate constant of 6.0 ± 1.5 × 104 m-1 min-1, a rate which is almost 10 times faster than that observed for analogous conditions using the [4Fe-4S] cluster-loaded form of IscU (29). Such rates are consistent only with transfer of intact cluster, as activation with equivalent amounts of Fe2+ and S2- ions is at least 15 times slower. Moreover, unlike IscU-directed apo-AcnA activation, which is optimal with a 3:1 stoichiometry, NfuA-directed activation of apo-AcnA occurs with a 1:1 stoichiometry. This feature is illustrated in Fig. 6B, which shows apo-aconitase activation after 20 min of incubation as a function of the molar ratio of [4Fe-4S] clusters on NfuA to apo-AcnA. The data indicate stoichiometric [4Fe-4S] cluster transfer from NfuA to apo-AcnA. The curvature is a consequence of incomplete cluster transfer after 20 min at near stoichiometric [4Fe-4S] cluster concentrations. Indeed, the observed data are well simulated by theoretical data constructed for [4Fe-4S] cluster transfer occurring with a 1:1 stoichiometry and a second order rate constant of 6.0 × 104 m-1 min-1 after 20 min of reaction. Hence, cluster transfer and activation of apo-aconitase is much faster and much more efficient with [4Fe-4S]-NfuA than with [4Fe-4S]-IscU.
FIGURE 6.
Activation of apo-AcnA activity using [4Fe-4S] cluster-loaded NfuA. A, apo-AcnA (4 μm) was incubated with [4Fe-4S] cluster-loaded NfuA (12 μm in [4Fe-4S]2+ clusters) at room temperature under anaerobic conditions. Aliquots containing 4 μm AcnA were withdrawn after 100, 460, 820, 1140, 1480, and 1800 s, and AcnA activity was immediately measured. The solid line is the best fit to second order kinetics based on the initial concentrations of [4Fe-4S]2+ clusters on NfuA and apo-AcnA, and corresponds to a rate constant of 6.0 × 104 m-1 min-1. B, apo-AcnA activation as a function of the concentration of NfuA-ligated [4Fe-4S]2+ clusters. The concentration of apo-AcnA was kept constant at 4 μm, and the concentration of NfuA-ligated [4Fe-4S]2+ clusters was varied as indicated on the x-axis. After 20 min, aliquots were withdrawn and assayed for AcnA activity. The solid line is theoretical data computed for [4Fe-4S] cluster transfer from NfuA to apo-AcnA occurring with a 1:1 stoichiometry and with a rate constant of 6.0 × 104 m-1 min-1 after 20 min of reaction. The data points in A and B correspond to the average values of three independent measurements, and the error bars represent the standard deviation.
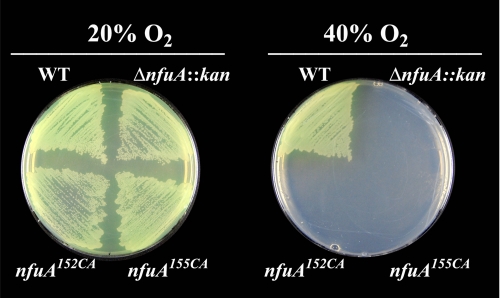
Phenotypic Analysis of Mutant Strains Defective in NfuA Function—A strain (designated DJ1707) was constructed that has the nfuA gene partially deleted and replaced by a kanamycin gene cartridge insertion. DJ1707 has no obvious phenotypic traits with respect to growth rate when cultured in liquid medium or colony size when cultured on agar plates. However, DJ1707 cell extracts have only ∼50% aconitase activity when compared with the isogenic wild-type strain. The diminished aconitase activity of the DJ1707 strain relative to the otherwise isogenic wild-type strain contrasted with the equivalent levels of isocitrate dehydrogenase in the two strains. Isocitrate dehydrogenase, like aconitase, is a tricarboxylic acid cycle enzyme, but it does not require a [Fe-S] cluster for its activity. Another phenotype associated with loss of NfuA function is a complete loss of capacity for growth under a 40% oxygen atmosphere (Fig. 7).
FIGURE 7.
Inactivation of nfuA and its conserved cysteines results in a null-growth phenotype under elevated oxygen conditions. Strains were cultured in Burk's medium supplemented with 13 mm ammonium acetate under ambient (∼20%) O2 or 40% O2 for 3 days. The standard laboratory strain designated as DJ was used as a wild-type control (WT). Strain DJ1707 has a deletion and kanamycin gene cartridge insertion within the nfuA gene (ΔnfuA::kan). Strains DJ1759 and DJ1769 have, respectively, NfuA Cys152 and NfuA Cys155 substituted by alanine.
The functional importance of the two cysteine residues located within the Cys-X-X-Cys motif was tested by constructing strains having these residues individually substituted by alanine. These strains, DJ1759 and DJ1769, exhibit the same oxygen-sensitive growth phenotype associated with deletion and insertional inactivation of nfuA (Fig. 7).
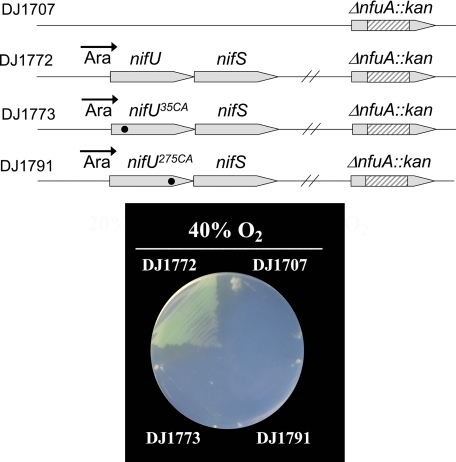
NifU Replacement of NfuA Requires Functional NifU N- and C-terminal Domains—DJ1707 exhibited the same sensitivity to elevated oxygen concentrations whether or not NifU is also expressed. Thus, NifU is not normally able to supplant the function of NfuA. However, when nifUS expression is placed under control of the strong ara regulatory elements (Fig. 8) the oxygen-sensitive phenotype associated with loss of NfuA function is fully corrected. Thus, there is a capacity for functional replacement of NfuA by NifU, but only when NifU is expressed at high levels. In separate experiments it was determined whether both the N- and C-terminal domains of NifU are required to rescue the phenotype associated with loss of NfuA function. This possibility was examined by construction of two different strains. One of these (DJ1773) has the NifU-Cys35 residue substituted by alanine (DJ1773) and the other (DJ1791) has the NifU-Cys275 residue substituted by alanine. Previous work has shown that such substitutions respectively eliminate the capacity for [Fe-S] cluster formation within the IscU-like or NfuA-like domains within NifU (8). Both strains DJ1773 and DJ1791 are also inactivated for nfuA. Results shown in Fig. 8 reveal that a capacity for the assembly of [Fe-S] clusters on both the IscU-like and NfuA-like domains of NifU is required for correction of the oxygen-sensitive phenotype associated with the loss of NfuA function.
FIGURE 8.
Rescue of the null-growth phenotype associated with the functional loss of NfuA by elevated ara-directed expression of NifUS. Top, schematic representation of the genetic organizations of DJ1707, DJ1772, DJ1773, and DJ1791. Bottom, growth of strains when cultured in Burk's medium supplemented with 13 mm ammonium acetate and 20 mm arabinose and cultured under a 40% O2 atmosphere. NifU and NifS expression are under the control of the ara-regulatory elements, resulting in accumulation of high levels when arabinose is added to the growth medium.
DISCUSSION
NfuA is a modular protein that has an N-terminal domain similar to IscA and a C-terminal domain similar to the C-terminal domain of NifU. Because both IscA and NifU are involved in the maturation of [Fe-S] proteins, such sequence conservation indicates a likely role for NfuA in [Fe-S] protein maturation in A. vinelandii, but what is that role? The most pertinent observations from previous work and this study that bear on this question can be summarized as follows: (i) IscU is essential for the in vivo maturation of [Fe-S] proteins. (ii) The [4Fe-4S] cluster-loaded form of IscU is a poor in vitro donor for aconitase maturation (29), whereas the [4Fe-4S] cluster loaded form of NfuA is very effective for in vitro activation of aconitase. (iii) NfuA is not essential for in vivo [Fe-S] protein maturation but loss of NfuA results in lower activity of at least one key [4Fe-4S] protein, i.e. aconitase. (iv) The phenotypic relationship observed between IscU and NfuA is also observed for the IscU-like and NfuA-like domains of NifU. Specifically, functional loss of the IscU-like domain of NifU nearly eliminates the capacity for the in vivo maturation of nitrogenase, whereas functional loss of the NfuA-like domain has no obvious effect on nitrogenase maturation (8). Furthermore, the IscU-like domain of NifU is a relatively poor [4Fe-4S] donor for maturation of the nitrogenase iron protein (9). (v) The ability of NifU to correct the oxygen-sensitive phenotype associated with the loss of NfuA requires functionality of both the IscU-like and NfuA-like domains within NifU.
These results lead us to propose a model where IscU, and the IscU-like domain within NifU, have essential roles in the de novo formation of [Fe-S] clusters, whereas NfuA and the NfuA-like domain of NifU could have auxiliary roles related to the effective transfer of [Fe-S] clusters to specific target proteins. This model suggests that [Fe-S] clusters initially formed on the IscU type of scaffolds in A. vinelandii are subsequently transferred to NfuA-type proteins. Thus, NfuA-type proteins would be considered to serve as intermediate [Fe-S] cluster carriers or, perhaps, as [Fe-S] cluster reservoirs. Given that IscU appears to be required for the maturation of housekeeping [Fe-S] proteins in A. vinelandii, the participation of intermediate carriers is a reasonable possibility. Specifically, it is difficult to imagine that IscU would have the capacity to directly and effectively interact with the large variety of cellular [Fe-S] proteins present in cells. This proposed lack of direct physiological delivery of [Fe-S] clusters from IscU to various client proteins is supported by the relatively poor capacity for cluster-loaded forms of IscU to effect in vitro [Fe-S] protein maturation. Nevertheless, the observation that NfuA is dispensable indicates that IscU can serve at some level as a primary [Fe-S] cluster donor to client proteins, or that IscU is able to deliver [Fe-S] clusters to a suite of different intermediate carriers, some of which have overlapping functions. We favor the latter possibility and suggest that a variety of proteins, recently shown to have the capacity to harbor [Fe-S] clusters, for example, Nfu (14-17), IscA (11, 12, 37, 38), and glutaredoxins (39-41), could collectively serve as a physiological [Fe-S] cluster reservoir. This possibility is in line with the manifestation of a clear phenotype for NfuA and IscA (3) in A. vinelandii under conditions of oxygen stress, a situation that could increase the demand for [Fe-S] clusters. Also, the fact that both the N-terminal and C-terminal domains within NifU are required to replace the function of NfuA, indicates the two domains have functional interdependence. Specifically, the observed phenotype is consistent with the possibility that [Fe-S] clusters assembled on the N-terminal (IscU-like) domain within NifU can be transferred to the C-terminal (NfuA-like) domain. The converse is unlikely to occur because maturation of nitrogenase does not require an intact NfuA-like domain within NifU.
Another interesting feature of NfuA is that it shares sequence identity with the IscA family of proteins, but this sequence conservation does not include cysteine residues that are conserved among the IscA family. This feature suggests that amino acid sequence conservation between the IscA family and NfuA could be related to target specificity, rather than [Fe-S] cluster assembly, and supports the possibility that IscA and NfuA could have some overlapping functions. Given the large number of [Fe-S] proteins within the cell, as well as the variety of potential environmental conditions a cell might encounter, a hierarchy of intermediate [Fe-S] cluster carriers that could control the distribution of [Fe-S] clusters would be a reasonable strategy for maximizing metabolic capacity. There is already precedent for such a strategy in the case of E. coli, because there are at least two different systems that can function for [Fe-S] protein maturation under different conditions. One of these, the ISC system, operates under standard laboratory conditions whereas the SUF system operates under conditions of iron limitation or oxygen stress (42). It should be noted that A. vinelandii does not encode a SUF system for [Fe-S] cluster assembly. Other cases of apparent specialized targeting of preassembled [Fe-S] clusters involve the role of ErpA, an IscA-like protein, necessary for the maturation of IspG (43), and the roles of NifU and NifS in maturation of nitrogenase (44). Future studies will be aimed at testing the hypothesis that NfuA and the NfuA-like domain of NifU function as intermediate carriers of [Fe-S] clusters preformed on the respective IscU and IscU-like scaffolds.
A final important feature to emerge from this and other studies is the apparent organizational and physiological plasticity of [Fe-S] protein maturation systems from different organisms. This aspect is highlighted in Fig. 1, which compares a variety of proteins having Nfu-like modules. It is particularly noteworthy that the cluster types that can be assembled on Nfu-like modules, as well as phenotypes associated with loss of Nfu-like function, are reported to be different for different organisms. For example, in vitro reconstitution of a chloroplastic Nfu-like protein from plants has been shown to result in formation of a [2Fe-2S] cluster, and this protein is essential for maturation of chloroplastic [Fe-S] proteins (16, 17). Thus, it appears that while Nfu-type modules have auxiliary or specialized functions in certain organisms, such as A. vinelandii, they have primary functions in [Fe-S] protein maturation in other organisms. Such variations in the [Fe-S] protein maturation process in different organisms are probably linked to physiological conditions, most significantly, intracellular redox conditions. Results similar to those reported here using A. vinelandii have also been found for the NfuA protein from E. coli (45).
Acknowledgments
We thank B. Py, F. Barras, and M. Fontecave for providing their results on the E. coli NfuA homolog prior to publication.
This work was supported, in whole or in part, by National Institutes of Health Grants GM47295 (to B. H. H.) and GM62524 (to M. K. J.). This work was also supported by Grant MCB0717710 (to D. R. D.) from the National Science Foundation. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: NIF, nitrogen fixation; ISC, iron sulfur cluster; MOPS, 4-morpholinepropanesulfonic acid; VTMCD, variable temperature magnetic circular dichroism; AcnA, aconitase A.
References
- 1.Johnson, D. C., Dean, D. R., Smith, A. D., and Johnson, M. K. (2005) Annu. Rev. Biochem. 74 247-281 [DOI] [PubMed] [Google Scholar]
- 2.Zheng, L., Cash, V. L., Flint, D. H., and Dean, D. R. (1998) J. Biol. Chem. 273 13264-13272 [DOI] [PubMed] [Google Scholar]
- 3.Johnson, D. C., Unciuleac, M. C., and Dean, D. R. (2006) J. Bacteriol. 188 7551-7561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnson, D. C., Dos Santos, P. C., and Dean, D. R. (2005) Biochem. Soc. Trans. 33 90-93 [DOI] [PubMed] [Google Scholar]
- 5.Dos Santos, P. C., Johnson, D. C., Ragle, B. E., Unciuleac, M. C., and Dean, D. R. (2007) J. Bacteriol. 189 2854-2862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agar, J. N., Krebs, C., Frazzon, J., Huynh, B. H., Dean, D. R., and Johnson, M. K. (2000) Biochemistry 39 7856-7862 [DOI] [PubMed] [Google Scholar]
- 7.Agar, J. N., Yuvaniyama, P., Jack, R. F., Cash, V. L., Smith, A. D., Dean, D. R., and Johnson, M. K. (2000) J. Biol. Inorg. Chem. 5 167-177 [DOI] [PubMed] [Google Scholar]
- 8.Dos Santos, P. C., Smith, A. D., Frazzon, J., Cash, V. L., Johnson, M. K., and Dean, D. R. (2004) J. Biol. Chem. 279 19705-19711 [DOI] [PubMed] [Google Scholar]
- 9.Smith, A. D., Jameson, G. N., Dos Santos, P. C., Agar, J. N., Naik, S., Krebs, C., Frazzon, J., Dean, D. R., Huynh, B. H., and Johnson, M. K. (2005) Biochemistry 44 12955-12969 [DOI] [PubMed] [Google Scholar]
- 10.Ollagnier-de-Choudens, S., Mattioli, T., Takahashi, Y., and Fontecave, M. (2001) J. Biol. Chem. 276 22604-22607 [DOI] [PubMed] [Google Scholar]
- 11.Krebs, C., Agar, J. N., Smith, A. D., Frazzon, J., Dean, D. R., Huynh, B. H., and Johnson, M. K. (2001) Biochemistry 40 14069-14080 [DOI] [PubMed] [Google Scholar]
- 12.Abdel-Ghany, S. E., Ye, H., Garifullina, G. F., Zhang, L., Pilon-Smits, E. A., and Pilon, M. (2005) Plant. Physiol. 138 161-172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ding, H., Clark, R. J., and Ding, B. (2004) J. Biol. Chem. 279 37499-37504 [DOI] [PubMed] [Google Scholar]
- 14.Tong, W. H., Jameson, G. N., Huynh, B. H., and Rouault, T. A. (2003) Proc. Natl. Acad. Sci. U. S. A. 100 9762-9767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nishio, K., and Nakai, M. (2000) J. Biol. Chem. 275 22615-22618 [DOI] [PubMed] [Google Scholar]
- 16.Leon, S., Touraine, B., Ribot, C., Briat, J. F., and Lobreaux, S. (2003) Biochem. J. 371 823-830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yabe, T., Morimoto, K., Kikuchi, S., Nishio, K., Terashima, I., and Nakai, M. (2004) Plant. Cell 16 993-1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Touraine, B., Boutin, J. P., Marion-Poll, A., Briat, J. F., Peltier, G., and Lobreaux, S. (2004) Plant. J. 40 101-111 [DOI] [PubMed] [Google Scholar]
- 19.Schilke, B., Voisine, C., Beinert, H., and Craig, E. (1999) Proc. Natl. Acad. Sci. U. S. A. 96 10206-10211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Balasubramanian, R., Shen, G., Bryant, D. A., and Golbeck, J. H. (2006) J. Bacteriol. 188 3182-3191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jacobson, M. R., Brigle, K. E., Bennett, L. T., Setterquist, R. A., Wilson, M. S., Cash, V. L., Beynon, J., Newton, W. E., and Dean, D. R. (1989) J. Bacteriol. 171 1017-1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Strandberg, G. W., and Wilson, P. W. (1968) Can. J. Microbiol. 14 25-31 [DOI] [PubMed] [Google Scholar]
- 23.Saas, J., Ziegelbauer, K., von Haeseler, A., Fast, B., and Boshart, M. (2000) J. Biol. Chem. 275 2745-2755 [DOI] [PubMed] [Google Scholar]
- 24.Cribbs, R., and Englesberg, E. (1964) Genetics 49 95-108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fish, W. W. (1988) Methods Enzymol. 158 357-364 [DOI] [PubMed] [Google Scholar]
- 26.Cosper, M. M., Jameson, G. N., Hernandez, H. L., Krebs, C., Huynh, B. H., and Johnson, M. K. (2004) Biochemistry 43 2007-2021 [DOI] [PubMed] [Google Scholar]
- 27.Johnson, M. K. (2000) in Physical Methods in Bioinorganic Chemistry. Spectroscopy and Magnetism (Que, L., Jr., ed), pp. 233-285, University Science Books, Sausalito, CA
- 28.Ravi, N., Bollinger, J. M., Huynh, B. H., Edmondson, D. E., and Stubbe, J. (1994) J. Am. Chem. Soc. 116 8007-8014 [Google Scholar]
- 29.Unciuleac, M. C., Chandramouli, K., Naik, S., Mayer, S., Huynh, B. H., Johnson, M. K., and Dean, D. R. (2007) Biochemistry 46 6812-6821 [DOI] [PubMed] [Google Scholar]
- 30.Kennedy, M. C., and Beinert, H. (1988) J. Biol. Chem. 263 8194-8198 [PubMed] [Google Scholar]
- 31.Czernuszewicz, R. S., Macor, K. A., Johnson, M. K., Gewirth, A., and Spiro, T. G. (1987) J. Am. Chem. Soc. 109 7178-7187 [Google Scholar]
- 32.Spiro, T. G., Czernuszewicz, R. S., and Han, S. (1988) in Biological Applications of Raman Spectroscopy (Spiro, T. G., ed), Vol. 3, pp. 523-553, John Wiley & Sons, New York [Google Scholar]
- 33.Brereton, P. S., Duderstadt, R. E., Staples, C. R., Johnson, M. K., and Adams, M. W. W. (1999) Biochemistry 38 10594-10605 [DOI] [PubMed] [Google Scholar]
- 34.Sen, S., Igarashi, R., Smith, A., Johnson, M. K., Seefeldt, L. C., and Peters, J. W. (2004) Biochemistry 43 1787-1797 [DOI] [PubMed] [Google Scholar]
- 35.Onate, Y. A., Finnegan, M. G., Hales, B. J., and Johnson, M. K. (1993) Biochim. Biophys. Acta 1164 113-123 [DOI] [PubMed] [Google Scholar]
- 36.Chandramouli, K., Unciuleac, M. C., Naik, S., Dean, D. R., Huynh, B. H., and Johnson, M. K. (2007) Biochemistry 46 6804-6811 [DOI] [PubMed] [Google Scholar]
- 37.Ollagnier-de Choudens, S., Sanakis, Y., and Fontecave, M. (2004) J. Biol. Inorg. Chem. 9 828-838 [DOI] [PubMed] [Google Scholar]
- 38.Morimoto, K., Yamashita, E., Kondou, Y., Lee, S. J., Arisaka, F., Tsukihara, T., and Nakai, M. (2006) J. Mol. Biol. 360 117-132 [DOI] [PubMed] [Google Scholar]
- 39.Rouhier, N., Unno, H., Bandyopadhyay, S., Masip, L., Kim, S. K., Hirasawa, M., Gualberto, J. M., Lattard, V., Kusunoki, M., Knaff, D. B., Georgiou, G., Hase, T., Johnson, M. K., and Jacquot, J. P. (2007) Proc. Natl. Acad. Sci. U. S. A. 104 7379-7384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lillig, C. H., Berndt, C., Vergnolle, O., Lonn, M. E., Hudemann, C., Bill, E., and Holmgren, A. (2005) Proc. Natl. Acad. Sci. U. S. A. 102 8168-8173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rodriguez-Manzaneque, M. T., Tamarit, J., Belli, G., Ros, J., and Herrero, E. (2002) Mol. Biol. Cell 13 1109-1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Outten, F. W., Djaman, O., and Storz, G. (2004) Mol. Microbiol. 52 861-872 [DOI] [PubMed] [Google Scholar]
- 43.Loiseau, L., Gerez, C., Bekker, M., Ollagnier-de Choudens, S., Py, B., Sanakis, Y., Teixeira de Mattos, J., Fontecave, M., and Barras, F. (2007) Proc. Natl. Acad. Sci. U. S. A. 104 13626-13631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jacobson, M. R., Cash, V. L., Weiss, M. C., Laird, N. F., Newton, W. E., and Dean, D. R. (1989) Mol. Gen. Genet. 219 49-57 [DOI] [PubMed] [Google Scholar]
- 45.Angelini, S., Gerez, C., Ollagnier-de Choudens, S., Sanakis, Y., Fontecave, M., Barras, F., and Py, B. (2008) J. Biol. Chem. 283 14084-14091 [DOI] [PubMed] [Google Scholar]