Abstract
Skeletal muscle plays a major role in glucose and lipid metabolism. Active hepatocyte growth factor (HGF) is present in the extracellular matrix in skeletal muscle. However, the effects of HGF on glucose and lipid metabolism in skeletal muscle are completely unknown. We therefore examined the effects of HGF on deoxyglucose uptake (DOGU), glucose utilization, and fatty acid oxidation (FAO) in skeletal muscle cells. HGF significantly enhanced DOGU in mouse soleus muscles in vitro. Furthermore, HGF significantly increased: (i) DOGU in a time- and dose-dependent manner; (ii) glucose utilization; and (iii) plasma membrane expression of Glut-1 and Glut-4 in the rat skeletal muscle model of L6 myotubes. HGF-mediated effect on DOGU was dependent on the activation of phosphatidylinositol 3-kinase signaling pathway. On the other hand, HGF markedly and significantly decreased FAO in L6 myotubes without affecting the activities of carnitine palmitoyltransferase I and II. Collectively, these results indicate that HGF is a potent activator of glucose transport and metabolism and also a strong inhibitor of FAO in rodent myotubes. HGF, through its ability to stimulate glucose transport and metabolism and to impair FAO, may participate in the regulation of glucose disposal in skeletal muscle.
Hepatocyte growth factor (HGF),5 originally identified as a circulating factor that promotes hepatic regeneration after liver injury (1), displays pleiotropic cellular activities, including angiogenesis, anti-apoptosis, and mitogenesis, in a wide variety of cell types expressing the HGF receptor c-Met (2). HGF overexpression in mouse pancreatic beta cells in vivo increases Glut-2 and glucokinase mRNA expression and enhances glucose transport and metabolism in these cells (3). Conversely, adult knock-out mice in which HGF/c-Met signaling has been deleted from the beta cell display markedly decreased Glut-2 expression, impaired glucose tolerance, and reduced glucose-stimulated insulin secretion (4). HGF has also been shown to up-regulate the Na+/glucose co-transporter SGLT1 and the facilitative glucose transporter Glut-5 in rat intestine epithelial cells (5). Furthermore, HGF promotes Glut-4 translocation to the cellular membrane and enhances glucose uptake through phosphatidylinositol 3-kinase (PI3K) activation in 3T3-L1 adipocytes (6). Taken together, these studies demonstrate HGF-mediated regulatory effects on glucose transport in three different cells types: beta cells, intestinal epithelial cells, and adipocytes.
Skeletal muscle plays a major role in carbohydrate and lipid metabolism at rest and during exercise (7). Muscle metabolism is profoundly altered by obesity. It has been proposed that the elevated plasma free fatty acid (FFA) levels in obesity lead to increased muscle lipid accumulation and decreased insulin sensitivity, the so-called “lipotoxic” model of insulin resistance in skeletal muscle (8–10). HGF is present in adult mature muscle fibers, where it participates in skeletal muscle development and regeneration after injury (11–14). However, whether HGF can affect glucose transport, glucose metabolism, and fatty acid oxidation (FAO) in differentiated skeletal muscle myotubes is unknown.
In the present study, we have found that HGF substantially enhances glucose transport and metabolism in skeletal muscle myotubes. This effect is mediated by PI3K/Akt and by increased presence of Glut-1 and Glut-4 in the plasma membrane of these cells. In addition, HGF potently inhibits FAO in skeletal muscle cells. Taken together, these results suggest that circulating or endogenous HGF may participate in the regulation of glucose uptake and metabolism in skeletal muscle.
EXPERIMENTAL PROCEDURES
Materials—Cell culture reagents were from Mediatech, Inc., (Herndon, VA). [9,10-3H]Palmitic acid, [3H]H2O, and 2-[3H]deoxyglucose were from PerkinElmer Life Sciences. l-[N-methyl-14C]Carnitine and d-[1-14C]mannitol were from American Radiolabeled Chemicals, Inc. (St. Louis, MO). d-[5-3H]Glucose and d-[U-14C]glucose were from Amersham Biosciences. Recombinant human HGF was from Research Diagnostics, Inc. (Flanders, NJ) and bovine insulin from Sigma. Antibodies against Akt, Akt-(pS473), ERK1/2, ERK1/2-(pThr-202/Tyr-204), p38 MAPK, p38 MAPK-(pThr-180/Tyr-182), Gab-1, phospho-acetyl-CoA carboxylase (Ser-79) were from Cell Signaling (Beverly, MA); against c-Met and actin from Sigma; against IRS-1 and PI3K from Santa Cruz Biotechnology (Santa Cruz, CA); against the α1-subunit of Na+/K+-ATPase from Abcam (Cambridge, MA); and against HGF from R&D Systems (Minneapolis, MN). Wortmannin was from Sigma, and SB202190 and PD98059 were from Calbiochem (La Jolla, CA).
2-[3H]Deoxyglucose Uptake in Mouse Soleus Muscle—Following an overnight fast, male C57BL/6J mice (2–3 months old) were anesthetized and soleus muscles were quickly excised. All studies were performed with the approval of, and in accordance with guidelines established by, the University of Pittsburgh Institutional Animal Care and Use Committee.
Analysis of HGF effects on glucose uptake in soleus muscles ex vivo was performed as previously reported (15). Briefly, muscles were preincubated for 30 min with gentle agitation in continuously gassed (95% O2-5% CO2) Krebs-Ringer-Henseleit buffer (KRHB) containing 0.1% bovine serum albumin, 32 mm mannitol, and 8 mm glucose, with or without HGF. Next, muscles were placed in fresh KRHB containing 40 mm mannitol with or without HGF for 10 min. For determining 2-deoxyglucose uptake (DOGU), soleus muscles were incubated with 1 mm 2-[3H]deoxyglucose (25 Ci/mmol) and 39 mm [14C]mannitol (53 Ci/mmol) in the presence or absence of HGF and/or insulin for 30 min. Muscles were removed rapidly, rinsed, blotted, digested in 1N NaOH, and analyzed for 14C and 3H.
L6 Cell Culture—L6 cells, kindly provided by Dr. A. Klip (Hospital for Sick Children, Toronto, ON, Canada), were grown in α-minimum Eagle's medium (α-MEM) containing 10% fetal bovine serum, 100 units/ml penicillin, and 100 μg/ml streptomycin (growth medium) and differentiated into myotubes as previously described elsewhere (16). After 6 days, myotube differentiation was complete, and experimental procedures were initiated.
Glucose Uptake in L6 Cells—Glucose transport in L6 myotubes was performed as previously reported (16, 17). Briefly, L6 myotubes were re-fed with differentiation medium with HGF, vehicle, and/or inhibitors for different time points. Medium was changed to serum-free α-MEM with the same additions and cells cultured for 4 h. Uptake of 2-[3H]deoxyglucose was determined over 5 min in the presence of 10 μm (0.5 μCi/ml) 2-deoxyglucose. Protein content was measured by the bicinchoninic acid method (Pierce).
Glucose Utilization in L6 Cells—Glucose utilization was determined as previously described with some modifications (3). Briefly, L6 myotubes were treated with HGF or vehicle for 2 h in differentiation medium containing 6.5 μCi/ml [5-3H]glucose. Conditioned medium was then collected and [3H]H2O determined by the vapor-phase equilibration method. Protein content was measured as above.
[14C]Glucose Incorporation into Glycogen in L6 Cells—Glucose incorporation into glycogen was analyzed as previously described (16). Briefly, L6 myotubes were treated with HGF or vehicle in serum-free α-MEM for 2 h and a further 1 h in serum-free α-MEM containing 2 μCi/ml d-[U-14C]glucose in the presence or absence of insulin. After incubation with the isotope, cells were washed with ice-cold phosphate-buffered saline and solubilized in 1N NaOH at 50 °C for 1 h. An aliquot was then collected and unlabeled glycogen added as carrier. Glycogen was precipitated by the addition of pre-chilled ethanol and incubation at –20 °C for 30 min. Samples were centrifuged at 18,000 × g for 10 min at 4 °C, the pellets digested in 1N NaOH at 60 °C for 30 min, and an aliquot counted in a liquid scintillation counter. Protein content was measured as above.
FAO in L6 Cells—FAO was measured as described elsewhere (16). Briefly, L6 myotubes were incubated for 3 h with HGF or vehicle in differentiation medium containing 0.5 μCi/ml [9,10-3H]palmitic acid and 0.1 mm palmitate preconjugated with 0.625% (w/v) essentially fatty acid-free bovine serum albumin. FAO was determined by determining the [3H]H2O content in the conditioned medium by vapor-phase equilibration. Protein content was measured as above.
3H-Fatty Acid Incorporation into Total Lipids in L6 Cells—L6 myotubes were incubated for 3 h with HGF or vehicle in differentiation medium containing 0.5 μCi/ml [9,10-3H]palmitic acid and 0.1 mm palmitate pre-conjugated with 0.625% (w/v) essentially fatty acid-free bovine serum albumin. Total lipids were extracted as previously reported (16). Briefly, cell monolayers were harvested by scraping. After centrifugation, pellets were resuspended in phosphate-buffered saline and an aliquot was removed for protein assay. Methanol:chloroform (2:1) was added to the remaining material and vortexed. After centrifugation, chloroform:water (1:1) was added to supernatant and vortexed again. Phases were separated by centrifugation at 18,000 × g at room temperature, and the lower phase was collected. Samples were then dried and the pellets dissolved in chloroform and taken for liquid scintillation counting.
Carnitine Palmitoyltransferase (CPT) Assay in L6 Cells—Activities of CPT-I and CPT-II were determined by a radiochemical assay in the direction of acylcarnitine formation as described previously (18). Briefly, L6 myotubes, incubated for 3 h with HGF or vehicle, were harvested in a buffer containing 150 mm KCl, 5 mm Tris-HCl, pH 7.2, and broken with a glass homogenizer. Cell homogenates were used for CPT-I assays. For CPT-II assays, a portion of the homogenate was incubated for 5 min at 4 °C with 1% (w/v) octylglucoside, which inactivates CPT-I and releases CPT-II from the mitochondrial matrix in an active form.
Immunoblot Analysis—Soleus muscles obtained from 3-month-old C57BL/6J male mice and L6 myotubes incubated with HGF or vehicle for different time points in serum-free α-MEM were homogenized in lysis buffer (20 mm Tris-HCl, pH 7.5, 150 mm NaCl, 1 mm EDTA, 1 mm EGTA, 1% (v/v) Triton X-100, 2.5 mm sodium pyrophosphate, 1 mm β-glycerophosphate, 1 mm Na3VO4, 1 μg/ml leupeptin, 1 mm phenylmethylsulfonyl fluoride). After 10 min on ice, extracts were sonicated and centrifuged at 18,000 × g for 10 min at 4 °C. Pellets were discarded, and the solubilized proteins (∼20 μg/sample) were resolved by SDS-PAGE and electrotransferred onto polyvinylidene difluoride filters for immunoblotting by conventional means. Signals were detected by chemiluminescence (ECL Plus detection system; Amersham Biosciences), and band densitometry was quantitated with the NIH Image software. Immunoprecipitation experiments were performed with 1-mg protein extracts incubated overnight at 4 °C with the appropriate antibody and then with protein-G-agarose (Invitrogen) for 4 h before resuspension in Laemmli buffer and separation by SDS-PAGE.
Total membranes from L6 myotubes were prepared as described before (19). Briefly, cells were harvested by scraping. After centrifugation, pellets were resuspended in homogenization buffer (255 mm sucrose, 20 mm HEPES, pH 7.4, 2 mm EDTA with protease inhibitors), homogenized with 15 strokes, and then centrifuged at 700 × g for 5 min at 4 °C to remove nuclei and unbroken cells. The supernatant from this low speed spin was then centrifuged at 195,000 × g for 75 min at 4 °C to obtain total membranes. The final pellet was resuspended in homogenization buffer and used in Western blot analysis with specific antibodies against Glut-1 and Glut-4, kindly provided by Dr. A. Klip (Hospital for Sick Children, Toronto, ON, Canada).
Statistical Analysis—Data are expressed as means ± S.E. Unpaired two-tailed Student's t-tests or one-way analysis of variance followed by all pairwise multiple comparison procedures (Student-Newman-Keuls method) were used to determine statistical significance. Differences were considered significant at p < 0.05.
RESULTS
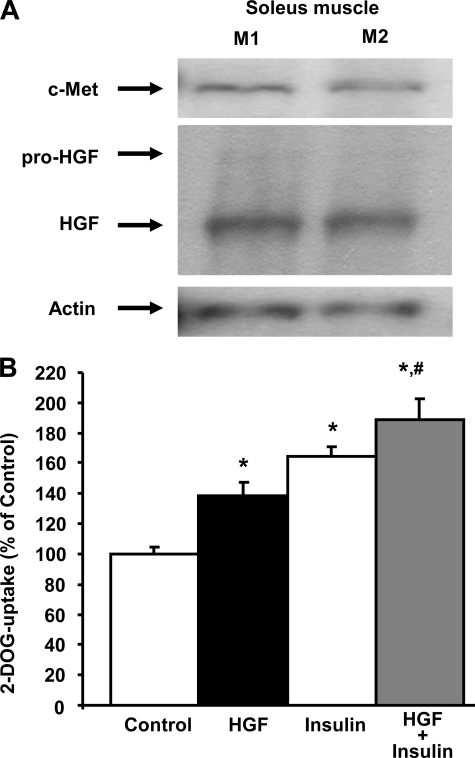
HGF Increases DOGU in Mouse Soleus Muscle—Western blot analysis of c-Met and HGF expression revealed that both receptor and ligand are present in mouse soleus muscle (Fig. 1A). Importantly, acute incubation of mouse soleus muscle ex vivo with HGF significantly increased DOGU, and this effect was similar and not significantly different from the effect of insulin (Fig. 1B). HGF and insulin combined significantly increased DOGU compared with the effect of these peptides alone (Fig. 1B). These results indicate that HGF can increase glucose transport in mouse soleus muscle ex vivo.
FIGURE 1.
HGF stimulates glucose uptake in mouse soleus muscle. A, Western blot analysis of HGF and c-Met expression in soleus muscle from two C57BL/6J male mice (M1 and M2). Actin expression was determined to ensure similar protein loading. B, effect of 25 ng/ml HGF and/or 1 milliunit/ml insulin on 2-[3H]deoxyglucose (DOG) uptake in mouse soleus muscle. Values are means ± S.E. of 6–8 muscles per condition. *, p < 0.05 versus control uptake; #, p < 0.05 versus uptake in muscles stimulated with HGF or insulin alone.
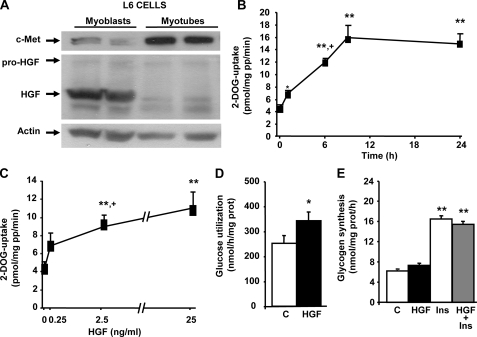
HGF Increases DOGU and Glucose Metabolism in L6 Myotubes—To analyze whether the HGF effect on DOGU was observed in differentiated skeletal muscle cells and to elucidate the mechanisms employed by HGF for this effect, we used rat L6 myotubes, a muscle-derived cell line known to be responsive to insulin (20). Analysis of c-Met and HGF expression in L6 myoblast and L6 myotubes indicated that both receptor and ligand are expressed in these cells (Fig. 2A). Interestingly, c-Met expression was increased, whereas HGF expression was decreased, in myotubes compared with their expression in undifferentiated myoblasts (Fig. 2A).
FIGURE 2.
HGF stimulates glucose uptake and utilization but does not alter glucose incorporation into glycogen in L6 myotubes. A, Western blot analysis of HGF and c-Met expression in two different cultures of L6 myoblasts before (left two lanes) and after differentiation into L6 myotubes (right two lanes). Actin expression was determined to ensure similar protein loading. HGF time (B) and dose (C) dependently stimulated glucose transport in L6 myotubes. Cells were incubated with HGF (25 ng/ml) for several time periods and with various concentrations for 24 h as indicated in the figure. Uptake of 2-[3H]deoxyglucose (DOG) was measured during a 5-min period as described under “Experimental Procedures.” D, HGF (25 ng/ml) increased glucose utilization in L6 myotubes. E, effect of HGF (25 ng/ml) and/or 100 nm insulin on [U-14C]glucose incorporation into glycogen in L6 myotubes. Values are means ± S.E. of at least four experiments performed in triplicate. *, p < 0.025 and **, p < 0.01 versus control or 0 value; +, p < 0.05 versus uptake in the previous time point or concentration.
HGF significantly increased DOGU in a time-dependent manner in L6 myotubes (Fig. 2B). DOGU significantly increased within 1 h of incubation (40–50%), and the effect was maximal at 9–24 h (∼4-fold) (Fig. 2B). Moreover, HGF increased DOGU dose dependently in L6 myotubes (Fig. 2C). Importantly, 2.5 ng/ml HGF, a dose similar to that present in the circulation of obese subjects (21), significantly increased (∼2-fold) glucose uptake in skeletal muscle cells, an effect not significantly enhanced by a higher HGF concentration (25 ng/ml) (Fig. 2C). HGF effect on DOGU was specific of L6 myotubes because no effect was observed in L6 myoblasts (not shown).
HGF-mediated increase in DOGU was accompanied by a significant augmentation in glucose utilization in L6 myotubes (Fig. 2D). On the other hand, HGF did not significantly alter lactate production (not shown) or glycogen synthesis in basal or insulin-stimulated conditions in these cells (Fig. 2E). Collectively, these results indicate that HGF is a potent stimulator of glucose transport and metabolism, but not storage, in skeletal muscle myotubes.
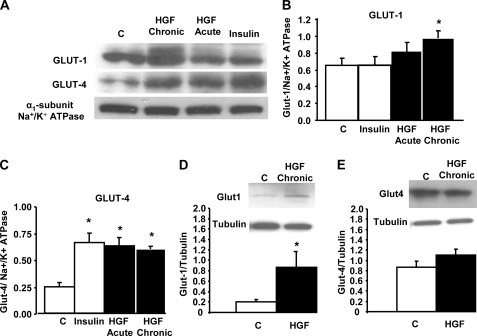
HGF Increases Glut-1 and Glut-4 Levels in Plasma Membranes of L6 Myotubes—To determine whether HGF-induced increase in DOGU correlated with an increase in the presence of glucose transporters in the plasma membrane of L6 myotubes, we analyzed Glut-1 and Glut-4 levels in membrane protein extracts prepared from these cells after treatment with HGF or insulin (Fig. 3, A–C). As shown in Fig. 3A, Glut-4 levels in L6 myotube membranes were increased following acute (1 h) or chronic (24 h) treatment with HGF, and this increase was similar to that induced by insulin. Quantitation of four different experiments revealed that Glut-4 levels in L6 myotube membranes were 2- to 3-fold increased by HGF treatment (Fig. 3C). On the other hand, Glut-1 levels were not significantly increased by insulin or acute treatment with HGF (Fig. 3B). Equally, acute treatment with HGF did not alter the expression of caveolin-3, another membrane marker, in L6 myotube membranes (not shown). However, chronic treatment with HGF significantly enhanced Glut-1 levels in L6 myotube membranes (Fig. 3B). Taken together, these results suggest that chronic HGF exposure enhances glucose transport in skeletal muscle cells by increasing the abundance of both Glut-1 and Glut-4 in the membrane of these cells. The increase in membrane-associated Glut-1 induced by chronic HGF treatment correlated with an up-regulation in Glut-1 total protein levels (Fig. 3D). However, total Glut-4 protein levels did not significantly increase following chronic HGF treatment (Fig. 3E), suggesting that the augmentation in membrane-associated Glut-4 protein is due to protein translocation and not to increased total protein expression.
FIGURE 3.
HGF increases the presence of glucose transporters in the plasma membrane of L6 myotubes. A, Western blot analysis of Glut-1 and Glut-4 expression in membrane protein extracts from L6 myotubes treated with 25 ng/ml HGF for 1 h (Acute) or 24 h (Chronic) and with 100 nm insulin for 30 min. Expression of the α1-subunit Na+/K+ ATPase was determined to ensure similar membrane protein loading. Densitometric analysis of Glut-1 (B) and Glut-4 (C) in four different experiments. Western blot analysis of Glut-1 (D) and Glut-4 (E) expression in total protein extracts from L6 myotubes treated with 25 ng/ml HGF for 24 h. Expression of tubulin was determined to ensure similar protein loading. The y-axis represents arbitrary units. Results are means ± S.E. *, p < 0.05 versus control (C).
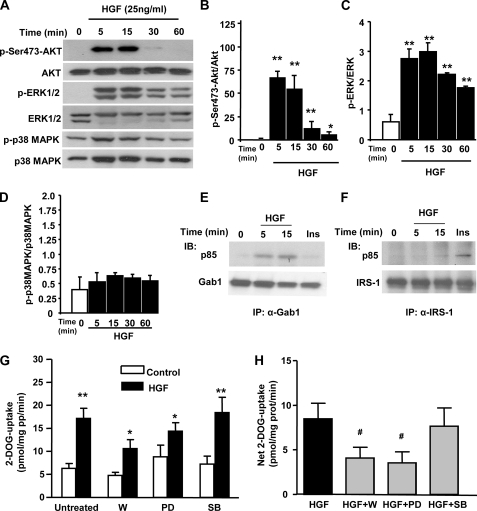
HGF-mediated DOGU in L6 Myotubes Is PI3K- and ERK1/2-dependent—We next explored signaling pathways downstream of c-Met in L6 myotubes (Fig. 4A). HGF rapidly, potently, and time dependently stimulated Akt phosphorylation in these cells (Fig. 4, A and B). Similarly, HGF also increased the phosphorylation of ERK1/2 in a time-dependent manner (Fig. 4, A and C). In contrast, HGF did not increase p38 MAPK phosphorylation in L6 myotubes (Fig. 4, A and D). As previously shown in adipocytes (6), HGF increased the association of PI3K to Gab-1 but not to IRS-1 (Fig. 4, E and F).
FIGURE 4.
Activation of PI3K and ERK1/2 is essential for HGF-induced stimulation on glucose transport in L6 myotubes. A, representative Western blot depicting the effects of HGF (25 ng/ml) on Ser-473-Akt, ERK1/2, and p38 MAPK phosphorylation in L6 myotubes. Densitometric analysis of Ser-473-Akt (B), ERK1/2 (C), and p38 MAPK (D) phosphorylation in four different experiments. The y-axis represents the ratio of phosphorylated versus total protein in arbitrary units. Results are means ± S.E. *, p < 0.05 and **, p < 0.01 versus time 0. Gab-1, in response to 25 ng/ml HGF (E), and IRS-1, in response to 100 nm insulin (20 min) (F), recruit the p85 subunit of PI3K. Representative autoradiographs of two independent experiments with identical results. G, uptake of 2-[3H]deoxyglucose (DOG) in L6 myotubes incubated for 24 h with 25 ng/ml HGF with or without 100 nm wortmannin (W), 10 μm PD98059 (PD), or 10 μm SB202190 (SB). H, net DOG uptake was calculated subtracting the corresponding basal from the HGF-stimulated uptake. Values are means ± S.E. of four different experiments in triplicate. *, p < 0.05 and **, p < 0.01 versus corresponding control. #, p < 0.05 versus HGF.
To analyze whether the inhibition of these signaling pathways could affect HGF-mediated DOGU in L6 myotubes, we incubated these cells with 100 nm wortmannin (PI3K inhibitor), 10 μm PD98059 (ERK1/2 inhibitor), or 10 μm SB202190 (p38 MAPK inhibitor) for 30 min before and during chronic incubation with HGF. As shown in Fig. 4, G and H, wortmannin and PD98059, but not SB202190, significantly inhibited the net increase in DOGU induced by HGF in L6 myotubes. However, the results with the ERK1/2 inhibitor should be taken with caution because basal glucose uptake was increased by the inhibitor, reducing the net glucose uptake induced by HGF. Collectively, these results indicate that HGF-induced activation of Akt, and potentially ERK1/2, is involved in HGF-mediated DOGU in skeletal muscle cells.
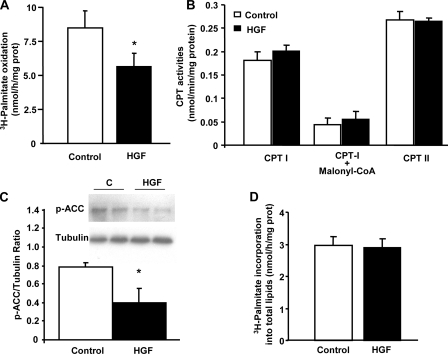
HGF Decreases FAO in L6 Myotubes—To determine whether HGF has any effect on FFA metabolism in skeletal muscle cells, we analyzed FAO rates in L6 myotubes incubated with HGF. As shown in Fig. 5A, HGF strongly and significantly inhibited FAO in L6 myotubes. HGF-induced inhibition in FAO was not the result of alterations in the intrinsic activity of the enzymes CPT-I and CPT-II (Fig. 5B), suggesting that the increase in glucose metabolism and potential malonyl-CoA formation could mediate the FAO inhibitory effect. Indeed, phosphorylation of acetyl-CoA carboxylase, the enzyme that catalyzes the ATP-dependent carboxylation of acetyl-CoA to form malonyl-CoA, was significantly diminished (∼50%) by HGF (Fig. 5C). Because phosphorylation of acetyl-CoA carboxylase inhibits its enzymatic activity, these results support the hypothesis that FAO is diminished by HGF through decreased acetyl-CoA carboxylase phosphorylation and enhanced malonyl-CoA levels in L6 myotubes. Interestingly, HGF-mediated inhibition of FAO did not result in detectable changes in total lipid formation in these cells (Fig. 5D).
FIGURE 5.
HGF inhibits fatty acid oxidation in L6 myotubes. A, oxidation of palmitate in L6 myotubes treated with 25 ng/ml HGF for 3 h. Values are means ± S.E. of four different experiments in triplicate. *, p < 0.05 versus control. B, CPT-I and CPT-II activity in total cellular extracts of L6 myotubes treated with 25 ng/ml HGF for 3 h. CPT-I activity was equally inhibited by 1 mm malonyl-CoA in protein extracts of L6 myotubes treated with or without HGF. Values are means ± S.E. of three different experiments in triplicate. C, Western blot analysis of acetyl-CoA carboxylase phosphorylation (Ser-79) in protein extracts from L6 myotubes treated with 25 ng/ml HGF for 3 h. The y-axis represents the ratio of phosphorylated acetyl-CoA carboxylase versus tubulin in arbitrary units. Values are means ± S.E. of four different experiments. *, p < 0.05 versus control. D, effect of 25 ng/ml HGF on palmitate-induced lipid accumulation in L6 myotubes. Values are means ± S.E. of four different experiments in triplicate. No significant differences were found following HGF treatment.
DISCUSSION
These studies clearly demonstrate a novel role for physiological or pathophysiological levels of HGF in modulating glucose transport, glucose metabolism, and FFA oxidation in skeletal muscle cells. Because HGF is locally produced and stored in skeletal muscle fibers (11, 13) and because HGF circulating levels are increased in obese subjects (21), it is possible that HGF participates in skeletal muscle glucose disposal in normal and obese conditions.
Although it has been known for many years that HGF/c-Met axis is active in skeletal muscle, where it participates in muscle development and regeneration after damage (11–14), no study has addressed whether HGF might have metabolic actions on differentiated mature skeletal muscle cells. It has recently been reported that HGF enhances glucose transport in pancreatic beta cells and adipocytes (3, 6). The current studies demonstrate for the first time that HGF also acutely increases glucose uptake in mouse soleus muscle ex vivo and rat L6 myotubes in vitro and that this effect is translated into an augmentation of glucose utilization, but not storage, in L6 myotubes. Furthermore, prolonged incubation with HGF leads to a severalfold enhancement in glucose transport activity in L6 myotubes. Taken together, these studies demonstrate that, in addition to behaving as a growth factor, HGF has additional biological features as a modulator of glucose metabolic flux in at least three different cell types (beta cells, adipocytes, and skeletal muscle cells) engaged in glucose homeostasis control.
We have also shown that HGF increases the association of PI3K to Gab-1, but not to IRS-1, and activates Akt and ERK1/2, but not p38 MAPK, signaling pathways in L6 myotubes. The activation of PI3K/Akt, and potentially ERK1/2, signaling pathway contributes to a maximal HGF-mediated glucose uptake in these cells. It is widely known that activation of the PI3K/Akt signaling pathway is essential for insulin-mediated Glut-4 trafficking to the plasma membrane and glucose uptake in skeletal muscle cells (22). Importantly, HGF increases the level of Glut-4 expression in L6 myotube plasma membranes without altering total Glut-4 expression levels and with potency similar to that of insulin. Therefore, it is likely that HGF-induced activation of Akt may contribute to the increase in Glut-4 presence in the membrane of skeletal muscle cells exposed acutely or chronically to this growth factor. However, HGF-mediated glucose transport in L6 myotubes is not completely abolished by wortmannin, suggesting that other pathways and glucose transporters might be involved in HGF-mediated effect in these cells. In contrast to Glut-4, which moves to the cell surface in response to stimulation by insulin and other factors, Glut-1 is stably located on the cell surface irrespective of stimulation and it is principally considered to be involved in basal glucose uptake (23). Acute incubation with HGF or insulin did not significantly alter the level of expression of Glut-1 in L6 myotube plasma membranes. Interestingly, however, chronic incubation with HGF significantly enhanced total Glut-1 expression and Glut-1 presence in the plasma membrane of these cells. Activation of ERK has been reported to up-regulate Glut-1 expression, thereby augmenting glucose transport (24, 25). Therefore, it is likely that HGF-mediated activation of ERK1/2 can contribute to the increased expression of Glut-1 in the plasma membrane of L6 cells. Collectively, these and previous results indicate that HGF regulates the expression and/or membrane localization of four isoforms encoded by the glucose transporter gene family: Glut-1 (skeletal muscle cells), Glut-2 (pancreatic beta cells) (3), Glut-4 (adipocytes and skeletal muscle cells) (6), and Glut-5 (intestinal epithelial cells) (5), highlighting a broad and underappreciated physiological role for HGF in the control of glucose transporter function.
Exercise increases glucose uptake and the expression and/or membrane localization of Glut-4 in skeletal muscle cells (22, 26). However, whether extrinsic local factors participate in the regulation of these events has not been explored in detail. Recent studies have shown that neuregulin-1, a member of the epidermal growth factor family of proteins, is a glucose transport activator present in myocytes as an inactive form (27). Interestingly, muscle contraction triggers the proteolytic activation of neuregulin-1 in muscle cells, where its binding to Erb-4 is important for muscle contraction-induced glucose transport (28). HGF is present in the extracellular matrix of skeletal muscle fibers in an active form, whence it is released following mechanical stretch (11–13). Therefore, it is possible that HGF might also participate in the increased Glut-4 expression and glucose uptake that occur in skeletal muscle following exercise.
Obesity is associated with insulin resistance. Several studies have demonstrated a close relationship between the impairment of insulin-mediated glucose uptake and deregulated lipid metabolism (7–10). A potential cause for these effects has been ascribed to the high plasma FFA levels frequently observed in obese/insulin-resistant conditions and the accumulation of harmful FFA-derived metabolites that have negative effects on insulin signaling in skeletal muscle (29–31). Adipose tissue production of HGF has been shown to contribute to remarkably elevated serum HGF levels in obese subjects (21, 32, 33), and HGF levels significantly decline with the weight loss and reduced body fat mass that occurs after gastroplasty (34). Based on these data, we wondered whether HGF might have any impact on FAO in skeletal muscle cells. HGF markedly and significantly decreased FAO in L6 myotubes. Because HGF potently increased glucose uptake and glucose utilization without altering glycogen synthesis, because CPT-I and CPT-II activities were normal, and because HGF decreased acetyl-CoA carboxylase phosphorylation, one potential explanation for HGF inhibitory effect on FAO could be that malonyl-CoA levels increase in these cells inhibiting CPT-I action (35). However, a potential decrease in FFA uptake induced by HGF could also play a role in this effect. Future studies will determine the exact mechanism/s involved in HGF inhibitory effects on FAO in skeletal muscle. Interestingly, HGF did not modify the incorporation of [3H]palmitate into total lipids in these cells. This result suggests that HGF did not alter total lipid formation in L6 myotubes. Nevertheless, this result does not rule out the possibility that HGF alters the formation of specific lipid pools within skeletal muscle cells. Indeed, HGF increases the synthesis of triglycerides, phospholipids, and cholesterol in cultured rat hepatocytes in vitro and in the liver of rats in vivo (36). However, these effects were observed only with very high doses of HGF, 100–1000 ng/ml in vitro and 1 mg/kg per day in vivo (36). Taken together, these results indicate that HGF affects FAO, potentially deregulating lipid metabolism in skeletal muscle cells.
Collectively, these studies indicate that HGF is a novel and potent stimulator of glucose uptake and an inhibitor of FAO in muscle cells. These results raise several important questions such as: What is the role of local HGF in skeletal muscle glucose disposal in vivo? What is the role of circulating HGF in skeletal muscle glucose transport in obesity? Is c-Met expression in skeletal muscle altered in obesity or exercise situations? Future in vivo studies will clarify whether HGF participates in glucose disposal by the skeletal muscle in resting, exercise, and obese conditions.
Acknowledgments
We thank Dr. Andrew F. Stewart and Dr. Rupangi C. Vasavada for thoughtful discussions of the ideas in this report, Dr. Amira Klip (Hospital for Sick Children, Toronto, Ontario, Canada) for providing L6 cells and the Glut-4 and Glut-1 antisera, and Nick Dedousis, Taylor Rosa, Jennifer Roccisana, and Ian Sipula for superb technical assistance.
This work was supported, in whole or in part, by National Institutes of Health (NIH) Grants DK067351 (to A. G.-O.), DK069363 (to B. A. B.), and DK058855 (to R. M. O.) and by a Pilot and Feasibility grant from the Obesity and Nutrition Research Center, University of Pittsburgh (supported by NIH Grant DK46204 to G. P.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: HGF, hepatocyte growth factor; DOGU, deoxyglucose uptake; FAO, fatty acid oxidation; PI3K, phosphatidylinositol 3-kinase; ERK, extracellularly regulated kinase; MAPK, mitogen-activated protein kinase; CPT, carnitine palmitoyltransferase; PBS, phosphate-buffered saline; FFA, free fatty acid; α-MEM, α-minimal essential medium.
References
- 1.Nakamura, T., Nishizawa, T., Hagiya, M., Seki, T., Shimonishi, M., Sugimura, A., Tashiro, K., and Shimizu, S. (1989) Nature 342 440–443 [DOI] [PubMed] [Google Scholar]
- 2.Stuart, K. A., Riordan, S. M., Lidder, S., Crostella, L., Williams, R., and Skouteris, G. G. (2000) Int. J. Exp. Pathol. 81 17–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garcia-Ocana, A., Vasavada, R. C., Cebrian, A., Reddy, V., Takane, K. K., Lopez-Talavera, J. C., and Stewart, A. F. (2001) Diabetes 50 2752–2762 [DOI] [PubMed] [Google Scholar]
- 4.Roccisana, J., Reddy, V., Vasavada, R. C., Gonzalez-Pertusa, J. A., Magnuson, M. A., and Garcia-Ocana, A. (2005) Diabetes 54 2090–2102 [DOI] [PubMed] [Google Scholar]
- 5.Kato, Y., Yu, D., and Schwartz, M. Z. (1998) J. Pediatr. Surg. 33 13–15 [DOI] [PubMed] [Google Scholar]
- 6.Bertola, A., Bonnafous, S., Cormont, M., Anty, R., Tanti, J. F., Tran, A., Le Marchand-Brustel, Y., and Gual, P. (2007) J. Biol. Chem. 282 10325–10332 [DOI] [PubMed] [Google Scholar]
- 7.Blaak E. E. (2005) Best Pract. Res. Clin. Endocrinol. Metab. 19 391–403 [DOI] [PubMed] [Google Scholar]
- 8.Boden, G., Jadali, F., White, J., Liang, Y., Mozzoli, M., Chen, X., Coleman, E., and Smith, C. (1991) J. Clin. Investig. 88 960–966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Magnan, C., Gilbert, M., and Kahn, B. B. (1996) Lipids 31 1141–1149 [DOI] [PubMed] [Google Scholar]
- 10.Dresner, A., Laurent, D., Marcucci, M., Griffin, M. E., Dufour, S., Cline, G. W., Slezak L. A., Andersen, D. K., Hundal, R. S., Rothman, D. L., Petersen, K. F., and Shulman, G. I. (1999) J. Clin. Investig. 103 253–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tatsumi, R., Anderson, J. E., Nevoret, C. J., Halevy, O., and Allen, R. E. (1998) Dev. Biol. 194 114–128 [DOI] [PubMed] [Google Scholar]
- 12.Tatsumi, R., Hattori, A., Ikeuchi, Y., Anderson, J. E., and Allen, R. E. (2002) Mol. Biol. Cell 13 2909–2918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tatsumi, R., and Allen, R. E. (2004) Muscle Nerve 30 654–658 [DOI] [PubMed] [Google Scholar]
- 14.Bladt, F., Riethmacher, D., Isenmann, S., Aguzzi, A., and Birchmeier, C. (1995) Nature 376 768–771 [DOI] [PubMed] [Google Scholar]
- 15.Buettner, R., Newgard, C. B., Rhodes, C. J., and O'Doherty, R. M. (2000) Am. J. Physiol. 278 E563–E569 [DOI] [PubMed] [Google Scholar]
- 16.Perdomo, G., Commerford, S. R., Richard, A. M., Adams, S. H., Corkey, B. E., O'Doherty, R. M., and Brown, N. F. (2004) J. Biol. Chem. 279 27177–27186 [DOI] [PubMed] [Google Scholar]
- 17.Sinha, S., Perdomo, G., Brown, N. F., and O'Doherty, R. M. (2004) J. Biol. Chem. 279 41294–41301 [DOI] [PubMed] [Google Scholar]
- 18.Brown, N. F. (2003) Methods Mol. Biol. 228 281–301 [DOI] [PubMed] [Google Scholar]
- 19.Mitsumoto, Y., Burdett, E., Grant, A., and Klip, A. (1991) Biochem. Biophys. Res. Commun. 175 652–659 [DOI] [PubMed] [Google Scholar]
- 20.Niu, W., Huang, C., Nawaz, Z., Levy, M., Somwar, R., Li, D., Bilan, P. J., and Klip, A. (2003) J. Biol. Chem. 278 17953–17962 [DOI] [PubMed] [Google Scholar]
- 21.Rehman, J., Considine, R. V., Bovenkerk, J. E., Li, J., Slavens, C. A., Jones, R. M., and March, K. L. (2003) J. Am. Coll. Cardiol. 41 1408–1413 [DOI] [PubMed] [Google Scholar]
- 22.Huang, S., and Czech, M. P. (2007) Cell Metab. 5 237–252 [DOI] [PubMed] [Google Scholar]
- 23.Burant, C. F., Sivitz, W. I., Fukumoto, H., Kayano, T., Nagamatsu, S., Seino, S., Pessin, J. E., and Bell, G. I. (1991) Recent Prog. Horm. Res. 47 349–387 [DOI] [PubMed] [Google Scholar]
- 24.Birnbaum, M. J., Haspel, H. C., and Rosen, O. M. (1987) Science 235 1495–1498 [DOI] [PubMed] [Google Scholar]
- 25.Flier, J. S., Mueckler, M. M., Usher, P., and Lodish, H. F. (1987) Science 235 1492–1495 [DOI] [PubMed] [Google Scholar]
- 26.Hayashi, T., Wojtaszewski, J. F., and Goodyear, L. J. (1997) Am. J. Physiol. 273 E1039–E1051 [DOI] [PubMed] [Google Scholar]
- 27.Canto, C., Suarez, E., Lizcano, J. M., Grino, E., Shepherd, P. R., Fryer, L. G., Carling, D., Bertran, J., Palacin, M., Zorzano, A., and Guma, A. (2004) J. Biol. Chem. 279 12260–12268 [DOI] [PubMed] [Google Scholar]
- 28.Canto, C., Chibalin, A. V., Barnes, B. R., Glund, S., Suarez, E., Ryder, J. W., Palacin, M., Zierath, J. R., Zorzano, A., and Guma, A. (2006) J. Biol. Chem. 281 21690–21697 [DOI] [PubMed] [Google Scholar]
- 29.Schmitz-Peiffer, C., Browne, C. L., Oakes, N. D., Watkinson, A., Chisholm, D. J., Kraegen, E. W., and Biden, T. J. (1997) Diabetes 46 169–178 [DOI] [PubMed] [Google Scholar]
- 30.Turinsky, J., Bayly, B. P., and O'Sullivan, D. M. (1990) J. Biol. Chem. 265 7933–7938 [PubMed] [Google Scholar]
- 31.Hulver, M. W., Berggren, J. R., Cortright, R. N., Dudek, R. W., Thompson, R. P., Pories, W. J., MacDonald, K. G., Cline, G. W., Shulman, G. I., Dohm, G. L., and Houmard, J. A. (2003) Am. J. Physiol. 284 E741–E747 [DOI] [PubMed] [Google Scholar]
- 32.Bell, L. N., Ward, J. L., Degawa-Yamauchi, M., Bovenkerk, J. E., Jones, R., Cacucci, B. M., Gupta, C. E., Sheridan, C., Sheridan, K., Shankar, S. S., Steinberg, H. O., March, K. L., and Considine, R. V. (2006) Am. J. Physiol. 291 E843–E848 [DOI] [PubMed] [Google Scholar]
- 33.Fain, J. N., Madan, A. K., Hiler, M. L., Cheema, P., and Bahouth, S. W. (2004) Endocrinology 145 2273–2282 [DOI] [PubMed] [Google Scholar]
- 34.Swierczynski, J., Korczynska, J., Goyke, E., Adrych, K., Raczynska, S., and Sledzinski, Z. (2005) Obes. Surg. 15 803–808 [DOI] [PubMed] [Google Scholar]
- 35.McGarry, J. D., and Brown, N. F. (1997) Eur. J. Biochem. 244 1–14 [DOI] [PubMed] [Google Scholar]
- 36.Shiota, A., Yamashita, Y., Fujise, N., Masunaga, H., Yasuda, H., and Higashio, K. (2000) Pharmacol. Res. 42 443–452 [DOI] [PubMed] [Google Scholar]