Abstract
Photosynthetic (Ps) growth of purple non-sulfur bacteria such as Rhodobacter capsulatus depends on the cyclic electron transfer (ET) between the ubihydroquinone (QH2): cytochrome (cyt) c oxidoreductases (cyt bc1 complex), and the photochemical reaction centers (RC), mediated by either a membrane-bound (cyt cy) or a freely diffusible (cyt c2) electron carrier. Previously, we constructed a functional cyt bc1-cy fusion complex that supported Ps growth solely relying on membrane-confined ET (Lee, D.-W., Ozturk, Y., Mamedova, A., Osyczka, A., Cooley, J. W., and Daldal, F. (2006) Biochim. Biophys. Acta 1757 ,346 -352). In this work, we further characterized this cyt bc1-cy fusion complex, and used its derivatives with shorter cyt cy linkers as “molecular rulers” to probe the distances separating the Ps components. Comparison of the physicochemical properties of both membrane-embedded and purified cyt bc1-cy fusion complexes established that these enzymes were matured and assembled properly. Light-activated, time-resolved kinetic spectroscopy analyses revealed that their variants with shorter cyt cy linkers exhibited fast, native-like ET rates to the RC via the cyt bc1. However, shortening the length of the cyt cy linker decreased drastically this electronic coupling between the cyt bc1-cy fusion complexes and the RC, thereby limiting Ps growth. The shortest and still functional cyt cy linker was about 45 amino acids long, showing that the minimal distance allowed between the cyt bc1-cy fusion complexes and the RC and their surrounding light harvesting proteins was very short. These findings support the notion that membrane-bound Ps components form large, active structural complexes that are “hardwired” for cyclic ET.
Gram-negative, purple, non-sulfur, facultative phototrophic α-proteobacteria of Rhodobacter species provide excellent model systems for studying photosynthetic (Ps)4 and respiratory electron transfer (ET) chains (1-4). Among them, Rhodobacter capsulatus uses both a freely diffusible and a membrane-anchored cytochrome (cyt) (c2 and cy, respectively) electron carrier between the ubihydroquinone (QH2):cyt c oxidoreductase (cyt bc1 complex) and either the photochemical reaction center (RC) or the cbb3-type cyt c oxidase (Cox) under Ps or respiratory growth conditions, respectively (5-7). In contrast, the closely related Rhodobacter sphaeroides relies exclusively on the soluble cyt c2 for its Ps growth (8), even though it has a membrane-bound cyt cy that is functional only under respiratory growth conditions (9). R. capsulatus cyt cy is 199 amino acids long, with its NH2-terminal 28 residues corresponding to an unprocessed signal sequence-like membrane anchor and COOH-terminal 100 residues (Thr99 to Arg199) to a monoheme cyt c domain (10). The remaining 70-residues (Asn28 to Thr98) long Ala and Pro-rich region of cyt cy forms a linker attaching the cyt c domain to the membrane anchor and allowing its observed mobility (11). Noticeably, the linker of R. capsulatus cyt cy is markedly longer than that of R. sphaeroides and other non-Ps bacterial cyt cy (Fig. 1A). A seminal finding was that R. sphaeroides mutants lacking cyt c2 can be complemented for Ps growth by R. capsulatus cyt cy (12), pointing out that the cyt cy linker length might be critical for its electron carrier function during Ps cyclic ET.
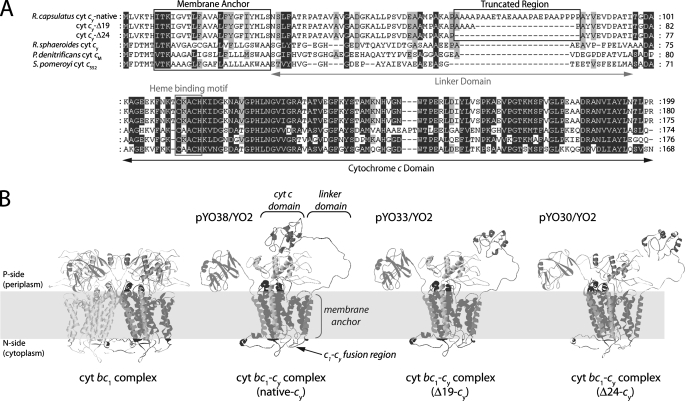
FIGURE 1.
A, amino acid sequence alignments of R. capsulatus cyt cy and its shorter linker variants with its homologues in other species, R. capsulatus cyt cy (native linker) (CAA79860); R. capsulaturs cyt cy (Δ19); R. capsulatus cyt cy (Δ24); R. sphaeroides cyt cy (AAC26877); P. denitrificans cyt cM (CAA49830); and S. pomeroyi DSS-3 cyt c552 (AAV96763). B, hypothetical three-dimensional structural models of the R. capsulatus cyt bc1-cy fusion complex and its shorter linker derivatives. The cyt c domain of R. capsulatus cy was modeled using SWISS-MODEL, and the overall structures were visualized using the R. capsulatus cyt bc1 (Protein Data Bank 1ZRT) and yeast cyt bc1:cyt c co-crystal (PDB 1NTK) structures.
Earlier studies, regarding the rate of ET mediated by cyt cy from the cyt bc1 complex to the RC, and the correlative presence of the cyt bc1 complex with cyt cy in membranes (7, 13), suggested that these proteins must be in close proximity to one another and to the RC and its light harvesting (LH) complexes. Occurrence of larger, nontransient macromolecular structures, ensuring efficient substrate channeling, catalytic enhancement, and sequestration of reactive intermediates during electron transport have been proposed to occur in membranes of purple bacteria (14), plants (15), and mitochondria (16). However, as the membrane-embedded components can diffuse independently from one another within lipid bilayers, differentiating between static “hardwired” electron transport complexes from those undergoing random collisions (17) is difficult to document in Ps membranes (18). In the accompanying work (38), we have demonstrated by creating soluble variants of cyt cy (cyt S-cy) that this electron carrier needs not be membrane anchored to support Ps growth of R. capsulatus. In this work, we have exploited the functional cyt bc1-cy fusion complex that we constructed earlier by fusing genetically cyt cy to the cyt bc1 complex (19) to probe the physical proximities of the Ps components to one another in R. capsulatus. We surmised that if the native length of the cyt cy linker is optimized for efficient electronic coupling across the distance between the cyt bc1 complex and the RC, then progressively shortening it might divulge the minimal distances separating these physiological partners in Ps membranes. Characterization of the physicochemical properties of both membrane-embedded and highly purified cyt bc1-cy fusion complex variants indicate that the shortest functional cyt cy linker is about 45 residues long. This short linker still insures rapid ET from the cyt bc1 to the RC, but exhibits decreased electronic coupling to the RC, thereby limiting cyclic ET and Ps growth. Thus, Ps cyclic ET components appear to be tightly packed together, forming membrane-embedded large structural complexes.
EXPERIMENTAL PROCEDURES
Bacterial Strains and Growth Conditions—Bacterial strains and plasmids used in this study are listed in Table 1. R. capsulatus strains were grown at 35 °C in mineral-peptone-yeast extract-enriched media (MPYE) supplemented with antibiotics as needed (10, 2.5, and 10 μg/ml kanamycin, tetracycline, and spectinomycin, respectively) under either semiaerobic/dark (respiratory), or photoheterotrophic/light (Ps) conditions using anaerobic jars and H2 + CO2 generating gas packs (BD Biosciences), as described previously (20). Ps growth curves were obtained using screw-cap tubes, incubated at 35 °C in an aquarium filled with water and illuminated by tungsten (Lumiline, Sylvania) lamps at an intensity of 150 μE/m2/s. Culture turbidity was monitored with a Klett-Summerson photometer equipped with a red (number 66) filter as described (21).
TABLE 1.
R. capsulatus strains used
| Strain R. capsulatus | Genotype | Phenotype | Ref. |
|---|---|---|---|
| MT-G4/S4 | crtD121 RifR, Δ (cycA::kan) | Cyt c2−, KanR, Ps+ | 5 |
| YO2 | crtD121 RifR, Δ(petABC::gen), Δ(cycA::kan), Δ(cycY::spe) | Cyt c2−, cyt cy−, cyt bc1−, Gen R, Kan R, Spe R, Ps− | 19 |
| pMTS1/MT-RBC1 | MT-RBC1 strain (crtD121 RifR, Δ(petABC::spe), cyt bc1−, SpeR, Ps-) with an expression plasmid carrying petABC | Cyt bc1+, KanR, Ps+ | 37 |
| pYO38/YO2 | YO2 strain with an expression plasmid carrying petABC::cycY-FLAG (native cyt cy) | Cyt bc1-cy+, TetR, Ps+ | 19 |
| pYO33/YO2 | YO2 strain with an expression plasmid carrying petABC::cycY (ΔAla67-Pro87)-FLAG | Cyt bc1-cy+ (Δ19 amino acid), TetR, Ps+ | This work |
| pYO30/YO2 | YO2 strain with an expression plasmid carrying petABC::cycY (ΔAla63-Pro88)-FLAG | Cyt bc1-cy+ (Δ24 amino acid), TetR, Ps+ | This work |
Molecular Genetic Techniques—PCR amplification of a 0.7-kb carboxyl-terminal FLAG epitope-tagged allele of cycY on plasmid pHM7 (10) using mutagenic primers YO5EcoRV (5′-GCCGGGGGATATCTGCTCGTCAAGACGCACATC-3′) and YO6HindIII (5′-GCCGGGGCAAGCTTGCAAAGATGTGAGGGC-3′) replaced the initiating methionine (ATG) of cycY with leucine (CTG). This PCR product was then digested with EcoRV and HindIII restriction enzymes and ligated into the StuI and HindIII sites of plasmid pMTS1 (20), to yield pYO37 containing a petABC::cycY fusion without the stop codon (TGA) of petC (cyt c1). The BamHI-HindIII fragment of pYO37 was transferred into the same sites of the broad host range plasmid pRK415, yielding pYO38 (Table 1). Similarly, two additional in-frame deletion derivatives of pYO38 (Δ19 and Δ24 in the linker region of cyt cy) of the cycY-FLAG fusion were obtained by appropriate PCR amplification of the 0.7-kb carboxyl-terminal FLAG epitope-tagged version of cycY, yielding pYO32 (petABC::cycY-FLAG-Δ19) and pYO29 (petABC::cycY-FLAG-Δ24), respectively, and transferred to the BamHI-HindIII sites of pRK415 to yield pYO33 and pYO30, respectively. All constructs were subsequently verified by DNA sequencing.
A Δ(petABC::gen) insertion-deletion allele was constructed by replacing the 2.4-kb ApaLI-StuI fragment of petABC in plasmid pMTS1 with the 1.2-kb HindIII-BamHI fragment containing the gentamycin resistance gene by blunt end ligation, yielding pYO34. This Δ(petABC::gen) allele was then transferred using Gene Transfer Agent into the chromosome of R. capsulatus strain FJ2 (ΔcycA, ΔcycY) (5) to yield the triple mutant YO2, lacking both the cyt bc1 complex and electron carrier cyts c2 and cy.
Biochemical Techniques—Intracytoplasmic (chromatophore) membranes were prepared as described previously (19), except where noted 1 mm ε-aminocaproic acid and 100 mm EDTA were added to minimize proteolysis following cell disruption. The R. capsulatus cyt bc1 complex was purified as described previously (22). Protein concentrations were determined using the bicinchoninic acid method (23) with bovine serum albumin as a standard. SDS-PAGE (15%) were run as described in Ref. 24, and prior to loading, samples were solubilized in 62.5 mm Tris-HCl (pH 6.8), 2% SDS, 0.1 m dithiothreitol, 25% glycerol, and 0.01% bromphenol blue with subsequent incubation at 60 °C for 10 min. Cytochromes c were visualized by their heme peroxidase activities using 3,3′,5,5′-tetramethylbenzidine (TMBZ) and H2O2 according to Thomas et al. (25).
Spectroscopic Techniques—Optical spectra were recorded on a PerkinElmer UV-visible spectrophotometer Lambda 20. Absorption difference spectra for the c- and b-type cytochromes were obtained using chromatophore membranes (0.3 mg of total protein/ml), oxidized by adding a crystal of potassium ferricyanide, and reduced by a few grains of either solid sodium ascorbate or sodium dithionite, as appropriate. Time-resolved, light-activated kinetic spectroscopy was performed on a dual wavelength kinetic spectrophotometer with chromatophore membranes resuspended in 50 mm MOPS buffer containing 100 mm KCl (pH 7.0) in the presence of the following redox mediators (with their respective midpoint redox potential, Em,7): 100 μm ferricyanide (430 mV), 8 μm 2,3,5,6-tetramethyl-p-phenylenediamine (260 mV), 6 μm 1,2-naphthoquinone (NQ, 145 mV), 1 μm phenazine methosulfate (80 mV), 1 μm phenazine ethosulfate (50 mV), 6 μm 2-hydroxy-1,4-naphthoquinone (HNQ, -145 mV), 6 μm benzyl viologen (-359 mV), and a membrane potential uncoupler (2.5 μm valinomycin), as described (26). The amount of chromatophore membranes used in each assay was normalized to the RC content, as determined by measuring the flash-induced optical absorbance difference between 605 and 540 nm at an Eh of 380 mV, and using an extinction coefficient of 29.8 mm-1 cm-1. Transient cyt c re-reduction and cyt b reduction kinetics at an ambient potential of 100 mV, initiated by a short saturating flash (∼8 μs) from a xenon lamp were followed at 550-540 and 560-570 nm, respectively. Antimycin, myxothiazol, and stigmatellin were used as indicated at 5, 5, and 1 μm, respectively.
Optical potentiometric titrations were performed with the purified cyt bc1-cy fusion complex (0.1 mg/ml) in 50 mm MOPS buffer (100 mm KCl, pH 7.0) with the following mediators: 20 μm tetrachlorohydroquinone (350 mV), 20 μm 2,3,5,6-tetramethyl-p-phenylenediamine, 20 μm 1,2-naphthoquinone 4-sulfonate (210 mV), 20 μm NQ, 10 μm phenazine methosulfate, 10 μm phenazine ethosulfate, 40 μm duroquinone (5 mV), 20 μm pyocyanine (PCN, -34 mV), 6 μm indigotrisulfonate (-90 mV), 20 μm HNQ, 20 μm anthroquinone 2-sulfonate (-225 mV). The optical changes that accompanied the Eh changes were recorded in the α-band region (500 to 600 nm), and the Em values were determined by fitting the normalized absorption data to a single component n = 1 Nernst equation. EPR spectroscopy was performed at sample temperatures of 10 or 20 K using a Bruker ESP 300E spectrometer (Bruker Biosciences), fitted with an Oxford instruments ESR-9 helium cryostat (Oxford Instrumentation Inc.). Spectrometer settings were as indicated in the appropriate figure legends.
Purification of the Cyt bc1-cy Fusion Complex—The R. capsulatus cyt bc1-cy fusion complex was purified using pYO38/YO2. Chromatophore membranes (5.9 g wet weight) were obtained from frozen washed cells (81 g wet weight) after two passages through a French pressure cell and collected by ultracentrifugation (20). Chromatophore pellets were resuspended to a final protein concentration of 18 mg/ml in 50 mm phosphate buffer (pH 8.0) containing 7.2 mm NaCl, 20% glycerol, 1 mm phenylmethylsulfonyl fluoride, 100 mm EDTA, 10 protease inhibitor mixture tablets (Roche Applied Science) and 1 mm ε-aminocaproic acid. Dodecyl maltoside (DDM, 20% w/v stock solution) was added dropwise to this suspension to a final concentration of 1 mg of DDM/mg of total proteins. The mixture was stirred gently for 1 h at 4 °C, and then ultracentrifuged (120,000 × g for 2 h) to eliminate non-dispersed membranes. The supernatant was loaded onto a Q-Sepharose ff column (2.6 × 32 cm) pre-equilibrated with 50 mm phosphate buffer (pH 8.0) containing 20% glycerol, 0.01% (w/v) DDM, and 7.2 mm NaCl (Buffer A). The column was washed with 5 to 6 column volumes (CVs) of Buffer A containing 400 mm NaCl, and then the remaining photosynthetic pigments were washed with 3 to 4 CVs of the same buffer containing 0.05% (w/v) DDM until a red band on top of the column became visible. The adsorbed cyt bc1-cy fusion proteins were eluted with 4 CVs of a linear 400-800 mm NaCl gradient in the presence of 0.05% (w/v) DDM. Fractions were monitored for their absorption at 280 and 420 nm, and 500 to 600 nm for their dithionite-reduced minus ferricyanide-oxidized optical difference spectra, and those containing the highest concentrations of c-type and b-type cytochromes were pooled, concentrated using an Amicon Diaflo apparatus equipped with a PM30 membrane. The concentrated sample (∼33 ml) was passed twice through an anti-FLAG M2 affinity column (5 ml), pre-equilibrated with 10 CVs of 50 mm phosphate buffer (pH 8.0) containing 150 mm NaCl and 0.01% (w/v) DDM. The column was then washed with 20 CVs of the same buffer, and eluted with 6 CVs of 100 mm glycine-HCl buffer (pH 3.5) containing 0.01% (w/v) DDM. Eluents were collected into vials containing 1 m Tris-HCl buffer (pH 8.0) to a final concentration of 50 mm. Fractions containing the cyt bc1-cy fusion complex were pooled, buffer exchanged with 50 mm phosphate buffer (pH 8.0) containing 0.01% (w/v) DDM using Amicon Ultra (50,000 Mr cut off) centrifugal filter devices (Millipore Co., Ireland), and stored at -80 °C in the presence of 20% glycerol until further use.
RESULTS
Design of the Cyt bc1-cy Fusion Complexes with Shorter Cyt cy Linker Lengths—The availability of a functional cyt bc1-cy fusion complex (19) allowed us to probe whether the cyt cy linker could be used as a “molecular ruler” to estimate the distances between the Ps ET components. Comparison of R. capsulatus cyt cy with its counterparts from other species indicated that the cyt c domain is highly similar to that from Silicibacter pomeroyi (71%), Paracoccus denitrificans (70%), and R. sphaeroides (65%). On the other hand, the linker regions of P. denitrificans, R. sphaeroides, and S. pomeroyi are about 20 to 30 residues shorter than that of R. capsulatus (Fig. 1A). Generation of computer-assisted hypothetical three-dimensional structures of the cyt bc1-cy fusion complexes with shorter linkers directed us to the region between amino acids 65 and 90 of cyt cy to mimic its non-functional variants as Ps electron carriers (Fig. 1B). Considering that the R. sphaeroides cyt cy linker is 26 amino acids shorter than its R. capsulatus counterpart, plasmids pYO30 (with a 24-amino acid long deletion between positions Ala63 and Pro88 of cyt cy) (Δ24-cy) and pYO33 (with a 19-amino acid long deletion between the positions Ala67 and Pro88 of cyt cy) (Δ19-cy) were constructed to determine the Ps ET of each electron carrier (Table 1).
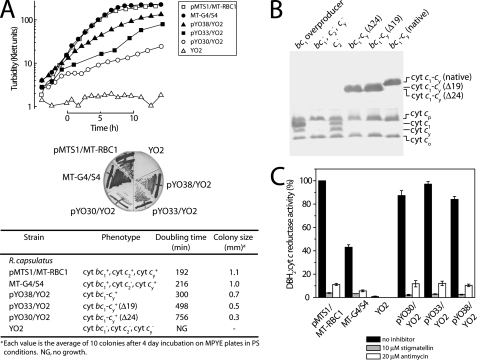
Phenotypic Characterization of R. capsulatus Strains Harboring Cyt bc1-cy Fusion Complex Variants with Shorter Cyt cy Linkers—Plasmids pYO30 (Δ24-cy) and pYO33 (Δ19-cy) were introduced into the R. capsulatus mutant YO2 (lacking the cyts bc1, c2, and cy), and the Ps growth abilities of the resulting strains were examined (Fig. 2A). On enriched MPYE medium under Ps conditions, R. capsulatus strain pMTS1/MT-RBC1 overproducing cyt bc1 complex, MT-G4/S4 lacking the cyt c2, and pYO38/YO2 harboring an intact cyt bc1-cy fusion complex exhibited doubling times of about 192, 216, and 300 min, respectively (Fig. 2A). However, under similar conditions, pYO33/YO2 (Δ24-cy) and pYO30/YO2 (Δ19-cy) grew markedly slower than pYO38/YO2 (native-cy) (756 and 498 versus 300 min, respectively). As the mutant YO2 cannot be complemented for Ps growth by either the cyt bc1 complex or cyt cy alone, the data indicated that the cyt bc1-cy fusion complexes with shorter cyt cy linkers provided both the oxidoreductase and electron carrier functions required for Ps growth. However, the slower growth rates suggested that shortening the cyt cy linker hampered the growth abilities of PS.
FIGURE 2.
A, photosynthetic growth properties on liquid and solid media (enriched MPYE) of various R. capsulatus strains (YO2 lacking the cyt bc1, cyt cy, and cyt c2; pMTS1/MT-RBC1 overproducing the cyt bc1 complex, MT-G4/S4 lacking the cyt c2, and pYO38/YO2, pYO33/YO2, and pYO30/YO2 containing the cyt bc1-cy fusion complexes with the native 19 amino acids and 24-amino acid shorter cyt cy linkers, respectively) were determined by monitoring the turbidity of the cultures, as described under “Experimental Procedures.” B, the c-type cytochrome profiles of the same strains were revealed using chromatophore membranes (100 μg of total proteins per lane) and 15% SDS-PAGE/TMBZ analyses. C, DBH2:cyt c reductase activities of chromatophore membranes (20 μg of total proteins) derived from the same strains described above were determined as in Atta-Asafo-Adjei and Daldal (20), in the absence (no inhibitor) or presence (10 μm stigmatellin or 20 μm antimycin), and for comparative purposes the steady-state enzymatic activities are represented as % of the overproduced native cyt bc1 complex.
Prosthetic Group Insertion, Enzymatic Activity, and Subunit Assembly of the Cyt bc1-cy Fusion Complexes—As the slower Ps growth might also be attributed to decreased amounts of cyt bc1-cy fusion complexes, chromatophore membranes of appropriate strains were examined by TMBZ/SDS-PAGE analyses (Fig. 2B). Typical membrane-associated c-type cytochrome profiles comprised of the cyts cp (32 kDa), c1 (30 kDa), cy (29 kDa), and co (28 kDa) were observed for pMTS1/MT-RBC1 and MT-G4/S4, whereas the YO2-derived samples contained only cyts cp and co (both subunits of Cox) as expected. On the other hand, pYO38/YO2, pYO33/YO2, and pYO30/YO2 harboring cyt bc1-cy fusion complexes with shorter cyt cy linkers had both cyts c1 and cy bands replaced by a single peroxidase-active band at 61, 59, and 58 kDa, respectively, corresponding to their cyt c1-cy fusion subunits.
Steady-state enzymatic activities of the cyt bc1 complexes were also assayed by measuring DBH2-dependent reduction of horse heart cyt c (Fig. 2C). pYO38/YO2, pYO33/YO2, and pYO30/YO2 harboring cyt bc1-cy fusion complexes with different lengths of cyt cy linkers showed very similar levels of DBH2: cyt c reductase activities as compared with that of the pMTS1/MT-RBC1 overproducing a native cyt bc1 complex. Therefore, neither fusing cyt cy to cyt c1, nor changing the length of the cyt cy linker region significantly affected the cyt bc1 complex enzymatic activity of various cyt bc1-cy fusion complexes with different cyt cy linker lengths.
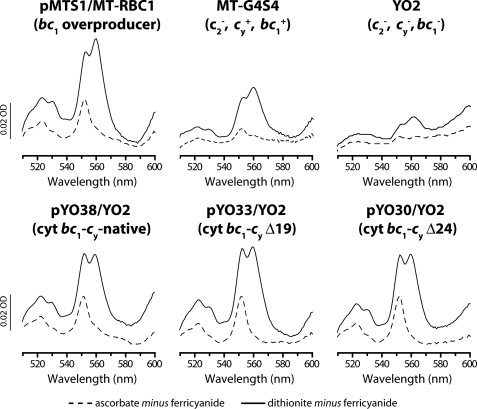
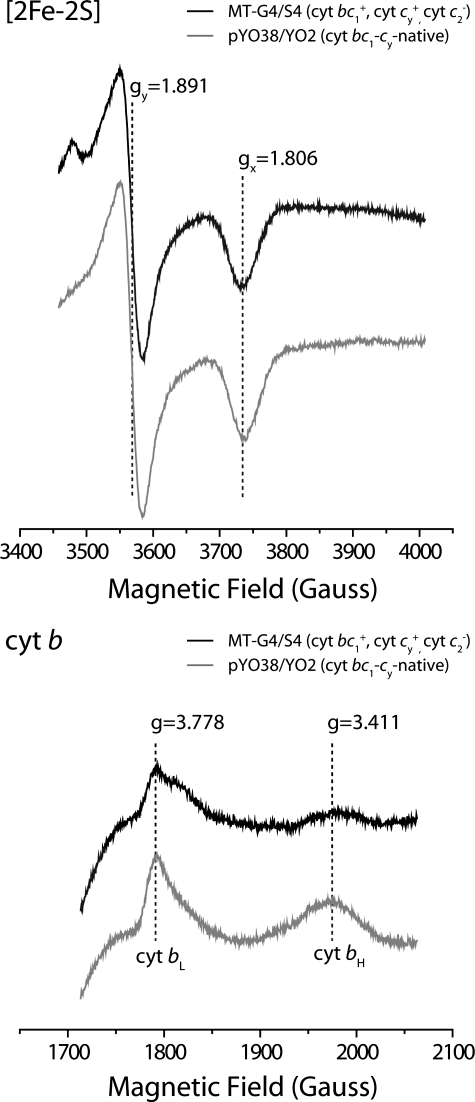
The presence of b-type cyts and cyts c to b ratios for all strains were also determined using optical difference spectroscopy to assess the relative amounts of the cyt bc1 complexes. Changes in the amounts of the c-type (α peakmax at 551 nm, ε551-542 of 20 mm-1 cm-1) and b-type (α peakmax at 560 nm, ε560-574 of 28 mm-1 cm-1) cytochromes were monitored after reduction by ascorbate and dithionite, respectively, of ferricyanide-oxidized chromatophore membranes from appropriate strains (Fig. 3). Strains pMTS1/MT-RBC1 and MT-G4/S4 exhibited cyt c to b ratios of ∼1:2, whereas very small amounts of c or b peaks were detected in YO2. On the other hand, strains pYO38/YO2, pYO33/YO2, and pYO30/YO2 had similar amounts of b-type cytochromes with cyt c to b ratios of about 1:1 (Fig. 3). The data indicated that all strains contained similar amounts of cyt bc1-cy fusion complexes regardless of the linker length of their cyt cy. Furthermore, to confirm that the Fe-S subunits of fusion complexes had native-like physicochemical properties, EPR spectroscopy was used. Chromatophore membranes prepared from pYO38/YO2 exhibited the [2Fe-2S] cluster gy and gx signals of 1.891 and 1.806, respectively, which were identical to those seen with a native cyt bc1 complex (e.g. MT-G4/S4) (Fig. 4A). Moreover, under appropriate conditions, the EPR signals with gz values of 3.778 and 3.411 assigned to cyts bL and bH, respectively, were observed with the same membrane preparations (Fig. 4B). The overall data established that all cyt bc1-cy fusion complexes assembled similarly, and exhibited similar enzymatic activities, indicating that the slower growth rates observed with pYO33/YO2 and pYO30/YO2 could not be correlated with the amounts of fusion complexes.
FIGURE 3.
Reduced minus oxidized optical difference spectra of b-type and c-type cytochromes in chromatophore membranes (0.4 mg of total proteins) from various R. capsulatus strains. Strains were as described in the legend to Fig. 2, and grown on enriched MPYE medium by respiration.
FIGURE 4.
EPR spectra of the cyt bc1 complex [2Fe-2S] clusters (upper panel) and the cyt b hemes (lower panel) of R. capsulatus MT-G4/S4 lacking cyt c2 and pYO38/YO2 containing a cyt bc1-cy fusion complex with a native cyt cy linker, as described in the legend to Fig. 2. For EPR spectroscopy of the [2Fe-2S] cluster or cyt b hemes (bL and bH), chromatophore membranes were reduced with ascorbate or air oxidized, respectively, as described in Refs. 35 and 36. Spectra were recorded at 20 K, 9.443 GHz, 12 G and 10 K, 9.59 GHz, 10 G temperature, microwave frequencies, and modulation amplitudes, respectively.
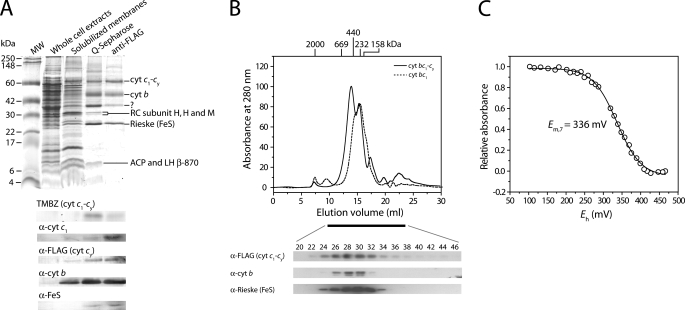
Purification and Characterization of Purified Cyt bc1-cy Fusion Complexes—Purification of cyt bc1-cy fusion complexes was pursued to establish that they existed as intact physical entities in the membranes. Earlier, we partially purified the cyt bc1-cy fusion complex (19) using the procedure described by Valkova-Valchanova et al. (22). However, as detailed characterization required purer samples, we developed a new procedure. About 8 mg of purified cyt bc1-cy fusion complex was obtained starting with about 2.5 g of chromatophore membranes derived from 97 g (wet weight) of cells, followed by detergent solubilization, Q-Sepharose ff ion-exchange and FLAG affinity column chromatographies (Table 2). The final preparations of the cyt bc1-cy fusion complex contained less than 5% of Ps pigments associated with the LH complexes, as estimated by optical spectra (data not shown). The purified cyt bc1-cy fusion complex was highly active, reducing horse heart cyt c as an electron acceptor with decylhydroquinone as an electron donor (“Experimental Procedures”). Its specific activity (about 27.2 nmol/mg of protein/min under the assay conditions used) was comparable with that of the purified cyt bc1 complex (41.0 nmol/mg of protein/min) (22). Optical difference spectra indicated that the purified cyt bc1-cy fusion complex had a cyt b to c ratio of ∼1:1, similar to that seen in chromatophore membranes from pYO38/YO2 (data not shown). Potentiometric redox equilibrium titration of the heme groups of the cyt c1-cy fusion subunit in the presence of 100 mm KCl at pH 7.0 indicated a single component with an Em,7 value of +336 mV (Fig. 5B). Considering that this subunit is a diheme c-type cyt (cyts c1 and cy of Em,7 of 320 and 365 mV, respectively (10, 27), the data suggested that the two hemes were in rapid equilibrium with each other, with a mean Em,7 value high enough to convey electrons efficiently from the cyt bc1 complex to the RC.
TABLE 2.
Purification of the cyt bc1-cy fusion complex
| Step | Protein | Total activity | Specific activity | Yield | -Fold |
|---|---|---|---|---|---|
| mg | unitsa | units/mg | % | ||
| Chromatophore + DDM | 592 | ||||
| Solubilized chromatophore | 518 | 2,577 | 4.9 | 100 | 1.0 |
| Q-Sepharose ff | 33 | 634 | 19.3 | 25 | 3.9 |
| FLAG | 8b | 204 | 27.2 | 8 | 5.5 |
One unit of DBH2-dependent cyt c reduction activity was defined as the amount of enzyme that produced 1 μmol of reduced cyt c per min under the assay conditions.
FLAG-purified samples contained 70 and 87 nmol of cyt c (extinction coefficient, ε551-542 of 20 mm−1 cm−1) and cyt b (ε560-574 of 28 mm−1 cm−1) per milligram of protein, respectively.
FIGURE 5.
Purification of the cyt bc1-cy fusion complex. A, SDS-PAGE, TMBZ, and immunoblot analyses. Approximately 50 μg of total proteins per lane were used in each case, except the pool from anti-FLAG, which had only 10 μg. Column fractions obtained during the purification procedure, and α-cyt c1, α-FLAG (i.e. cyt cy), α-cyt b, and α-Fe-S antibodies were as described under “Experimental Procedures” and in the text. B, size exclusion chromatography (upper panel) of the purified cyt bc1-cy fusion complex and cyt bc1 complex in the presence of 150 mm NaCl and 0.05% DDM. Gel filtration chromatography was performed using a Superose 6 HR 10/30 column, which was run at a flow rate of 0.3 ml/min, and the elution profile was monitored at 280 nm. The column was calibrated with blue dextran (2,000 kDa), thyroglobulin (669 kDa), apoferritin (440 kDa), catalase (232 kDa), and aldolase (158 kDa) as standards, run in the presence of 150 mm NaCl and 0.05% DDM, and their elution positions are indicated at the top of the chromatograph. Aliquots of each fraction (0.5 ml) were concentrated and subjected to 15% SDS-PAGE and immunoblot analyses (lower panel) using subunit-specific antibodies as described in A. C, dark equilibrium redox titration of the cyt c1-cy subunit of the purified cyt bc1-cy fusion complex (0.1 mg/ml). The titration was performed in 50 mm MOPS buffer (pH 7.0) containing 0.1 m KCl and 1 mm EDTA in the presence of 0.01% (w/v) DDM. Redox mediators were as described under “Experimental Procedures” (26). The Em,7 value indicated was determined by fitting the normalized data to a n = 1 Nernst equation.
SDS-PAGE analysis of purified cyt bc1-cy fusion complex showed three major bands with 61, 41, and 24 kDa, assigned to the cyt c1-cy, cyt b, and the Fe-S protein subunits, respectively, by TMBZ staining and immunoblot analyses with specific monoclonal antibodies (Fig. 5A). Additional bands of higher Mr seen with the cyt bc1-cy fusion complexes were attributed to their aggregated forms, based on TMBZ staining and immunoblot data, as they were also seen with native cyt bc1 complexes (27). Finally the non-stoichiometric band running between the cyt b and Fe-S protein subunits, and detected weakly by cyt c1-specific antibodies, in FLAG affinity purified samples reflected degradation products of the cyt c1-cy fusion subunit. The oligomeric state of purified cyt bc1-cy fusion complex was determined by size exclusion chromatography using an analytical grade Superose 6 HR 10/30 (GE Healthcare Inc.) column calibrated with high molecular weight standard markers in the presence of 0.05% (w/v) DDM and 150 mm NaCl (Fig. 5B). Under these conditions, the purified cyt bc1 complex runs as one major peak around 240 kDa (estimated to correspond to its dimeric form), whereas the purified cyt bc1-cy fusion complex exhibited two major peaks at about 417 and 257 kDa, respectively. Immunoblot analyses with subunit-specific antibodies showed that both peaks had homogeneous constituents, suggesting that purified cyt bc1-cy fusion complexes consisted of a mixture of tetrameric and dimeric forms under the conditions used.
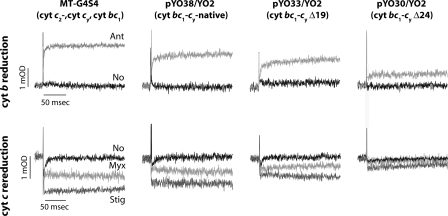
Light-activated Cyt b Reduction and Cyt c Re-reduction Kinetics—A major aim being to probe the extent of ET from the cyt bc1-cy fusion complexes with shorter cyt cy linkers to the RC in situ, appropriate strains were examined using light-activated, time-resolved optical spectroscopy (Fig. 6). In all cases, chromatophore membranes were normalized to the same amounts of photooxidized RC by measuring the absorbance changes at 605-540 nm at an Eh of 380 mV after a train of 10 saturating actinic flashes spaced 50 ms apart. Transient cyt b reduction and cyt c re-reduction kinetics, initiated by rapid (microsecond time scale) light activation of the RC, were monitored on the millisecond time scale, at an ambient redox potential Eh of 100 mV. At this Eh, the membrane Q pool contains Q and QH2, and the RC as well as cyts c, and the [2Fe-2S] cluster of the Fe-S protein subunits of cyt bc1 or the cyt bc1-cy fusion complexes are fully reduced. First, light-induced time-resolved single turnover cyt b reduction kinetics were examined at 560-570 nm, in the presence and absence of antimycin A as a specific cyt bc1 complex Qi site inhibitor. In MT-G4/S4, which has only cyt cy as the sole electron carrier between the cyt bc1 complex and the RC, and in pYO38/YO2 producing a cyt bc1-cy fusion complex with a native cyt cy linker, cyt b was reduced by oxidation of a QH2 via the Qo site, and immediately re-oxidized by Q bound at the Qi site with an expected rate faster than the available time resolution. However, in the presence of antimycin A (2 μm), cyt b oxidation was abolished to reveal the light-induced transient reduction phase (Fig. 6, top row). In the case of pYO33/YO2 (Δ19-cy) and pYO30/YO2 (Δ24-cy) with shorter cyt cy linkers, fast cyt b oxidation/reduction rates were quasi-similar to those seen with MT-G4/S4 and pYO38/YO2, but the amplitudes of these changes (per RC) were significantly smaller (Fig. 6, top row). As similar amounts of cyt bc1-cy fusions complexes were present in all strains tested (Figs. 2 and 3) the smaller cyt b reduction amplitudes cannot be interpreted as lower amounts of these complexes in the membranes.
FIGURE 6.
Light-induced, time-resolved cyt b reduction and cyt c re-reduction kinetics of various R. capsulatus strains. In each case, chromatophore membranes containing an amount of RC equal to 0.45 μm were resuspended in 50 mm MOPS buffer (pH 7.0) containing 100 mm KCl and 100 mm EDTA at an Eh of 100 mV. The amount of RC was determined based on the extent of its photooxidation by a train of 10 flashes separated by 50 ms at an Eh of 380 mV, and using an extinction coefficient ε605-540 of 29 mm-1 cm-1, as described under “Experimental Procedures.” The traces for cyt b reduction (upper panel) were monitored in the absence (No) and the presence of the Qi site inhibitor antimycin (Ant, 5 μm), and those for cyt c re-reduction (lower panel) were in the absence of inhibitor or in the presence of myxothiazol (Myx, 5 μm), where no QH2 oxidation takes place at the Qo site, or in the presence of stigmatellin (Stig, 1 μm), where no electron is transferred from the [2Fe2S] cluster to the c1 heme. All samples contained the same amounts of the cyt bc1-cy complexes as documented in Figs. 2 and 3.
The cyt c oxidation/re-reduction kinetics exhibited by the cyt bc1 complexes were subsequently examined using the specific Qo site inhibitors myxothiazol and stigmatellin to probe ET to the RC. Myxothiazol blocks cyt c re-reduction by displacing QH2 without immobilizing the Fe-S protein, whereas stigmatellin not only displaces QH2 but also immobilizes the Fe-S protein to block ET to the c1 heme as well. In the presence of stigmatellin, MT-G4/S4 and pYO38/YO2 showed typical cyt c oxidation without any cyt c re-reduction (Fig. 6, bottom row), due to the absence of ET from the Fe-S protein subunit to the cyt c1 heme. In the presence of myxothiazol, cyt c re-reduction reached about half of the amplitude seen in the presence of stigmatellin (i.e. full oxidation), revealing the pre-flash, chemically derived electron localized in the Fe-S protein despite the absence of QH2 oxidation at the Qo site (Fig. 6, bottom row) (see e.g. Refs. 28 and 29 for a detailed explanation of these ET kinetics). Cyt c re-reduction kinetics exhibited by pYO33/YO2 (Δ19-cy) and pYO30/YO2 (Δ24-cy) were quasi-similar to those seen with MT-G4/S4 and pYO38/YO2 (native-cy) in the presence and absence of the Qo site inhibitors. However, in the presence of shorter cyt cy linkers, significant differences in the cyt c oxidation/re-reduction amplitudes were observed. These amplitude decreases could not reflect lower amounts of the cyt bc1-cy fusion complexes with shorter cyt cy linkers in the strains examined (Figs. 2 and 3). Thus, they indicated decreased electronic couplings to the RC, revealing the limits of their physical abilities to reach and convey electrons to the photooxidized RCs (Fig. 6). Comparative kinetic data indicated that a cyt cy linker of about 45 amino acids long (i.e. Δ24-cy) seems to be the shortest one able to sustain cyclic ET and Ps growth of R. capsulatus.
DISCUSSION
In this work, first, we have characterized the physicochemical properties of a functional cyt bc1-cy complex that we constructed earlier (19) by fusing the COOH-terminal last amino acid of the cyt c1 subunit of the cyt bc1 complex to the NH2-terminal first amino acid of its physiological membrane-bound electron acceptor cyt cy (Fig. 1A). Using membrane preparations, detailed analyses demonstrated that the cyt bc1-cy fusion complex contained its prosthetic groups ([2Fe-2S] cluster and b- and c-type hemes) in appropriate amounts and assembled properly. Purification of the cyt bc1-cy fusion complex was needed to establish that it is an intact physical entity able to conduct membrane-confined Ps cyclic ET. This task was challenging because the fusion complex was susceptible to proteolytic degradation, and its chromatographic properties were distinct from the previously purified cyt cy (10) and cyt bc1 complexes (22). We developed a new procedure, using tandem Q-Sepharose ff ion-exchange and FLAG affinity column chromatographies, to obtain highly pure samples with good yields to initiate crystallization efforts. Unlike the dimeric cyt bc1 complex, the purified cyt bc1-cy complex was a mixture of dimeric and tetrameric populations, with different oligomerization properties in vitro, possibly due to the presence of cyt cy. The FLAG affinity purified samples contained small amounts of cyt c1-cy degradation products, running as an additional band around 30 kDa, and the Q-Sepharose column fractions were enriched in RC-LH subunits, usually absent in purified cyt bc1 complexes (nLC-MS/MS data, not shown). During purification, although most of the Ps components are usually washed out from the cyt bc1 complex in the presence of 0.01% DDM by a buffer of 0.2 m ionic strength, they remained with the cyt bc1-cy fusion complex. The significance, if any, of these apparent tighter associations remains to be seen.
Interestingly, both cyt b reduction and cyt c re-reduction rates exhibited by the cyt bc1-cy fusion complexes with shorter linkers were quasi-similar to those seen with strains harboring unconnected or fused cyt cy and cyt bc1 complexes with native cyt cy linkers. These fast rates further evidenced that the monitored ETs were mediated by the membrane-bound cyts cy, and not by their soluble versions (cyt S-cy), somehow generated by proteolysis. Otherwise, as described in the accompanying work (38), the ET rates would have become much slower. Sharply contrasting the rates, the amplitudes of ET to the RCs were slightly smaller that the unconnected systems even in the presence of a cyt bc1-cy complex with a native cyt cy linker, and became progressively smaller with shorter linkers. That only a fraction of all light-oxidized RC complexes can be reduced by cyt cy has been well documented earlier using native systems (5, 12). This work therefore demonstrated that the amounts of electronically coupled RC-LH-cyt bc1-cy fusion complexes diminished further with shorter cyt cy linkers, reflecting the distance dependence of efficient cyclic electron transport between the donor and acceptor complexes in Ps membranes. Furthermore, the progressive decrease suggested that membranes might contain a distribution of such closely associated photosynthetic units.
Even though the cyt bc1-cy fusion complexes with shorter cyt cy linkers were produced in comparable amounts irrespective of the linker sizes, they supported Ps growth at different degrees. Comparison of the shortest (Δ24-cy) and barely functional cyt cy linker with that of non-Ps competent R. sphaeroides cyt cy, which is 26 residues shorter than that of its R. capsulatus counterpart (Fig. 1A), suggested that at least a linker of about 44-46 amino acids long (provided that the native linker is about 70 residues long) is required to couple electronically the cyt bc1-cy fusion complex and the RC surrounded with its LH complexes. Assuming that both the RC (30) and the cyt bc1 complex (31) extend into the periplasm by ∼30 Å, then half of the remaining linker would be consumed to bring the cyt c domain of cyt cy to the same plane with its physiological partners. The remaining 20-25-residue long linker controlling its electronic coupling ability then suggest that the RC-LH complexes and the cyt bc1-cy complex must be very close to each other, possibly forming large structural complexes. Furthermore, it is noteworthy that both the R. sphaeroides cyt cy (9) and R. capsulatus cyt bc1-cy fusion complexes with shorter linkers convey electrons to their cognate cyt c oxidases.5 Thus, Ps supercomplexes apparently require longer cyt cy linkers than the respiratory counterparts, possibly due to the LH complexes surrounding the RCs.
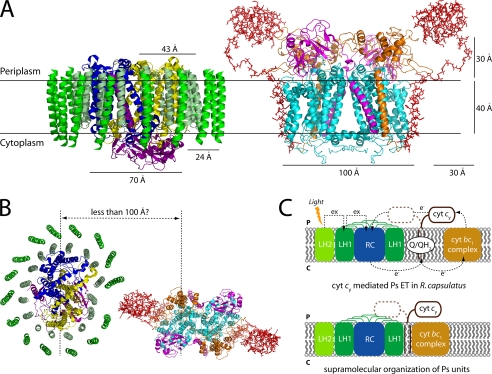
Kinetic behaviors of functional supercomplexes in R. sphaeroides Ps membranes between the RC and the “trapped” cyt c2 acting in a locally confined manners (14, 32-34), strongly suggest that the RC-LH and the cyt bc1 complexes must be very close to each other. The data presented here provide complementary structural and kinetic information for such Ps supercomplexes, with a ratio of the RC-LH:cyt bc1:cyt c2 (or cy) being 2:1:1 (13, 33). Joliot et al. (33) proposed that in the RC-LH complexes dimerized via LH-PufX interactions, two RC dimers interact with a single cyt bc1 complex dimer. If this is also the case with the cyt bc1-cy fusion complexes in the Ps membranes, then cy cyts might be located between the cyt bc1 complexes and the RC-LH complexes with their membrane anchors in the vicinity of the quasi-closed LH rings, promoting tighter associations between the Ps components. The motion constraints thus imposed on cyt cy might then only be compensated by a linker long enough to allow its movement between the cyt bc1 complex dimers and the opposing RC-LH complexes of R. capsulatus (Fig. 7).
FIGURE 7.
A, crystal structure of the RC-LH1 core complex (PDB 1PYH) from Rhodopseudomonas palustris and hypothetical three-dimensional structural model of R. capsulatus cyt bc1-cy fusion complex (pYO30/YO2, Δ24-cy) (with the transmembrane helices of cyt bc1 shown as ribbons, and cyt cy (Δ24-cy) shown as sticks), are drawn using the program PyMOL. The narrow section of the RC (subunits L, yellow; M, blue; H, purple) surrounded by the LH1 complex (chains α, pale green and β, green) and the cyt bc1-cy fusion complex (subunits cyt b, cyan; cyt c1, orange; cyt cy, red; and the Fe-S protein, magenta) are viewed parallel to the membrane plane. B, top view (perpendicular to the membrane plane) of the RC-LH1 core complex and the cyt bc1-cy fusion complex with the shortest cyt cy linker (Δ24-cy) is shown. Note that to reach the central part of RC subunits L and M in the RC-LH1 and cyt bc1-cy fusion complexes, the cyt c domain of cyt cy (assuming that its linker is stretched out in a fashion parallel to the membrane) needs to reach out for about 100 Å. C, a schematic representation of the major membrane proteins involved in cyclic ET of purple bacterial photosynthesis (upper panel), and a proposed mechanism for cyt cy-mediated cyclic ET via supraorganization of R. capsulatus photosynthetic unit (lower panel). cyt bc1 complex, hydroquinone cyt c oxidoreductase; cyt cy, membrane-associated cytochrome cy. Arrows indicate directions of electron (e-) and excitation (ex) flows.
In summary, the availability of functional cyt bc1-cy complexes with shorter linkers affecting the coupling to the RC-LH complexes now provide compelling indications that hardwired Ps units occur in membranes. Future purification of these Ps units will initiate studies addressing how the membrane supercomplexes are formed and regulated in vivo in response to changing environmental conditions, and why some organisms contain both a soluble cyt c2 and a membrane-anchored cyt cy.
Acknowledgments
We thank C. Sanders for helpful discussions and O. Onder and N. Selamoglu for mass spectrometry analyses.
This work was supported, in whole or in part, by National Institutes of Health Grant GM38237. This work was also supported by Department of Energy Grant ER9120053. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: Ps, photosynthetic; cyt, cytochrome; RC, photochemical reaction center; LH, light harvesting; ET, electron transfer; TMBZ, 3,3′,5,5′-tetramethylbenzidine; Q/QH2, quinone/hydroquinone pool; NQ, 1,2-naphthoquinone; HNQ, 2-hydroxy-1,4-naphthoquinone; DDM, dodecyl maltoside; MOPS, 4-morpholinepropanesulfonic acid; CV, column volume.
Y. Öztürk, D. Zannoni, and F. Daldal, unpublished data.
References
- 1.Berry, E. A., Guergova-Kuras, M., Huang, L. S., and Crofts, A. R. (2000) Annu. Rev. Biochem. 691005 -1075 [DOI] [PubMed] [Google Scholar]
- 2.Darrouzet, E., Cooley, J. W., and Daldal, F. (2004) Photosynth. Res. 7925 -44 [DOI] [PubMed] [Google Scholar]
- 3.Crofts, A. R., and Meinhardt, S. W. (1982) Biochem. Soc. Trans. 10201 -203 [DOI] [PubMed] [Google Scholar]
- 4.Gennis, R. B., Barquera, B., Hacker, B., Van Doren, S. R., Arnaud, S., Crofts, A. R., Davidson, E., Gray, K. A., and Daldal, F. (1993) J. Bioenerg. Biomembr. 25 195-209 [DOI] [PubMed] [Google Scholar]
- 5.Jenney, F. E., Jr., and Daldal, F. (1993) EMBO J. 121283 -1292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zannoni, D., and Daldal, F. (1993) Arch. Microbiol. 160413 -423 [DOI] [PubMed] [Google Scholar]
- 7.Jenney, F. E., Jr., Prince, R. C., and Daldal, F. (1994) Biochemistry 332496 -2502 [DOI] [PubMed] [Google Scholar]
- 8.Donohue, T. J., McEwan, A. G., Van Doren, S., Crofts, A. R., and Kaplan, S. (1988) Biochemistry 271918 -1925 [DOI] [PubMed] [Google Scholar]
- 9.Myllykallio, H., Zannoni, D., and Daldal, F. (1999) Proc. Natl. Acad. Sci. U. S. A. 964348 -4353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Myllykallio, H., Jenney, F. E., Jr., Moomaw, C. R., Slaughter, C. A., and Daldal, F. (1997) J. Bacteriol. 1792623 -2631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schoepp-Cothenet, B., Schutz, M., Baymann, F., Brugna, M., Nitschke, W., Myllykallio, H., and Schmidt, C. (2001) FEBS Lett. 487372 -376 [DOI] [PubMed] [Google Scholar]
- 12.Jenney, F. E., Jr., Prince, R. C., and Daldal, F. (1996) Biochim. Biophys. Acta 1273159 -164 [DOI] [PubMed] [Google Scholar]
- 13.Myllykallio, H., Drepper, F., Mathis, P., and Daldal, F. (1998) Biochemistry 375501 -5510 [DOI] [PubMed] [Google Scholar]
- 14.Vermeglio, A., and Joliot, P. (1999) Trends Microbiol. 7435 -440 [DOI] [PubMed] [Google Scholar]
- 15.Eubel, H., Jansch, L., and Braun, H. P. (2003) Plant Physiol. 133274 -286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schagger, H., and Pfeiffer, K. (2000) EMBO J. 191777 -1783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hackenbrock, C. R., Chazotte, B., and Gupte, S. S. (1986) J. Bioenerg. Biomembr. 18 331-368 [DOI] [PubMed] [Google Scholar]
- 18.Crofts, A. R. (2000) Trends Microbiol. 8105 -106 [DOI] [PubMed] [Google Scholar]
- 19.Lee, D. W., Ozturk, Y., Mamedova, A., Osyczka, A., Cooley, J. W., and Daldal, F. (2006) Biochim. Biophys. Acta 1757346 -352 [DOI] [PubMed] [Google Scholar]
- 20.Atta-Asafo-Adjei, E., and Daldal, F. (1991) Proc. Natl. Acad. Sci. U. S. A. 88 492-496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Daldal, F., Cheng, S., Applebaum, J., Davidson, E., and Prince, R. C. (1986) Proc. Natl. Acad. Sci. U. S. A. 832012 -2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Valkova-Valchanova, M. B., Saribas, A. S., Gibney, B. R., Dutton, P. L., and Daldal, F. (1998) Biochemistry 3716242 -16251 [DOI] [PubMed] [Google Scholar]
- 23.Smith, P. K., Krohn, R. I., Hermanson, G. T., Mallia, A. K., Gartner, F. H., Provenzano, M. D., Fujimoto, E. K., Goeke, N. M., Olson, B. J., and Klenk, D. C. (1985) Anal. Biochem. 15076 -85 [DOI] [PubMed] [Google Scholar]
- 24.Laemmli, U. K. (1970) Nature 277680 -685 [DOI] [PubMed] [Google Scholar]
- 25.Thomas, P. E., Ryan, D., and Levin, W. (1976) Anal. Biochem. 75168 -176 [DOI] [PubMed] [Google Scholar]
- 26.Dutton, P. L. (1978) Methods Enzymol. 54411 -435 [DOI] [PubMed] [Google Scholar]
- 27.Robertson, D. E., Ding, H., Chelminski, P. R., Slaughter, C., Hsu, J., Moomaw, C., Tokito, M., Daldal, F., and Dutton, P. L. (1993) Biochemistry 321310 -1317 [DOI] [PubMed] [Google Scholar]
- 28.Darrouzet, E., Moser, C. C., Dutton, P. L., and Daldal, F. (2001) Trends Biochem. Sci. 26 445-451 [DOI] [PubMed] [Google Scholar]
- 29.Darrouzet, E., Valkova-Valchanova, M., Moser, C. C., Dutton, P. L., and Daldal, F. (2000) Proc. Natl. Acad. Sci. U. S. A. 974567 -4572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roszak, A. W., Howard, T. D., Southall, J., Gardiner, A. T., Law, C. J., Isaacs, N. W., and Cogdell, R. J. (2003) Science 3021969 -1972 [DOI] [PubMed] [Google Scholar]
- 31.Berry, E. A., Huang, L. S., Saechao, L. K., Pon, N. G., Valkova-Valchanova, M., and Daldal, F. (2004) Photosynth. Res. 81251 -275 [DOI] [PubMed] [Google Scholar]
- 32.Jungas, C., Ranck, J. L., Rigaud, J. L., Joliot, P., and Vermeglio, A. (1999) EMBO J. 18 534-542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Joliot, P., Joliot, A., and Vermeglio, A. (2005) Biochim. Biophys. Acta 1706204 -214 [DOI] [PubMed] [Google Scholar]
- 34.Joliot, P., Vermeglio, A., and Joliot, A. (1989) Biochim. Biophys. Acta 975336 -345 [DOI] [PubMed] [Google Scholar]
- 35.Darrouzet, E., Valkova-Valchanova, M., and Daldal, F. (2000) Biochemistry 3915475 -15483 [DOI] [PubMed] [Google Scholar]
- 36.Cooley, J. W., Ohnishi, T., and Daldal, F. (2005) Biochemistry 4410520 -10532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gray, K. A., Dutton, P. L., and Daldal, F. (1994) Biochemistry 33723 -733 [DOI] [PubMed] [Google Scholar]
- 38.Öztürk, Y., Lee, D.-W., Mandaci, S., Osyczka, A., Prince, R. C., and Daldal, F. (2008) J. Biol. Chem. 28313964 -13972 [DOI] [PMC free article] [PubMed] [Google Scholar]