Abstract
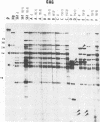
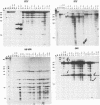
We have studied the extent of genetic and phenotypic diversification of human immunodeficiency virus type 1 (HIV-1) upon 15 serial passages of clonal viral populations in MT-4 cell cultures. Several genetic and phenotypic modifications previously noted during evolution of HIV-1 in infected humans were also observed upon passages of the virus in cell culture. Notably, the transition from non-syncytium-inducing to syncytium-inducing phenotype (previously observed during disease progression) and fixation of amino acid substitutions at the main antigenic loop V3 of gp120 were observed in the course of replication of the virus in MT-4 cell cultures in the absence of immune selection. Interestingly, most genetic and phenotypic alterations occurred upon passage of the virus at a low multiplicity of infection (0.001 infectious particles per cell) rather than at a higher multiplicity of infection (0.1 infectious particles per cell). The degree of genetic diversification attained by HIV-1, estimated by the RNase A mismatch cleavage method and by nucleotide sequencing, is of about 0.03% of genomic sites mutated after 15 serial passages. This value is not significantly different from previous estimates for foot-and-mouth disease virus when subjected to a similar process and analysis. We conclude that several genetic and phenotypic modifications of HIV-1 previously observed in vivo occur also in the constant environment provided by a cell culture system. Dilute passage promotes in a highly significant way the expression of deviant HIV-1 genomes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asjö B., Sharma U. K., Morfeldt-Månson L., Magnusson A., Barkhem T., Albert J., Olausson E., Von Gegerfelt A., Lind B., Biberfeld P. Naturally occurring HIV-1 isolates with differences in replicative capacity are distinguished by in situ hybridization of infected cells. AIDS Res Hum Retroviruses. 1990 Oct;6(10):1177–1182. doi: 10.1089/aid.1990.6.1177. [DOI] [PubMed] [Google Scholar]
- Benmansour A., Brahimi M., Tuffereau C., Coulon P., Lafay F., Flamand A. Rapid sequence evolution of street rabies glycoprotein is related to the highly heterogeneous nature of the viral population. Virology. 1992 Mar;187(1):33–45. doi: 10.1016/0042-6822(92)90292-w. [DOI] [PubMed] [Google Scholar]
- Chao L. Fitness of RNA virus decreased by Muller's ratchet. Nature. 1990 Nov 29;348(6300):454–455. doi: 10.1038/348454a0. [DOI] [PubMed] [Google Scholar]
- Cheng-Mayer C., Homsy J., Evans L. A., Levy J. A. Identification of human immunodeficiency virus subtypes with distinct patterns of sensitivity to serum neutralization. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2815–2819. doi: 10.1073/pnas.85.8.2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffin J. M. Genetic diversity and evolution of retroviruses. Curr Top Microbiol Immunol. 1992;176:143–164. doi: 10.1007/978-3-642-77011-1_10. [DOI] [PubMed] [Google Scholar]
- Coffin J. M. Genetic variation in AIDS viruses. Cell. 1986 Jul 4;46(1):1–4. doi: 10.1016/0092-8674(86)90851-2. [DOI] [PubMed] [Google Scholar]
- Delassus S., Cheynier R., Wain-Hobson S. Nonhomogeneous distribution of human immunodeficiency virus type 1 proviruses in the spleen. J Virol. 1992 Sep;66(9):5642–5645. doi: 10.1128/jvi.66.9.5642-5645.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domingo E., Escarmis C., Martinez M. A., Martinez-Salas E., Mateu M. G. Foot-and-mouth disease virus populations are quasispecies. Curr Top Microbiol Immunol. 1992;176:33–47. doi: 10.1007/978-3-642-77011-1_3. [DOI] [PubMed] [Google Scholar]
- Domingo E., Sabo D., Taniguchi T., Weissmann C. Nucleotide sequence heterogeneity of an RNA phage population. Cell. 1978 Apr;13(4):735–744. doi: 10.1016/0092-8674(78)90223-4. [DOI] [PubMed] [Google Scholar]
- Duarte E., Clarke D., Moya A., Domingo E., Holland J. Rapid fitness losses in mammalian RNA virus clones due to Muller's ratchet. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):6015–6019. doi: 10.1073/pnas.89.13.6015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díez J., Dávila M., Escarmís C., Mateu M. G., Dominguez J., Pérez J. J., Giralt E., Melero J. A., Domingo E. Unique amino acid substitutions in the capsid proteins of foot-and-mouth disease virus from a persistent infection in cell culture. J Virol. 1990 Nov;64(11):5519–5528. doi: 10.1128/jvi.64.11.5519-5528.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emini E. A., Schleif W. A., Nunberg J. H., Conley A. J., Eda Y., Tokiyoshi S., Putney S. D., Matsushita S., Cobb K. E., Jett C. M. Prevention of HIV-1 infection in chimpanzees by gp120 V3 domain-specific monoclonal antibody. Nature. 1992 Feb 20;355(6362):728–730. doi: 10.1038/355728a0. [DOI] [PubMed] [Google Scholar]
- Fenyö E. M., Albert J., Asjö B. Replicative capacity, cytopathic effect and cell tropism of HIV. AIDS. 1989;3 (Suppl 1):S5–12. doi: 10.1097/00002030-198901001-00002. [DOI] [PubMed] [Google Scholar]
- Fenyö E. M., Morfeldt-Månson L., Chiodi F., Lind B., von Gegerfelt A., Albert J., Olausson E., Asjö B. Distinct replicative and cytopathic characteristics of human immunodeficiency virus isolates. J Virol. 1988 Nov;62(11):4414–4419. doi: 10.1128/jvi.62.11.4414-4419.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudsmit J., Debouck C., Meloen R. H., Smit L., Bakker M., Asher D. M., Wolff A. V., Gibbs C. J., Jr, Gajdusek D. C. Human immunodeficiency virus type 1 neutralization epitope with conserved architecture elicits early type-specific antibodies in experimentally infected chimpanzees. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4478–4482. doi: 10.1073/pnas.85.12.4478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenink M., Fouchier R. A., de Goede R. E., de Wolf F., Gruters R. A., Cuypers H. T., Huisman H. G., Tersmette M. Phenotypic heterogeneity in a panel of infectious molecular human immunodeficiency virus type 1 clones derived from a single individual. J Virol. 1991 Apr;65(4):1968–1975. doi: 10.1128/jvi.65.4.1968-1975.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn B. H., Shaw G. M., Taylor M. E., Redfield R. R., Markham P. D., Salahuddin S. Z., Wong-Staal F., Gallo R. C., Parks E. S., Parks W. P. Genetic variation in HTLV-III/LAV over time in patients with AIDS or at risk for AIDS. Science. 1986 Jun 20;232(4757):1548–1553. doi: 10.1126/science.3012778. [DOI] [PubMed] [Google Scholar]
- Harada S., Koyanagi Y., Yamamoto N. Infection of HTLV-III/LAV in HTLV-I-carrying cells MT-2 and MT-4 and application in a plaque assay. Science. 1985 Aug 9;229(4713):563–566. doi: 10.1126/science.2992081. [DOI] [PubMed] [Google Scholar]
- Holland J. J., De La Torre J. C., Steinhauer D. A. RNA virus populations as quasispecies. Curr Top Microbiol Immunol. 1992;176:1–20. doi: 10.1007/978-3-642-77011-1_1. [DOI] [PubMed] [Google Scholar]
- Holland J., Spindler K., Horodyski F., Grabau E., Nichol S., VandePol S. Rapid evolution of RNA genomes. Science. 1982 Mar 26;215(4540):1577–1585. doi: 10.1126/science.7041255. [DOI] [PubMed] [Google Scholar]
- Jansen R. W., Siegl G., Lemon S. M. Molecular epidemiology of human hepatitis A virus defined by an antigen-capture polymerase chain reaction method. Proc Natl Acad Sci U S A. 1990 Apr;87(8):2867–2871. doi: 10.1073/pnas.87.8.2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javaherian K., Langlois A. J., McDanal C., Ross K. L., Eckler L. I., Jellis C. L., Profy A. T., Rusche J. R., Bolognesi D. P., Putney S. D. Principal neutralizing domain of the human immunodeficiency virus type 1 envelope protein. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6768–6772. doi: 10.1073/pnas.86.17.6768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Galindez C., Lopez J. A., Melero J. A., de la Fuente L., Martinez C., Ortin J., Perucho M. Analysis of genetic variability and mapping of point mutations in influenza virus by the RNase A mismatch cleavage method. Proc Natl Acad Sci U S A. 1988 May;85(10):3522–3526. doi: 10.1073/pnas.85.10.3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Galíndez C., Rojas J. M., Nájera R., Richman D. D., Perucho M. Characterization of genetic variation and 3'-azido-3'-deoxythymidine- resistance mutations of human immunodeficiency virus by the RNase A mismatch cleavage method. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4280–4284. doi: 10.1073/pnas.88.10.4280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meloen R. H., Liskamp R. M., Goudsmit J. Specificity and function of the individual amino acids of an important determinant of human immunodeficiency virus type 1 that induces neutralizing activity. J Gen Virol. 1989 Jun;70(Pt 6):1505–1512. doi: 10.1099/0022-1317-70-6-1505. [DOI] [PubMed] [Google Scholar]
- Meyerhans A., Cheynier R., Albert J., Seth M., Kwok S., Sninsky J., Morfeldt-Månson L., Asjö B., Wain-Hobson S. Temporal fluctuations in HIV quasispecies in vivo are not reflected by sequential HIV isolations. Cell. 1989 Sep 8;58(5):901–910. doi: 10.1016/0092-8674(89)90942-2. [DOI] [PubMed] [Google Scholar]
- Palker T. J., Clark M. E., Langlois A. J., Matthews T. J., Weinhold K. J., Randall R. R., Bolognesi D. P., Haynes B. F. Type-specific neutralization of the human immunodeficiency virus with antibodies to env-encoded synthetic peptides. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1932–1936. doi: 10.1073/pnas.85.6.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratner L., Haseltine W., Patarca R., Livak K. J., Starcich B., Josephs S. F., Doran E. R., Rafalski J. A., Whitehorn E. A., Baumeister K. Complete nucleotide sequence of the AIDS virus, HTLV-III. Nature. 1985 Jan 24;313(6000):277–284. doi: 10.1038/313277a0. [DOI] [PubMed] [Google Scholar]
- Rocha E., Cox N. J., Black R. A., Harmon M. W., Harrison C. J., Kendal A. P. Antigenic and genetic variation in influenza A (H1N1) virus isolates recovered from a persistently infected immunodeficient child. J Virol. 1991 May;65(5):2340–2350. doi: 10.1128/jvi.65.5.2340-2350.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruano G., Kidd K. K. Coupled amplification and sequencing of genomic DNA. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2815–2819. doi: 10.1073/pnas.88.7.2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saag M. S., Hahn B. H., Gibbons J., Li Y., Parks E. S., Parks W. P., Shaw G. M. Extensive variation of human immunodeficiency virus type-1 in vivo. Nature. 1988 Aug 4;334(6181):440–444. doi: 10.1038/334440a0. [DOI] [PubMed] [Google Scholar]
- Shaw G. M., Hahn B. H., Arya S. K., Groopman J. E., Gallo R. C., Wong-Staal F. Molecular characterization of human T-cell leukemia (lymphotropic) virus type III in the acquired immune deficiency syndrome. Science. 1984 Dec 7;226(4679):1165–1171. doi: 10.1126/science.6095449. [DOI] [PubMed] [Google Scholar]
- Sobrino F., Dávila M., Ortín J., Domingo E. Multiple genetic variants arise in the course of replication of foot-and-mouth disease virus in cell culture. Virology. 1983 Jul 30;128(2):310–318. doi: 10.1016/0042-6822(83)90258-1. [DOI] [PubMed] [Google Scholar]
- Sundqvist V. A., Albert J., Ohlsson E., Hinkula J., Fenyö E. M., Wahren B. Human immunodeficiency virus type 1 p24 production and antigenic variation in tissue culture of isolates with various growth characteristics. J Med Virol. 1989 Nov;29(3):170–175. doi: 10.1002/jmv.1890290305. [DOI] [PubMed] [Google Scholar]
- Temin H. M. Is HIV unique or merely different? J Acquir Immune Defic Syndr. 1989;2(1):1–9. [PubMed] [Google Scholar]
- Tersmette M., Gruters R. A., de Wolf F., de Goede R. E., Lange J. M., Schellekens P. T., Goudsmit J., Huisman H. G., Miedema F. Evidence for a role of virulent human immunodeficiency virus (HIV) variants in the pathogenesis of acquired immunodeficiency syndrome: studies on sequential HIV isolates. J Virol. 1989 May;63(5):2118–2125. doi: 10.1128/jvi.63.5.2118-2125.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentin A., Albert J., Fenyö E. M., Asjö B. HIV-1 infection of normal human macrophage cultures: implication for silent infection. Virology. 1990 Aug;177(2):790–794. doi: 10.1016/0042-6822(90)90551-2. [DOI] [PubMed] [Google Scholar]
- Wain-Hobson S. Human immunodeficiency virus type 1 quasispecies in vivo and ex vivo. Curr Top Microbiol Immunol. 1992;176:181–193. doi: 10.1007/978-3-642-77011-1_12. [DOI] [PubMed] [Google Scholar]
- Willey R. L., Smith D. H., Lasky L. A., Theodore T. S., Earl P. L., Moss B., Capon D. J., Martin M. A. In vitro mutagenesis identifies a region within the envelope gene of the human immunodeficiency virus that is critical for infectivity. J Virol. 1988 Jan;62(1):139–147. doi: 10.1128/jvi.62.1.139-147.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolinsky S. M., Wike C. M., Korber B. T., Hutto C., Parks W. P., Rosenblum L. L., Kunstman K. J., Furtado M. R., Muñoz J. L. Selective transmission of human immunodeficiency virus type-1 variants from mothers to infants. Science. 1992 Feb 28;255(5048):1134–1137. doi: 10.1126/science.1546316. [DOI] [PubMed] [Google Scholar]
- Wong-Staal F., Shaw G. M., Hahn B. H., Salahuddin S. Z., Popovic M., Markham P., Redfield R., Gallo R. C. Genomic diversity of human T-lymphotropic virus type III (HTLV-III). Science. 1985 Aug 23;229(4715):759–762. doi: 10.1126/science.2992084. [DOI] [PubMed] [Google Scholar]
- de la Torre J. C., Holland J. J. RNA virus quasispecies populations can suppress vastly superior mutant progeny. J Virol. 1990 Dec;64(12):6278–6281. doi: 10.1128/jvi.64.12.6278-6281.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Torre J. C., Martínez-Salas E., Diez J., Villaverde A., Gebauer F., Rocha E., Dávila M., Domingo E. Coevolution of cells and viruses in a persistent infection of foot-and-mouth disease virus in cell culture. J Virol. 1988 Jun;62(6):2050–2058. doi: 10.1128/jvi.62.6.2050-2058.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]