Abstract
Obesity increases the risk for metabolic and cardiovascular disease, and adipose tissue plays a central role in this process. Ceramide, the key intermediate of sphingolipid metabolism, also contributes to obesity-related disorders. We show that a high fat diet increased ceramide levels in the adipose tissues and plasma in C57BL/6J mice via a mechanism that involves an increase in gene expression of enzymes mediating ceramide generation through the de novo pathway (e.g. serine palmitoyltransferase) and via the hydrolysis of sphingomyelin (acid sphingomyelinase and neutral sphingomyelinase). Although the induction of total ceramide in response to the high fat diet was modest, dramatic increases were observed for C16, C18, and C18:1 ceramides. Next, we investigated the relationship of ceramide to plasminogen activator inhibitor-1 (PAI-1), the primary inhibitor of plasminogen activation and another key player in obesity. PAI-1 is consistently elevated in obesity and thought to contribute to increased artherothrombotic events and more recently to obesity-mediated insulin resistance. Interestingly, the changes in ceramide were attenuated in mice lacking PAI-1. Mechanistically, mice lacking PAI-1 were protected from diet-induced increase in serine palmitoyltransferase, acid sphingomyelinase, and neutral sphingomyelinase mRNA, providing a mechanistic link for decreased ceramide in PAI-1–/– mice. The decreases in plasma free fatty acids and adipose tumor necrosis factor-α in PAI-1–/– mice may have additionally contributed indirectly to improvements in ceramide profile in these mice. This study has identified a novel link between sphingolipid metabolism and PAI-1 and also suggests that ceramide may be an intermediary molecule linking elevated PAI-1 to insulin resistance.
Insulin resistance, type 2 diabetes, and accelerated arthrosclerosis are common health complications associated with obesity, which has now reached epidemic proportions in the United States (1, 2). Despite this, the molecular changes in obesity that promote these conditions are not completely understood. Increasing evidence suggests that adipose inflammation and abnormalities in sphingolipid metabolism may contribute to both the metabolic and cardiovascular risk associated with obesity.
Sphingolipids, such as ceramide, sphingosine, and sphingosine 1-phosphate, have been implicated in the pathogenesis of obesity, insulin resistance (3, 4), and cardiovascular disease (5–8). Ceramide production is mediated by the hydrolysis of membrane sphingomyelin by acid sphingomyelinase (ASMase)2 or neutral sphingomyelinase (NSMase) or by de novo synthesis via serine palmitoyltransferase (SPT) and ceramide synthase (9, 10). Ceramide is subsequently metabolized into sphingosine by ceramidases, and sphingosine can be further converted to S1P via sphingosine kinase (9, 10). Numerous studies unequivocally demonstrate a role for sphingolipids, specifically ceramide, in the development of insulin resistance (3, 4, 11). In fact, ceramide is now considered to be a putative intermediate linking both excess nutrients, such as saturated free fatty acids (FFA), and inflammatory cytokines, such as tumor necrosis factor α (TNF-α), to the induction of insulin resistance (3). Ceramide and sphingosine inhibit insulin action and signaling in cultured cells (3). Inhibiting de novo ceramide synthesis prevented palmitate-mediated ceramide accumulation and inhibition of insulin signaling (3, 12, 13). Moreover, inhibiting ceramide degradation augmented palmitate-mediated inhibition of insulin signaling (3, 12). More recently, Holland et al. (4) demonstrated that inhibition of ceramide synthesis by preventing de novo ceramide synthesis using the specific SPT inhibitor myriocin ameliorated glucocorticoid, saturated fat, and obesity-induced insulin resistance. Similarly, inhibition of de novo ceramide synthesis in apolipoprotein E knock-out mice, which are susceptible to atherosclerosis, with the SPT inhibitor myriocin dramatically reduced the progression of arthrosclerosis (6, 14).
In addition to abnormalities in sphingolipid metabolism, adipose inflammation is also emerging as a hallmark of obesity and a potential contributor to both the metabolic and cardiovascular risk associated with obesity (15–19). Adipose tissue synthesizes a large number of proinflammatory and prothrombotic proteins that affect glucose homeostasis and insulin sensitivity, including plasminogen activator inhibitor-1 (PAI-1), TNF-α, MCP-1 (monocyte chemoattractant protein 1), interleukin-6, and leptin, and the expressions of these molecules are elevated in adipose tissues in obesity (17–20). PAI-1 is the primary physiological inhibitor of plasminogen activation in vivo, and increased PAI-1 compromises normal fibrin clearance mechanisms and promotes thrombosis (21). PAI-1 is consistently increased in obesity and correlates strongly with parameters of the metabolic syndrome, including body mass index, insulin resistance, and hyperinsulinemia (22–24). Recent evidence suggests that PAI-1 contributes directly to the complications of obesity, including insulin resistance, type 2 diabetes, and atherothrombosis (22). The picture that is thus emerging is that elevated PAI-1 may be central to both the development of the obese phenotype and its metabolic consequences. Previously, we demonstrated that ceramide induced PAI-1 expression in adipocytes (25). These observations, together with studies demonstrating a role for PAI-1 in the development of insulin resistance, support the hypothesis that ceramide and PAI-1 are by some means interrelated, and increases in these molecules may be potentially important in the pathogenesis of obesity-associated disorders, including insulin resistance and cardiovascular risk.
Several studies have demonstrated increased ceramide levels in tissues, such as the muscle and liver of insulin-resistant/obese rodents and humans (26–29), and have shown that these levels correlated negatively with insulin sensitivity. We previously demonstrated that adipose ceramide levels were decreased in genetically obese ob/ob mice compared with their lean counterparts (25). Since the ob/ob mice lack the satiety hormone leptin, and human obesity is characterized by increased levels of circulating leptin, the relevance of this model to human obesity is uncertain. Alternately, it is possible that the observed changes in ceramide in ob/ob mice may be due to an epistatic effect caused by the lack of leptin rather than obesity per se. To address these questions, we determined adipose and plasma ceramide in a more physiologically relevant model of diet-induced obesity in C57BL/6J mice. We show that in C57BL/6J mice, a high fat diet (HFD) leads to a significant increase in ceramide levels in the adipose tissues and plasma through a mechanism that involves the induction of enzymes that increase ceramide synthesis (SPT, ASMase, and NSMase). Our data also unexpectedly suggest that this chronic long term increase in ceramide in this model of diet-induced obesity may be related to increased levels of PAI-1. Mice lacking PAI-1 were protected from diet-induced increases in SPT, ASMase, and NSMase mRNA and in ceramide. Moreover, the improvements in the ceramide profile in PAI–/– mice may, at least in part, also be mediated by the decreased levels of plasma FFAs and adipose TNF-α expression observed in these mice. This study has not only demonstrated that an HFD leads to increased ceramide but also identified a novel link between sphingolipid metabolism and PAI-1, a molecule that is consistently elevated in obesity and thought to be a common denominator of obesity-mediated cardiovascular and metabolic abnormalities.
EXPERIMENTAL PROCEDURES
Mice—C57BL/6J (8-week-old) male wild type (WT) and age-matched PAI-1-deficient (PAI-1–/–) mice (Jackson Laboratory) were placed for 16 weeks on either an HFD (D12492; Research Diets, New Brunswick, NJ) in which 60% of the total calories were derived from fat (soybean oil and lard) or a control low fat diet (LFD; D12450B) in which 10% of the total calories were derived from fat. However, the total kcal % in the high and low fat diets was identical. This HFD induces obesity, hyperphagia, and insulin resistance in C57BL/6J mice (30). Body weights and food intake were monitored weekly.
RNA Analysis—Real time reverse transcription-PCR was performed in an iCycler (Bio-Rad) as previously described (25, 31, 32). For real time PCR, cDNA was prepared from 1 μg of total RNA by using random hexamers and Moloney murine leukemia virus reverse transcriptase (PerkinElmer Life Sciences) in a final reaction volume of 20 μl. Real time PCR amplifications were performed from 2.5 μl of cDNA diluted 1:2 by using gene-specific primer sets (Invitrogen). Each primer set was used at 150 nm in a final volume of 25 μl by using the SYBR Green Master Mix (PerkinElmer Life Sciences). All PCRs were performed in an iCycler (Bio-Rad). Quantification of a given gene, expressed as relative mRNA level compared with a control, was calculated after normalization to β-actin RNA and using the ΔΔCT formula as described by PerkinElmer Life Sciences. Individual CT values are means of duplicate measurements. Separate control experiments demonstrated that the efficiencies of target and reference (i.e. β-actin) amplifications were equal. The specificity of the PCR amplification was verified by melt curve analysis of the final products directly in the iCycler and by agarose-gel electrophoresis.
Measurement of Ceramide Levels—Ceramide levels were analyzed by high performance liquid chromatography-tandem mass spectroscopy as previously described (33) (Lipidomics Core, Medical University of South Carolina). Briefly, tissue homogenates (in buffer containing 0.25 m sucrose, 25 mm KCl, 50 mm Tris, 0.5 mm EDTA, pH 7.4) or plasma were fortified with internal standards and extracted into a one-phase neutral organic solvent system (ethyl acetate/isopropyl alcohol/water; 60:30:10 v/v/v), evaporated, and reconstituted in methanol and analyzed by a surveyor/TSQ 7000 liquid chromatography/mass spectrometry system. Quantitative analysis was performed in a positive multiple-reaction monitoring mode, based on calibration curves generated by adding to an artificial matrix known amounts of target analytes, synthetic standards, and an equal amount of internal standard. The ceramide levels were normalized to total protein levels (1 mg of protein/sample).
SMAse Assay—ASMase and NSMase in adipose tissue extracts were determined using the Amplex Red sphingomyelinase assay kit according to the manufacturer's protocol (Molecular Probes). Frozen tissue was homogenized in a buffer containing 100 mm Tris-HCl and 0.5% Triton X-100 (pH 7.4) and centrifuged at 10,000 × g for 5 min, and the supernatant (50 μg of protein; determined by the Bradford assay according to the manufacturer's protocol (Bio-Rad)) was used for the sphingomyelinase assay. NSMase activity was determined in a total volume of 100 μl containing 0.1 m Tris-HCl (pH 7.4), 10 mm MgCl2, 0.5 mm sphingomyelin, 0.2 units/ml choline oxidase, 2 units/ml horseradish peroxidase, 8 units/ml alkaline phosphatase, and 0.2 mm Amplex Red. For ASMase activity, the reaction was carried out in an acidic buffer (50 mm sodium acetate, pH 5.0) containing 0.5 mm sphingomyelin without Mg2+, and the reaction was detected with Amplex reagents in 100 mm Tris-HCl (pH 8.0, optimal pH for Amplex Red detection). The fluorescence was measured in a fluorescence microplate reader at the excitation and emission wavelengths of 560 and 590 nm, respectively. Sphingomyelinase activity was calculated based on a standard curve run parallel to sample reactions.
Metabolic Parameters—Plasma FFA concentrations were measured with an NEFA-C assay kit (Wako Pure Chemical Industries Inc.). Plasma insulin concentrations were measured with an insulin assay kit (Mercodia Ultrasensitive Insulin ELISA; Alpco Diagnostics, Windham, NH), and glucose levels were monitored with a Glucometer Elite blood glucose meter (Bayer, Elkhart, IN). Plasma levels of PAI-1 and TNF-α were determined using the LINCOplex adipokines assay system (Linco Diagnostic Services, St. Charles, MO).
Glucose and Insulin Tolerance Test—Glucose tolerance tests were performed on fasted (6 h) mice, whereas the insulin tolerance tests were performed on nonfasted mice. Mice were weighed and then injected intraperitoneally with either glucose (2 g/kg of body weight) or insulin (Himulin; Lilly; 0.75 units/kg of body weight). Blood samples were obtained via retro-orbital bleeds at base line and at 10, 30, 60, 90, and 120 min after glucose or insulin injection and analyzed for glucose levels.
Histological Analysis—Adipose tissues were removed from mice after 16 weeks on the respective high or low fat diet. Formalin-fixed, paraffin-embedded sections (6 μm) were cut and stained with hematoxylin and eosin.
Statistical Analysis—Statistical comparison of results was performed using the unpaired Student's t test.
RESULTS
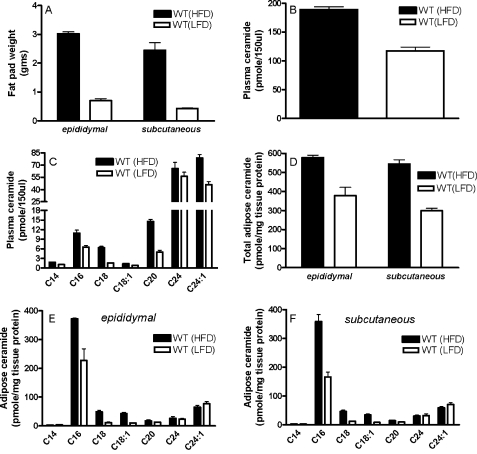
Ceramide Levels Are Increased in Plasma and Adipose Tissue of C57BL/6J Mice in Response to an HFD—As expected, after 16 weeks on the HFD, C57BL/6J WT mice become obese, gaining about 3 times as much weight as WT mice on the LFD. Mice on the HFD gained 22.7 ± 1.5 g, whereas mice on the LFD gained only 6.5 ± 1.6 g by the end of the feeding regime. However, the weight gain was not distributed evenly among the various fat depots in the mouse. Instead, abdominal subcutaneous fat increased about 6-fold compared with the 4-fold gain in the epididymal fat pads (Fig. 1A). However, in response to the same diet (i.e. either HFD or LFD), there appeared to be no significant difference between the weights of the epididymal and abdominal subcutaneous adipose tissues in WT mice.
FIGURE 1.
Weight gain and ceramide expression in plasma and adipose tissue of C57BL/6J mice in response to a HFD. Fat pad weight (A) and ceramide levels in plasma (total (B) and ceramide species (C)) and adipose tissues (total (D), ceramide species in epididymal fat (E), and ceramide species in subcutaneous fat (F)) were determined from C57BL/6J mice placed on a LFD or HFD for 16 weeks. For B and D, total ceramide is the sum of individual ceramide species. In all panels, data are means (n = 6) ± S.D. For B, plasma ceramide in LFD versus HFD, p < 0.01. For D, epididymal fat pad LFD versus HFD, p < 0.05; subcutaneous fat pad LFD versus HFD, p < 0.001.
Since sphingolipids, such as ceramide, have been implicated in the pathogenesis of obesity, we determined the effect of an HFD on ceramide levels in plasma and adipose tissues of C57BL/6J WT mice. In response to the HFD, total ceramide levels were significantly (p < 0.01) increased in plasma of WT mice on the HFD compared with those on an LFD (Fig. 1B), the most abundant being C24 and C24:1 ceramides. A general increase in response to the HFD was observed for all detectable individual ceramide species in the plasma, with the most dramatic and significant inductions observed for C16 (75%, p < 0.05), C18 (330%, p < 0.001), C20 (215%, p < 0.001), and C24:1 (71%, p < 0.05) ceramide (Fig. 1C). Total ceramide levels were also significantly elevated in epididymal (p < 0.05) and subcutaneous adipose tissues (p < 0.001) of these mice (Fig. 1D). Interestingly, in the adipose tissues, not all ceramide species were increased in response to the HFD (Fig. 1, E and F). The most abundant species of ceramide in adipose tissues was C16 ceramide, and in both fat depots, significant increases (p values ranging from p < 0.001 to 0.05) were observed for C16 ceramide (epididymal, 64%; subcutaneous, 115%). Significant increases were also seen for C18 ceramide (epididymal, 315%; subcutaneous, 289%) and C18:1 ceramide (epididymal, 332%; subcutaneous, 325%). In contrast, adipose expression of C14, C20, C24, and C24:1 ceramides were not modulated significantly by diet.
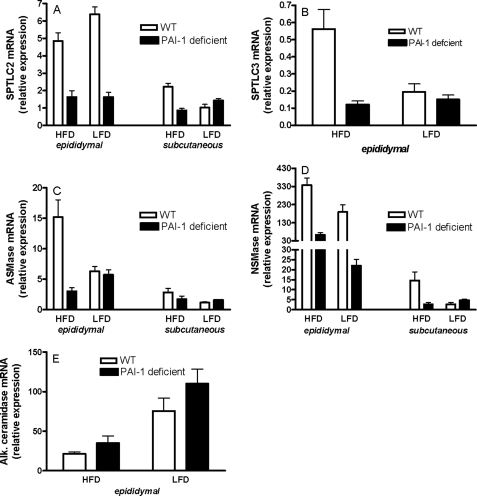
To determine the mechanism(s) by which the HFD leads to the chronic sustained increase in ceramide, we determined the effect of diet on the gene expression of enzymes involved in ceramide generation and metabolism. SPT is the primary enzyme involved in the initial rate-limiting step of de novo ceramide synthesis, whereas ASMase and NSMase lead to ceramide production via the hydrolysis of sphingomyelin. SPT is composed of three subunits, SPTLC1, SPTLC2, and SPTLC3 (34). Studies suggest that although both SPTLC2 and SPTLC3 subunits can independently increase SPT activity, SPTLC1 does not increase SPT activity and is not directly involved in the catalytic reaction (34). Moreover, the activities of SPTLC2 and SPTLC3 show a very close correlation to their mRNA expression (34). Compared with mice on the LFD, the HFD modestly reduced Sptlc2 mRNA expression in the epididymal fat pads but significantly increased (p < 0.05) its expression in the subcutaneous adipose tissues (Fig. 2A). However, a dramatic increase was observed for Sptlc3 mRNA expression in epididymal adipose tissues of mice in response to the HFD (Fig. 2B). Interestingly, Sptlc3 gene expression was very low or undetectable in the subcutaneous adipose tissues of WT mice both on the high and low fat diets. Sptlc1 gene expression was not modulated in the epididymal adipose tissues of mice on the HFD, but a modest increase was observed in subcutaneous fat (data not shown). HFD also affected expression of ASMase and NSMase mRNA. Both enzymes were increased in the epididymal and subcutaneous fat pads of WT mice on the HFD (Fig. 2, C and D). The gene expression of these enzymes in epididymal fat pads was, however, markedly higher than in the subcutaneous fat. ASMase activity also was significantly (p < 0.001) increased in both epididymal and subcutaneous fat pads of mice on the HFD compared with those fed an LFD (Fig. 2E). NSMase activity was significantly (p < 0.05) increased only in epididymal fad pads (Fig. 2E). A very modest increase in NSMase activity was observed in subcutaneous fat in response to the HFD; however, this increase was not significant (data not shown).
FIGURE 2.
Adipose tissue expression of enzymes involved in ceramide generation and hydrolysis. SPTLC2 (A), SPTLC3 (B), ASMase (C), NSMase (D), SMase activity (E) and alkaline ceramidase (F) mRNA levels were determined by real time reverse transcription-PCR from epididymal and subcutaneous fat pads of C57BL/6J mice after 16 weeks on an LFD or HFD. E, SMase activity in adipose tissues of C57BL/6J mice after 16 weeks on a LFD or HFD was determined using the Amplex Red sphingomyelinase assay kit. In all panels, data are means (n = 6) ± S.D.
We next determined the gene expression of enzymes involved in ceramide metabolism, leading to the formation of the downstream metabolite sphingosine and to complex sphingolipids, such as glucosylceramide. Ceramidases, which convert ceramide to sphingosine, were evaluated initially.
Alkaline ceramidase expression, which was only detectable in the epididymal fat pads, was significantly (p < 0.05) decreased in response to the HFD (Fig. 2F). No significant changes were observed in acid ceramidase gene expression in both the epididymal and subcutaneous adipose tissues in response to the HFD (data not shown). Likewise, the expression levels of GM3 synthase and glucosyl ceramide synthase (enzymes involved in conversion of ceramide to glucosylceramide and subsequent glycosphingolipids and gangliosides) showed no change in response to high fat feeding (data not shown). The above results suggest that in response to a HFD, coordinated changes are observed in the expression of enzymes involved in ceramide generation and metabolism in a manner that may lead to increased ceramide accumulation.
HFD-induced Weight Gain and Ceramide Is Attenuated in Mice Lacking PAI-1—Increased levels of PAI-1 is one of the biochemical hallmarks of obesity, and recent data suggest that PAI-1 may contribute directly to the health consequences of obesity, including insulin resistance, type 2 diabetes, and cardiovascular complications, and may even influence adipose tissue accumulation. We hypothesized that elevated PAI-1 in obesity may also contribute to obesity-mediated increases in ceramide. We initially characterized PAI-1 expression in response to the high and low fat diet in C57BL/6J mice. After 16 weeks, PAI mRNA levels were increased in both the subcutaneous and epididymal adipose tissues of mice on the HFD compared with those on the LFD (Fig. 3A), with PAI-1 expression significantly higher (p < 0.05) in the subcutaneous adipose tissue than in the epididymal fat pad. We next determined whether these changes in PAI-1 levels affected changes in weight. After 16 weeks on the LFD, the weight gains of the WT and PAI-1–/– mice were similar (Fig. 3B). However, although both the WT and PAI-1–/– mice gained weight on the HFD, the weight gain of the PAI-1–/– mice was significantly lower (p < 0.01) than that of the WT mice. To determine whether the changes in weight were associated with differences in adiposity, we compared changes in the abdominal subcutaneous fat and the epididymal fat pads in the WT and PAI-1–/– mice. The weight of the abdominal subcutaneous fat in the PAI-1–/– mice when compared with the WT mice was only modestly lower on the LFD but was dramatically reduced on the HFD to almost that of the WT mice on the LFD (Fig. 3C). The reduction in the weight of the epididymal fat pad in the PAI-1–/– mice fed a HFD when compared with WT mice was modest but significant (Fig. 3D, p < 0.05). Hematoxylin and eosinstained sections of adipose tissues showed that compared with WT mice, PAI-1-deficient mice on the HFD had decreased adipocyte size (Fig. 3E). This reduction was more pronounced in the subcutaneous fat (adipocyte diameter in WT mice, 123 ± 32 μm; PAI-1–/– mice, 63 ± 21 μm; lower panels) than in the epididymal fat (adipocyte diameter in WT mice, 131 ± 31 μm; PAI-1–/– mice, 103 ± 20 μm; upper panels).
FIGURE 3.
Weight gain and fat accumulation in WT and PAI-1–/– mice after 16 weeks on a LFD or HFD. A, PAI-1 mRNA expression as determined by real time reverse transcription-PCR in epididymal and subcutaneous fat pads. Data are means (n = 6) ± S.D. For both epididymal and subcutaneous fat pads, HFD versus LFD, p < 0.01. B, body weight gain in WT and PAI-1–/– mice. Data are means (n = 8) ± S.D. WT versus PAI-1–/– mice on HFD, p < 0.01. C, abdominal subcutaneous fat pad weights of WT and PAI-1–/– mice. Data are means (n = 8) ± S.D. WT versus PAI-1–/– mice on HFD, p < 0.01. D, epididymal fat pad weights of WT and PAI-1–/– mice. Data are means (n = 8) ± S.D. WT versus PAI-1–/– mice on HFD, p < 0.05. E, hematoxylin and eosin-stained histological sections (×200) of epididymal and subcutaneous fat pads from WT and PAI-1–/– mice. Results are representative of n = 6 in each group. Upper panels, epididymal fat; lower panels, subcutaneous fat.
Having shown that the diet-induced weight gain is ameliorated in mice lacking PAI, we next compared the effect of PAI-1 deficiency on ceramide levels in plasma and adipose tissues of mice fed an HFD or LFD. Although total ceramide levels were significantly increased in the plasma of WT mice on the HFD, this increase was not observed in mice lacking PAI-1 (Fig. 4A). Total plasma ceramide levels in PAI-1-deficient mice on the HFD closely approximated levels observed for WT mice on the LFD. Moreover, the lack of PAI-1 almost completely protected against the HFD-induced increases in C16 and C18 ceramide in the plasma and partially protected against increases in C20 (Fig. 4B).
FIGURE 4.
Plasma and adipose ceramide levels in WT and PAI-1–/– mice after 16 weeks on a LFD or HFD. Ceramide levels in plasma (total (A) and ceramide species (B)) and adipose tissues (total (C), ceramide species in epididymal fat (D), and ceramide species in subcutaneous fat (E)) were determined from WT and PAI-1–/– mice. F, Lass1/CerS1 gene expression in epididymal adipose tissues of WT and PAI-1-deficient mice. For A and C, total ceramide is the sum of individual ceramide species, and data are means (n = 6) ± S.D. For A, LFD versus HFD in WT mice, p < 0.01; WT versus PAI-1–/– mice on HFD, p < 0.05. For C, epididymal fat pad LFD versus HFD in WT mice, p < 0.05; epididymal fat pad WT versus PAI-1–/– on HFD, p < 0.001; subcutaneous fat pad LFD versus HFD in WT mice, p < 0.001; subcutaneous fat pad WT versus PAI-1–/– on HFD, p < 0.001; subcutaneous fat pad WT versus PAI–/–mice on LFD, p < 0.01.
As shown already (Fig. 1D), total ceramide levels in the epididymal and subcutaneous adipose tissues in WT mice were increased in response to an HFD. This increase in total ceramide was not observed in PAI-1–/– mice in the epididymal fat pad, but a modest blunted increase in ceramide was observed in the subcutaneous fat in PAI-1–/– mice fed a HFD (Fig. 4C). Interestingly, total ceramide expression was also lower in PAI-1–/– mice on the LFD compared with WT mice in both the epididymal and subcutaneous adipose tissues, with a larger reduction observed for the subcutaneous fat. The increases in individual ceramide species observed in the adipose tissue in WT mice in response to the HFD were significantly ameliorated in HFD-fed mice lacking PAI-1 (Fig. 4, D and E). The HFD-induced increase in C16 ceramide observed in the epididymal (Fig. 4D) and subcutaneous (Fig. 4E) adipose tissues in WT mice was not observed in mice lacking PAI-1. This was also striking for C18 and C18:1 ceramides (in epididymal fat, 315% in WT with 51% in PAI-1–/– for C18 and 332% with 58% for C18.1; in subcutaneous fat, 289% in WT with 50% in PAI-1–/– for C18 and 325% with 64% for C18.1). C18 and C18:1 ceramide levels were also lower in the epididymal and subcutaneous fat pads in PAI-1–/– mice on the LFD compared with WT mice.
The fatty acid composition of ceramide is regulated by a family of genes known as longevity assurance genes (Lass)/ceramide synthases (CerS), with six members (CerS1 to -6) identified thus far (35, 36). We measured the gene expression of ceramide synthase CerS1 to -6 in epididymal adipose tissues from WT mice on a high and LFD. A dramatic and significant (p < 0.05) increase was observed for CerS1 mRNA in adipose tissues of WT mice on the HFD compared with those on the LFD. (Fig. 4F). This increase was not observed in HFD fed PAI-1-deficient mice. The increase in CerS1 observed in this study is consistent with the HFD-induced increase in C18 ceramide in the adipose tissue, since overproduction of CerS1 is known to preferentially increase C18 ceramide. CerS3 and CerS5 appeared to be modestly increased in WT mice on the HFD, whereas CerS2, CerS4, and CerS6 showed no diet-induced changes (data not shown).
To determine the mechanism(s) by which PAI-1 deficiency ameliorates the HFD-mediated increase in ceramide, we initially compared the effect of diet on the gene expression of enzymes involved in ceramide generation and metabolism in WT and PAI-1–/– mice. In the absence of PAI-1, Sptlc2 gene expression was significantly lower (p < 0.01) in the epididymal fat pad and was not modulated by diet (Fig. 5A). The lack of PAI-1 also reduced Sptlc2 mRNA levels in the subcutaneous fat (p < 0.05) of the high fat-fed mice (Fig. 5A). Sptlc3 gene expression, which was primarily observed in epididymal adipose tissue, was also significantly reduced (p < 0.01) in PAI-1-deficient mice compared with WT mice (Fig. 5B). PAI-1 also influenced the expression of ASMase and NSMase mRNA. PAI-1-deficient mice on the HFD were completely and significantly (p < 0.001) protected against the HFD-induced increase in both ASMase (Fig. 5C) and NSMase (Fig. 5D) expression in epididymal fat pads. Although the relative expression levels of these enzymes were lower in the subcutaneous fat compared with epididymal fat, a similar trend was observed in this fat depot as well. Alkaline ceramidase expression, which was only detectable in the epididymal fat pads, was decreased in response to an HFD (p < 0.05), and the lack of PAI-1 had no effect on its expression (Fig. 5E). The above results suggest that the mechanism by which PAI-1 deficiency protects against increases in ceramide levels may be related to the decreased expression of ceramide-generating enzymes (SPT, ASMase, and NSMase) and increased expression of ceramide-metabolizing enzymes (e.g. alkaline ceramidase).
FIGURE 5.
Adipose tissue gene expression of enzymes involved in ceramide generation and hydrolysis in WT and PAI-1–/– mice. SPTLC2 (A), SPTLC3 (B), ASMase (C), NSMase (D), and alkaline ceramidase (E) mRNA levels were determined by real time reverse transcription-PCR from epididymal and subcutaneous fat pads of WT and PAI-1–/– mice after 16 weeks on a LFD or HFD. For all panels, data are means (n = 6) ± S.D.
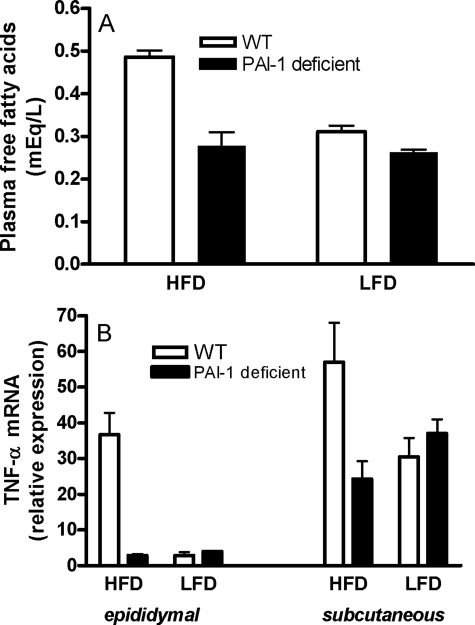
PAI-1 Deficiency Results in Reduced Plasma FFA Levels—PAI-1 deficiency may decrease ceramide levels directly through its effects on enzymes of sphingolipid metabolism or indirectly by changing other metabolic and inflammatory parameters. Plasma FFAs are elevated in obesity, and FFAs are known to drive the de novo pathway of ceramide generation via the induction of SPT activity, the rate-limiting step in this pathway (37). We therefore measured plasma FFA levels in WT and PAI-1–/– mice after 16 weeks on the HFD or the LFD (Fig. 6A). Although the HFD significantly (p < 0.001) increased plasma FFA levels in WT mice, this increase was significantly (p < 0.01) attenuated in mice lacking PAI-1. In fact, the lack of PAI-1 almost completely prevented the diet-induced increase in plasma FFA. The low levels of plasma FFA in PAI-1–/– mice may have indirectly contributed to the observed reduction in SPT expression and de novo ceramide synthesis observed in mice lacking PAI-1.
FIGURE 6.
Plasma FFA and adipose TNF-α expression in WT and PAI-1–/– mice. Plasma free fatty acids (A) and adipose TNF-α mRNA expression (B) were measured in WT and PAI-1–/– mice after 16 weeks on an LFD or HFD. For all groups in A and B, data are means (n = 6) ± S.D. For TNF-α expression, epididymal fat, LFD versus HFD in WT mice, p < 0.001; epididymal fat pad, WT versus PAI-1–/– on HFD, p < 0.01. Subcutaneous fat, LFD versus HFD in WT mice, p < 0.05; WT versus PAI-1–/– on HFD, p < 0.05.
PAI-1-deficient Mice Are Protected from High Fat Diet-induced Increase in Adipose TNF-α Expression—Obesity is associated with elevated TNF-α expression in adipose tissues, and in various cell types, TNF-α has been shown to activate ASMase, NSMase, and the de novo pathway of ceramide generation (9). Moreover, we previously demonstrated that in C57BL/6J mice adipose tissue, SPT, ASMase, and NSMase mRNA levels were significantly increased in response to an intraperitoneal injection of TNF-α, suggesting that TNF-α can contribute to elevated ceramide in obesity (25). We therefore sought to determine whether the expression of TNF-α levels was increased in mice on the HFD, and, if so, whether this increase is ameliorated in mice that lack PAI-1 and have reduced levels of ceramide. No diet-induced changes were observed in the plasma levels of TNF-α in WT or PAI-1-deficient mice (data not shown). However, the levels of TNF-α mRNA were significantly increased in both the epididymal and subcutaneous adipose tissues of WT mice on the HFD but not in PAI–/– mice (Fig. 6B), suggesting that there may be increased production of TNF-α protein within the local milieu of the adipose tissue that may act in an autocrine/paracrine manner. If this is indeed the case, then reduced levels of TNF-α in the local milieu of the adipose tissues of PAI-1–/– mice may be another mechanism that contributes to decreased ceramide expression in mice lacking PAI-1.
PAI-1-deficient Mice Show Increased Insulin Sensitivity—Both increased PAI-1 and ceramide have been implicated in the insulin resistance associated with obesity.
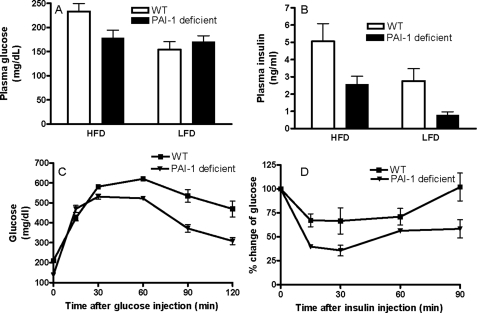
Although some studies have reported a protective effect of PAI-1 deficiency in the development of obesity and insulin resistance (38, 39), others have failed to observe these effects (40, 41). These inconsistencies are difficult to explain but may include effects of different diets, duration of experiments, the genetic background of the animals used, and other confounders. Using high and low fat diets matched in total kcal, we confirmed that PAI-1 deficiency partially protected the development of obesity in our diet-induced model of obesity in C57BL/6J mice (Fig. 3). In these next experiments, we investigated whether the lack of PAI-1 and the associated decrease in adipose and plasma ceramide would affect insulin sensitivity in this diet-induced model of obesity. WT mice on the HFD exhibited modest increases in plasma glucose (Fig. 7A) but a robust increase in plasma insulin (Fig. 7B) in the fasted state, indicating the emergence of insulin resistance. However, PAI-1–/– mice on the HFD showed a significant reduction in both plasma glucose and plasma insulin compared with WT mice (Fig. 7, A and B). The ameliorative effects of PAI-1 deficiency on insulin resistance were also confirmed by glucose and insulin tolerance tests. Glucose tolerance tests indicated that PAI-1–/– mice on the HFD were more efficient in their ability to clear an intraperitoneally injected bolus of glucose than WT mice on the same diet (Fig. 7C). The protection from diet-induced insulin resistance in PAI-1–/– mice was also confirmed in the insulin tolerance test (Fig. 7D). Insulin sensitivity as measured by the degree of reduction in plasma glucose after insulin administration, indicated that on the HFD, PAI-1–/– mice were more efficient at insulin-mediated suppression of plasma glucose than WT mice (Fig. 7D). These results demonstrate that the absence of PAI-1 clearly hindered the development of HFD-induced insulin resistance in this diet-induced obese model and suggest that the mechanisms by which it does so may be related to decreased ceramide.
FIGURE 7.
Glucose homeostasis in WT and PAI-1–/– mice. Plasma glucose (A) and plasma insulin (B) were measured after 16 weeks on the high or low fat diet in WT and PAI-1–/– mice. Data are means (n = 8) ± S.D. Plasma glucose, LFD versus HFD in WT mice, p < 0.05; WT versus PAI-1–/– mice on HFD, p < 0.05. Plasma insulin, LFD versus HFD in WT mice, p < 0.05; LFD versus HFD in PAI-1–/– mice, p < 0.01; WT versus PAI-1–/– mice on HFD, p < 0.01; WT versus PAI-1–/– mice on LFD, p < 0.05. C, glucose tolerance test. Mice fasted for ∼6 h were injected with a bolus of glucose, and blood samples were obtained at the indicated time and analyzed for glucose levels. D, insulin tolerance test. Mice were injected with insulin (0.75 units/kg of body weight), blood samples were obtained at the indicated times, and glucose levels were monitored. Data in A–D are means (n = 6) ± S.D.
DISCUSSION
This study demonstrates that plasma and adipose ceramide levels are increased in C57BL/6J mice fed an HFD via a mechanism that involves coordinated changes in the expression of ceramide-generating and -metabolizing enzymes. Moreover, this HFD-mediated increase in ceramide was attenuated in mice lacking PAI-1, providing an unexpected and novel link between sphingolipid metabolism and PAI-1 in the setting of obesity. Furthermore, our results suggest that the decrease in ceramide synthesis might be a possible contributing mechanism for both the improved insulin sensitivity observed in this study and previously (38, 39) and improved cardiovascular complications (42) observed in mice lacking PAI-1.
HFD-induced increase in total ceramide observed in this study was fairly modest and was not observed for all ceramide species. Instead, there were specific and striking increases in individual ceramide species in mice on the HFD, in particular in the long chain ceramides, with increases of more than 300% observed for C18 and C18.1 ceramide in adipose tissue and a similar increase of over 300% for C18 ceramide in plasma. Interestingly, in the plasma, although C24 and C24:1 ceramides constituted the major ceramide components that practically determine total ceramide levels, the most significant and dramatic changes in response to the HFD were observed for minor ceramide components, such as C18 (300%) and C20 (215%) ceramides. Similarly, whereas C16 was the major ceramide constituent in the adipose tissues, the most significant increases (∼300%) were observed for C18 and C18:1, which were relatively minor constituents in adipose tissues. In parallel with the dramatic increase in C18 ceramide species in adipose tissues in response to the HFD, CerS1 gene expression, which preferentially leads to the production of C18 ceramide, was also increased. Diversity in the behavior of individual ceramide species has been previously observed in various physiological and pathological conditions, (43, 44), and these studies suggest that changes in specific ceramide species rather than changes in total ceramide itself may underlie specific pathological events, a concept now widely accepted. The specific and striking increases in long chain ceramide, such as C18, observed in mice on the HFD may prove to be significant in the pathogenesis/progression of obesity and its associated health complications. These increases in both total ceramide and specific ceramide species observed in the adipose and plasma of mice on the HFD were significantly reduced in PAI-1-deficient mice, in some instances to levels similar to that observed in WT mice on the LFD. One possibility is that the reduction in ceramide in PAI-1–/– mice is a reflection of the general weight loss observed in these mice fed an HFD rather than a direct effect of the lack of PAI-1 itself. This, however, seems unlikely, since the lack of PAI-1 dramatically protects against the weight gain in subcutaneous fat and only very modestly in epididymal fat but is equally effective at protecting against ceramide increases in either fat depot. Moreover, a trend toward a decrease in adipose ceramide levels (although to a lesser extent) was also observed in PAI-1-deficient mice on the low fat diet as well. These data suggest that PAI-1 plays a direct role in ceramide generation, which is augmented in a setting of obesity, where PAI-1 levels are elevated. In light of these data, it is tempting to speculate that the improvements in the metabolic profile (reduced weight, improved insulin resistance, improved diabetes, etc.) observed in the PAI-1-deficient mice on the HFD may be mediated, at least in part, by the reduction of specific ceramide species, such as C18. Moreover, the selective changes observed in specific ceramide species in WT and PAI-1–/– mice in response to the HFD may be related to the expression of ceramide synthase/longevity assurance genes (CerS/Lass), a family of genes that regulate the fatty acid composition of ceramide (36). These studies are currently being explored.
Our results demonstrating that ceramide levels are reduced in response to a HFD in PAI-1–/– mice suggest a causal relationship between PAI-1 and ceramide. The loss of PAI-1 may decrease de novo ceramide synthesis by down regulating plasma FFA levels and SPT expression. The loss of PAI-1 may also decrease ceramide synthesis via the hydrolysis of sphingomyelin by down-regulating ASMase and NSMase expression. In previous studies, we demonstrated that injection of TNF-α increased the expression of SPT, NSMase, and ASMase in adipose tissues of C57BL/6J mice (25), and TNF-α has been shown to activate enzymes involved in ceramide generation in a number of other biological systems as well (9). Thus, the reduced levels of TNF-α observed in adipose tissue of PAI-1–/– mice on the HFD may have contributed to the decrease in the expression of enzymes involved in ceramide generation and thereby to reduced ceramide accumulation. Although this study clearly demonstrates that HFD induces the accumulation of ceramide in a PAI-1-dependent manner, the upstream events that may lead to changes in plasma FFA, TNF-α expression, or directly to enzymes involved in ceramide generation are currently unclear. However, several scenarios can be envisioned. Although a direct role for PAI-1 in signal transduction has not been demonstrated, it is well known that changes in the microenvironment and the extracellular matrix (ECM) surrounding cells can profoundly influence signal transduction events into and out of cells. PAI-1 via its interactions with ECM proteins and or via inhibiting proteases, such as matrix metalloproteinases, can modulate the ECM surrounding the cells and thereby indirectly regulate ceramide metabolism. PAI-1 is synthesized in an active form but is rapidly converted into an inactive form unless stabilized by binding to vitronectin, an adhesive glycoprotein found in the circulation and ECM of many tissues (21). PAI-1, through its interaction with vitronectin, can affect both integrin and urokinase receptor-dependent attachment of cells to vitronectin (45, 46). More importantly, recent studies suggest that the interaction of PAI-1 with vitronectin leads to inhibition of insulin-mediated AKT phosphorylation (47). PAI-1 can also inhibit insulin signaling by competing with αvβ3 integrin for vitronectin binding (47). Thus, it is tempting to speculate that PAI-1/ECM interactions in the local milieu of the adipose tissues in obesity, where PAI-1 levels are expected to be high, may also affect other signaling pathways, in particular those that regulate ceramide metabolism. This possibility remains to be determined and warrants further study.
PAI-1 is consistently elevated in obesity (18, 22–24, 48) and may directly contribute to complications of obesity, including insulin resistance, type 2 diabetes, and cardiovascular disease (22). Thus, increased PAI-1, in addition to being a consequence of obesity, may also have direct effects on the development of the obese/diabetic phenotype. Although the mechanisms by which PAI-1 contributes to increased cardiovascular risk are fairly clear, the molecular mechanism(s) by which PAI-1 contributes to insulin resistance is not as obvious. In this study, the improvements in weight gain and insulin resistance in PAI-1–/– mice on the HFD also correlated strongly with decreased levels of ceramide. Increasing evidence has now established a role for ceramide as an intermediate that links excess nutrients (e.g. saturated fatty acids) and inflammatory cytokines (e.g. TNF-α) to the induction of insulin resistance. For example, in vivo inhibition of de novo ceramide synthesis in various rodent models of obesity (4) improved insulin resistance. Thus, the reduction of adipose and plasma ceramide observed in the PAI-1-deficient mice on the HFD concurrent with the decrease in plasma FFA and adipose TNF-α may have contributed to the protection from diet-induced insulin resistance and the subsequent onset of diabetes in mice lacking PAI-1. These data thus support the hypothesis that ceramide may be a potential intermediary molecule linking elevated PAI-1 to insulin resistance.
The increase in ceramide observed in this study in response to an HFD may also be significant with respect to obesity-mediated cardiovascular disease. Studies have shown that plasma sphingomyelin and ceramide levels are closely related to the development of atherosclerosis (6, 14, 49, 50). Sphingomyelin carried into the arterial wall on atherogenic lipoproteins may be locally hydrolyzed to ceramide by sphingomyelinase, promoting lipoprotein aggregation and macrophage foam cell formation (51). Ceramide signaling may be responsible for certain inflammatory responses in the developing atherosclerotic lesions, such as smooth muscle cell proliferation (8). Moreover, ceramide also may contribute to the instability and rupture of atherosclerotic plaques because of its proapoptotic potential on macrophages and smooth muscle cells (52).
In vivo inhibition of de novo ceramide synthesis in various rodent models of atherosclerosis (6, 14) improved atherosclerotic lesion formation, thereby identifying a definitive role for ceramide in the pathogenesis of atherosclerosis. We previously demonstrated that ceramide can induce the expression of PAI-1 and inflammatory cytokines and chemokines from adipocytes (25); ceramide has also been shown to induce PAI-1 from endothelial cells (53). Our data showing that PAI-1 influences sphingolipid metabolism suggest that elevated PAI-1 levels may lead to increased ceramide expression. Thus, high levels of total ceramide and/or specific ceramide species may be a component of the cardiovascular risk profile attributed to elevated PAI-1.
In conclusion, this study provides information on the regulation of ceramide metabolism in response to a HFD and also suggests a novel role for PAI-1 in regulating ceramide metabolism. We propose that in the setting of obesity, ceramide may be a potential intermediary molecule linking elevated PAI-1 to insulin resistance and cardiovascular risk. Furthermore, these results may have broad implications in diseases where ceramide levels are altered, since PAI-1 may prove to be a novel target to improve ceramide profiles.
This work was supported, in whole or in part, by National Institutes of Health Grant R01HL071146 (to F. S.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: ASMase, acid sphingomyelinase; NSMase, neutral sphingomyelinase; SPT, serine palmitoyltransferase; FFA, free fatty acid; TNF-α, tumor necrosis factor α; PAI-1, plasminogen activator inhibitor-1; HFD, high fat diet; LFD, low fat diet; WT, wild type; ECM, extracellular matrix; GM3, N-acetylneuraminylgalactosylceramide.
References
- 1.Hill, J. O., Wyatt, H. R., Reed, G. W., and Peters, J. C. (2003) Science 299 853–855 [DOI] [PubMed] [Google Scholar]
- 2.Klein, S., Burke, L. E., Bray, G. A., Blair, S., Allison, D. B., Pi-Sunyer, X., Hong, Y., and Eckel, R. H. (2004) Circulation 110 2952–2967 [DOI] [PubMed] [Google Scholar]
- 3.Summers, S. A. (2006) Prog. Lipid Res. 45 42–72 [DOI] [PubMed] [Google Scholar]
- 4.Holland, W. L., Brozinick, J. T., Wang, L. P., Hawkins, E. D., Sargent, K. M., Liu, Y., Narra, K., Hoehn, K. L., Knotts, T. A., Siesky, A., Nelson, D. H., Karathanasis, S. K., Fontenot, G. K., Birnbaum, M. J., and Summers, S. A. (2007) Cell Metab. 5 167–179 [DOI] [PubMed] [Google Scholar]
- 5.Auge, N., Negre-Salvayre, A., Salvayre, R., and Levade, T. (2000) Prog. Lipid Res. 39 207–229 [DOI] [PubMed] [Google Scholar]
- 6.Hojjati, M. R., Li, Z., Zhou, H., Tang, S., Huan, C., Ooi, E., Lu, S., and Jiang, X. C. (2005) J. Biol. Chem. 280 10284–10289 [DOI] [PubMed] [Google Scholar]
- 7.Li, H., Junk, P., Huwiler, A., Burkhardt, C., Wallerath, T., Pfeilschifter, J., and Forstermann, U. (2002) Circulation 106 2250–2256 [DOI] [PubMed] [Google Scholar]
- 8.Auge, N., Maupas-Schwalm, F., Elbaz, M., Thiers, J. C., Waysbort, A., Itohara, S., Krell, H. W., Salvayre, R., and Negre-Salvayre, A. (2004) Circulation 110 571–578 [DOI] [PubMed] [Google Scholar]
- 9.Futerman, A. H., and Hannun, Y. A. (2004) EMBO Rep. 5 777–782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hannun, Y. A., and Obeid, L. M. (2002) J. Biol. Chem. 277 25847–25850 [DOI] [PubMed] [Google Scholar]
- 11.Aerts, J. M., Ottenhoff, R., Powlson, A. S., Grefhorst, A., van Eijk, M., Dubbelhuis, P. F., Kuipers, F., Serlie, M. J., Wennekes, T., Overkleeft, H. S., Sethi, J. K., and O'Rahilly, S. (2007) Diabetes 56 1341–1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chavez, J. A., Knotts, T. A., Wang, L. P., Li, G., Dobrowsky, R. T., Florant, G. L., and Summers, S. A. (2003) J. Biol. Chem. 278 10297–10303 [DOI] [PubMed] [Google Scholar]
- 13.Powell, D. J., Turban, S., Gray, A., Hajduch, E., and Hundal, H. S. (2004) Biochem. J. 382 619–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park, T. S., Panek, R. L., Mueller, S. B., Hanselman, J. C., Rosebury, W. S., Robertson, A. W., Kindt, E. K., Homan, R., Karathanasis, S. K., and Rekhter, M. D. (2004) Circulation 110 3465–3471 [DOI] [PubMed] [Google Scholar]
- 15.Weisberg, S. P., Hunter, D., Huber, R., Lemieux, J., Slaymaker, S., Vaddi, K., Charo, I., Leibel, R. L., and Ferrante, A. W. (2006) J. Clin. Invest. 116 115–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weisberg, S. P., McCann, D., Desai, M., Rosenbaum, M., Leibel, R. L., and Ferrante, A. W., Jr. (2003) J. Clin. Invest. 112 1796–1808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Axelsson, J., Heimburger, O., Lindholm, B., and Stenvinkel, P. (2005) J. Ren. Nutr. 15 131–136 [DOI] [PubMed] [Google Scholar]
- 18.Samad, F., and Loskutoff, D. J. (1996) Mol. Med. 2 568–582 [PMC free article] [PubMed] [Google Scholar]
- 19.Sartipy, P., and Loskutoff, D. J. (2003) Proc. Natl. Acad. Sci. U. S. A. 100 7265–7270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hotamisligil, G. S., Arner, P., Caro, J. F., Atkinson, R. L., and Spiegelman, B. M. (1995) J. Clin. Invest. 95 2409–2415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fearns, C., Samad, F., and Loskutoff, D. J. (1995) in Vascular Control of Hemostasis (van Hinsbergh, V. W. M., ed) pp. 207–226, Harwood Academic Publishers, Amsterdam, The Netherlands
- 22.De, T. B., Smith, L. H., and Vaughan, D. E. (2005) Curr. Opin. Pharmacol. 5 149–154 [DOI] [PubMed] [Google Scholar]
- 23.Samad, F., Yamamoto, K., and Loskutoff, D. J. (1996) J. Clin. Invest. 97 37–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Samad, F., and Loskutoff, D. J. (1997) Thromb. Haemost. 78 652–655 [PubMed] [Google Scholar]
- 25.Samad, F., Hester, K. D., Yang, G., Hannun, Y. A., and Bielawski, J. (2006) Diabetes 55 2579–2587 [DOI] [PubMed] [Google Scholar]
- 26.Turinsky, J., O'Sullivan, D. M., and Bayly, B. P. (1990) J. Biol. Chem. 265 16880–16885 [PubMed] [Google Scholar]
- 27.Zendzian-Piotrowska, M., Baranowski, M., Zabielski, P., and Gorski, J. (2006) J. Physiol. Pharmacol. 57 Suppl. 10, 101–114 [PubMed] [Google Scholar]
- 28.Adams, J. M., Pratipanawatr, T., Berria, R., Wang, E., DeFronzo, R. A., Sullards, M. C., and Mandarino, L. J. (2004) Diabetes 53 25–31 [DOI] [PubMed] [Google Scholar]
- 29.Unger, R. H., and Orci, L. (2001) FASEB J. 15 312–321 [DOI] [PubMed] [Google Scholar]
- 30.Van Heek, M., Compton, D. S., France, C. F., Tedesco, R. P., Fawzi, A. B., Graziano, M. P., Sybertz, E. J., Strader, C. D., and Davis, H. R., Jr. (1997) J. Clin. Invest. 99 385–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pandey, M., Loskutoff, D. J., and Samad, F. (2005) FASEB J. 19 1317–1319 [DOI] [PubMed] [Google Scholar]
- 32.Pandey, M., Tuncman, G., Hotamisligil, G. S., and Samad, F. (2003) Am. J. Pathol. 162 933–941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bielawski, J., Szulc, Z. M., Hannun, Y. A., and Bielawska, A. (2006) Methods 39 82–91 [DOI] [PubMed] [Google Scholar]
- 34.Hornemann, T., Richard, S., Rutti, M. F., Wei, Y., and von Eckardstein, A. (2006) J. Biol. Chem. 281 37275–37281 [DOI] [PubMed] [Google Scholar]
- 35.Mizutani, Y., Kihara, A., and Igarashi, Y. (2005) Biochem. J. 390 263–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pewzner-Jung, Y., Ben-Dor, S., and Futerman, A. H. (2006) J. Biol. Chem. 281 25001–25005 [DOI] [PubMed] [Google Scholar]
- 37.Merrill, A. H., Jr. (2002) J. Biol. Chem. 277 25843–25846 [DOI] [PubMed] [Google Scholar]
- 38.Schafer, K., Fujisawa, K., Konstantinides, S., and Loskutoff, D. J. (2001) FASEB J. 15 1840–1842 [DOI] [PubMed] [Google Scholar]
- 39.Ma, L.-J., Mao, S.-L., Taylor, K. L., Kanjanabuch, T., Guan, Y. F., Zhang, Y. H., Brown, N. J., Swift, L. L., McGuinness, O. P., Wasserman, D. H., Vaughan, D. E., and Fogo, A. B. (2004) Diabetes 53 336–346 [DOI] [PubMed] [Google Scholar]
- 40.Morange, P. E., Lijnen, H. R., Alessi, M. C., Kopp, F., Collen, D., and Juhan-Vague, I. (2000) Arterioscler. Thromb. Vasc. Biol. 20 1150–1154 [DOI] [PubMed] [Google Scholar]
- 41.Lijnen, H. R., Maquoi, E., Morange, P., Voros, G., van Hoef, B., Kopp, F., Collen, D., Juhan-Vague, I., and Alessi, M.-C. (2003) Arterioscler. Thromb. Vasc. Biol. 23 78–84 [DOI] [PubMed] [Google Scholar]
- 42.Megidish, T., White, T., Takio, K., Titani, K., Igarashi, Y., and Hakomori, S. (1995) Biochem. Biophys. Res. Commun. 216 739–747 [DOI] [PubMed] [Google Scholar]
- 43.Dobrzyn, A., and Gorski, J. (2002) Am. J. Physiol. 282 E277–E285 [DOI] [PubMed] [Google Scholar]
- 44.Koybasi, S., Senkal, C. E., Sundararaj, K., Spassieva, S., Bielawski, J., Osta, W., Day, T. A., Jiang, J. C., Jazwinski, S. M., Hannun, Y. A., Obeid, L. M., and Ogretmen, B. (2004) J. Biol. Chem. 279 44311–44319 [DOI] [PubMed] [Google Scholar]
- 45.Deng, G., Curriden, S. A., Hu, G., Czekay, R. P., and Loskutoff, D. J. (2001) J. Cell. Physiol. 189 23–33 [DOI] [PubMed] [Google Scholar]
- 46.Czekay, R.-P., Aertgeerts, K., Curriden, S. A., and Loskutoff, D. J. (2003) J. Cell Biol. 160 781–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lopez-Alemany, R., Redondo, J. M., Nagamine, Y., and Munoz-Canoves, P. (2003) Eur. J. Biochem. 270 814–821 [DOI] [PubMed] [Google Scholar]
- 48.Juhan-Vague, I., Alessi, M.-C., Mavri, A., and Morange, P. E. (2003) J. Thromb. Haemost. 1 1575–1579 [DOI] [PubMed] [Google Scholar]
- 49.Li, Z., Basterr, M. J., Hailemariam, T. K., Hojjati, M. R., Lu, S., Liu, J., Liu, R., Zhou, H., and Jiang, X. C. (2005) Biochim. Biophys. Acta. 1735 130–134 [DOI] [PubMed] [Google Scholar]
- 50.Jiang, X. C., Paultre, F., Pearson, T. A., Reed, R. G., Francis, C. K., Lin, M., Berglund, L., and Tall, A. R. (2000) Arterioscler. Thromb. Vasc. Biol. 20 2614–2618 [DOI] [PubMed] [Google Scholar]
- 51.Marathe, S., Choi, Y., Leventhal, A. R., and Tabas, I. (2000) Arterioscler. Thromb. Vasc. Biol. 20 2607–2613 [DOI] [PubMed] [Google Scholar]
- 52.Mitchinson, M. J., Hardwick, S. J., and Bennett, M. R. (1996) Curr. Opin. Lipidol. 7 324–329 [DOI] [PubMed] [Google Scholar]
- 53.Kimura, M., Soeda, S., Oda, M., Ochiai, T., Kihara, T., Ono, N., and Shimeno, H. (2000) J. Neurosci. Res. 62 781–788 [DOI] [PubMed] [Google Scholar]