Abstract
Dynamic changes in chromatin structure through ATP-dependent remodeling and covalent modifications on histones play important roles in transcription regulation. Among the many chromatin modifiers identified, the NuRD (nucleosome remodeling histone deacetylase) complex is unique because it possesses both nucleosome remodeling and histone deacetylase activities. To understand the biological function of the NuRD complex, we generated a knock-out mouse model of the Mta2 (metastasis-associated protein 2) gene, which encodes a NuRD-specific component. Mta2 null mice exhibited partial embryonic lethality. The surviving mice developed lupus-like autoimmune symptoms including skin lesions, bodyweight loss, glomerulonephritis, liver inflammation, and production of autoantibodies. Transplantation of bone marrow cells from Mta2 null mice recapitulated some of the symptoms including skin lesion and bodyweight loss in the recipient mice. Mta2 null T lymphocytes showed normal development but hyperproliferation upon stimulation, which correlates with hyperinduction of interleukin (IL)-2, IL-4, and interferon (IFN)-γ. T cell hyperproliferation, but not other autoimmune symptoms, was observed in T cell-specific Mta2 knock-out mice. Mta2 null T cells produced more IL-4 and IFN-γ under Th2 activation conditions, but normal levels of IL-4 and IFN-γ under Th1 activation conditions. Furthermore, we found that IL-4 is a direct target gene of Mta2. Our study suggests that Mta2/NuRD is involved in modulating IL-4 and IFN-γ expression in T cell immune responses, and gene expression in non-T cells plays an important role in controlling autoimmunity.
Dynamic changes in chromatin structure through ATP-dependent remodeling and covalent histone modifications play important roles in regulating gene expression. Studies in recent years have identified many ATP-dependent chromatin remodeling and histone modifying enzymes (1–3). Among them, the NuRD (nucleosome remodeling histone deacetylase) complex is of special interest because it possesses both nucleosome remodeling and histone deacetylase activities (4).
The major components of the mammalian NuRD complex include Mi-2β, Mta2 (metastasis-associated protein 2), HDAC1/2,3 RbAp46/48, and Mbd3 (5, 6). HDAC1/2 and RbAp46/48 form a deacetylase core complex that exists in both NuRD and the Sin3A histone deacetylase complex (6–8). However, Mi-2β, Mbd3, and Mta2 appear to be unique for the NuRD complex (4, 9). Mi-2β is an ATP-dependent nucleosome remodeling enzyme (10). Studies in Drosophila and Caenorhabditis elegans indicate that Mi-2β is involved in Hox gene silencing and somatic cell differentiation (11, 12). In mammalian cells, Mi-2β has been shown to interact with a master lymphocyte transcription factor Ikaros (13). Mbd3 is a member of the Mbd (methyl-CpG-binding domain) containing protein family. It interacts with Mbd2, which in turn can recruit the NuRD complex to repress transcription of methylated DNA (8, 14). Recent studies have also shown that Mbd3 is required for pluripotency of embryonic stem cells (15, 16).
In mammalian cells, Mta2 belongs to the Mta protein family, which also includes Mta1 and Mta3. In vitro studies demonstrated that MTA2 positively regulates HDAC activity (8). The C. elegans Mta2 homolog, egl-27 together with other NuRD component homologs have been shown to antagonize the Ras signaling pathway during vulval development (17). In addition to Mta2, Mta3 has also been shown to form a complex with other NuRD components and play important roles in invasive growth of breast cancer cells through repressing Snail gene transcription, and in B cell differentiation through the Bcl-6 transcription repressor (9, 18). Mta1 gene overexpression has been associated with cancer metastasis (19, 20), although whether it functions together with other NuRD components is not clear. Compared with Mta1 and Mta3, Mta2 is more ubiquitously expressed. It is likely that different Mta family members form different NuRD-like complexes with distinct functions.
Chromatin remodeling and histone modifications have been shown to play crucial roles in transcription regulation in the immune system (21). Previous studies have established that during T helper (Th) cell differentiation, expression of specific transcription factors, such as T-bet and GATA3, and cytokines, such as IFN-γ and IL-4, are regulated at the chromatin level (21–23). For example, histone hyperacetylation has been observed at the IFN-γ regulatory region in Th1 cells and at the IL-4 regulatory region in Th2 cells (24, 25). DNA demethylation at IL-4 promoters and the regulatory region have also been observed in Th2 differentiation (23, 26). Even though it is well established that histone modification and chromatin remodeling play important roles in lymphocyte differentiation and activation, little is known about the identity of the corresponding enzymes. Several recent reports have indicated a role of the NuRD complex in these processes. For example, deficiency in Mbd2, a NuRD-interacting methyl-CpG-binding protein, results in abnormal Th cell differentiation and abnormal IL-4 expression (27). Another NuRD interacting protein, Ikaros, has been shown to set thresholds for T cell activation and TCR-mediated T cell differentiation (8). However, a direct link between NuRD and T cell function has yet to be established.
To understand the in vivo function of Mta2/NuRD, we have generated Mta2 knock-out mice. Mta2 null mice exhibit multiple phenotypes including partial embryonic lethality, development defects, and more interestingly, a lupus-like autoimmune disease. This report focuses on characterizing the autoimmune phenotypes and T lymphocyte function in adult Mta2 null mice. Our data revealed the important role of Mta2/NuRD in regulating IL-4 and IFN-γ expression during Th2-prone immune response and in regulating gene expression in non-T cells to control autoimmunity.
EXPERIMENTAL PROCEDURES
Generation of Mice Carrying Mta22lox and Mta21lox Alleles and Manipulation of Mice—The Mta2 targeting vector included PGK-Neo and MC1-tk expression cassettes for positive and negative selection, respectively. Two FRT sites were inserted to each side of the PGK-Neo minigene for future removal of the expression cassette by FLP recombinase. Two loxP sites were inserted in introns 3 and 11, respectively, to flank the region including the PGK-Neo cassette and the genomic sequence from exons 4 to 11. Linearized Mta2 targeting vector was electroporated into J1 ES cells (129SvJ) and selected in the presence of G418 and FIAU (1-(2′-deoxy-2′-fluoro-β-d-arabinofuranosyl)-5′-iodouracil). Correctly targeted ES cell clones identified by PCR screens were expanded and injected into C57Bl/6 blastocysts to obtain chimeric mice, which were then bred to C57Bl/6 to produce C57Bl/6/129SvJ hybrid F1 progeny carrying the Mta22lox allele. Mice heterozygous for the Mta22lox allele were crossed with EIIa Cre transgenic mice to generate mice carrying the Mta21lox allele in which Cre recombinase-mediated loxP recombination had occurred and removed the PGK-Neo cassette as well as the genomic sequence from exons 4 to 11. The primers (set a in Fig. 1B) for genotyping Mta2 wild-type (WT) and Mta22lox alleles are the 5′ sequence 5′-GCTGAAGCAGACAGCAAAC-3′ and 3′ sequence, 5′-CATGCCAGGTTTTGAACCC-3′. PCR was performed as follows: 94 °C at 3 min followed by 35 cycles of: 94 °C for 30 s, 55 °C for 30 s, 72 °C for 45 s, and then one cycle at 72 °C for 7 min. A 390-bp fragment is derived from the mutant allele and a 335-bp fragment is derived from the wild-type allele. The primers (set b in Fig. 1B) for genotyping the Mta21lox allele are the 5′ sequence, 5′-GCTGACAGTAATGCTCGTGAGT-3′ and the 3′ sequence, 5′-ATGCTTCTCACTGAGCTACAGC-3′, and the fragment is 1.2-kb. PCR was performed as follows: 94 °C for 3 min followed by 35 cycles of: 94 °C for 30 s, 60 °C for 45 s, 72 °C for 90 s, and then one cycle at 72 °C for 7 min. A 1.5-kb fragment is derived from the Mta21lox allele.
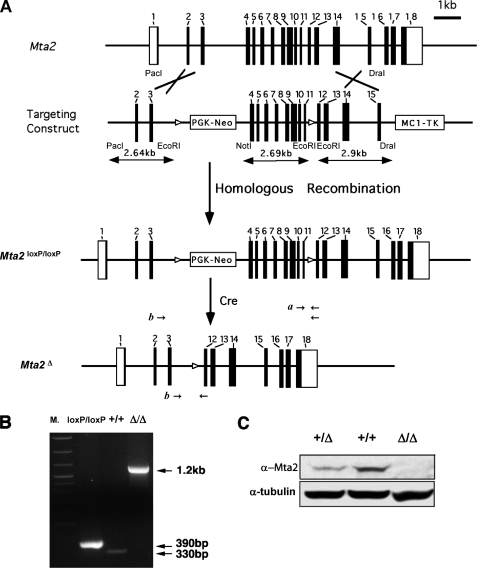
FIGURE 1.
Generation of Mta2 mutant mice. A, schematic representation of the Mta2 wild-type locus, the targeting vector, the Mta22lox allele, and the Mta21lox allele. The targeting vector contains two loxP sites (open triangle) that flank exons 4 to 11, and two expression cassettes, PGK-Neo and MC1-TK for positive and negative selection in ES cells. After the targeting construct is electroporated into ES cells, homologous recombination events were analyzed by PCR with two primer sets indicated as a and b. A third set of primers, c, is used to screen the Mta21lox allele, which is generated after Cre-mediated loxP recombination occurs in the Mta22lox allele. Cre-mediated excision of exons 4–11 generates a frameshift in exon 12. As a result, the mutant transcript only encodes the 63 N-terminal amino acids of Mta2. B, the three alleles of Mta2, namely, the wild-type allele (+/+), the Mta22lox allele, and the Mta21lox allele are distinguished by PCR. C, Western blot analysis with anti-Mta2 antibody using liver protein extracts from wild-type (+/+), heterozygous (+/Δ), and Mta2 knock-out mice (Δ/Δ). Mta2 protein was not detectable in Mta2 null mice. Tubulin was used as a loading control.
The Mta2 null and control mice described in this report were on a mixed genetic background (129SvJ and C57Bl/6). EIIa-Cre (28) transgenic mice were purchased from the Jackson Laboratory and the LckCre transgenic mice were obtained from the laboratory of Zhuang Yuan at Duke University (24). The mouse maintenance and experiments were done following the University of North Carolina Institutional Animal Care and Use Committee approved protocols. The skin and liver tissues from control and Mta2 knock-out mice were fixed in formalin for 20–24 h and embedded in paraffin. Tissue sections (5 μm) were stained with hematoxylin and eosin (H&E) for morphological examination.
ELISA—Serum was collected from Mta2 null and control mice of different ages. Serum anti-dsDNA, anti-Sm, anti-SSA, and anti-SSB antibodies levels were measured using ELISA kits from Alpha Diagnostics International Inc. (San Antonio, TX).
Flow Cytometry Analysis of Lymphoid Population—Age-matched conventional Mta2 null, T cell-specific null, and age- and sex-matched control mice were used for analysis. Single cell suspensions of thymocytes, spleen cells, and lymph node (LN) cells were stained with fluorescein isothiocyanate (FITC)-, phycoerythrin (PE)-, peridinin chlorophyll protein (PerCP)-, and allophycocyanin (APC)-conjugated monoclonal antibodies and analyzed with FACSCalibur (BD Biosciences). All the antibodies used for immunostaining were purchased from BD Biosciences. Data were analyzed using SUMMIT software.
T Cell Proliferation, Th Polarization, and Cytokine Assay—T cell proliferation assay was performed using a previously described protocol (29). Briefly, cervical, umbilical, and axial lymph nodes from each mouse were harvested and pooled. About 5 × 105 LN cells or 1 × 105 purified CD4+CD25- T cells were plated in each well of a 96-well plate. The plate was previously coated with goat anti-hamster antibody (10 μg/ml). Thirty-six to 48 h later, the cells were pulsed with [3H]thymidine and harvested after 12 h of incubation. For cytokine production assay, about 2 × 106 anti-CD3/anti-CD28 mAb-stimulated LN cells were plated in a 24-well plate and cultured for 4 days before harvesting of the supernatant. The cytometric bead array was performed according to manufacturer's instruction (BD Biosciences). For Th differentiation, CD4+ T cells were purified from pooled spleen and LN cells using the CD4+ purification kit follow up AutoMACS™ cell separation (Miltenyi Biotec Inc., Auburn, CA). 2 × 106 CD4+ cells were stimulated with anti-CD3 and anti-CD28 mAb, as described, in the presence of recombinant human IL-2 (100 units/ml) (nonpolarized condition). In addition, for Th1 differentiation, cells were stimulated in the presence of recombinant mouse IL-12 (5 ng/ml) and anti-IL-4 (4 μg/ml) (Pharmingen). For Th2 differentiation, cells were stimulated in the presence of mouse IL-4 (50 ng/ml), anti-IL-12 (5 μg/ml), and anti-IFN-γ (4 μg/ml) mAbs (Pharmingen). After 7 days of culture, cells were washed and stimulated with phorbol 12-myristate 13-acetate (10 ng/ml) and ionomycin (1 μm) for 6 h in the presence of BD GolgiPlug containing brefeldin A (Pharmingen) for the last 4 h. IL-4 and IFN-γ expression was assessed by intracellular staining. Harvested cells were stained with anti-CD4 mAb, permeabilized with BD FACS Permeabilizing Solution 2 (BD Bioscience), and stained with anti-IL4 and anti-IFN-γ mAbs. Samples were fixed with 1% paraformaldehyde and analyzed by FACSscan (BD Bioscience). 1 × 105 cells from each polarization condition were harvested and analyzed for IL-4 expression by real-time PCR.
Reconstituted SCID Mice—To reconstitute SCID mice, bone marrow (BM) cells from 11-week-old Mta2 WT and Mta2 null mice were injected into irradiated (250R) SCID-NOD mice (5 × 106 bone marrow cells per mouse). LN T cells in the reconstituted mice were harvested at 8 weeks post-reconstitution, and standard FACS and T cell proliferation assays were performed.
Quantitative Real-time PCR— Total RNA was extracted using the Qiagen RNeasy mini kit as per the manufacturer's instructions. RNAs were denatured for 3 min at 70 °C and cDNAs were synthesized in Ambion RT buffer with random decamers and 100 units of Moloney murine leukemia virus reverse transcriptase (Invitrogen) for 1 h at 42 °C followed by 10 min at 95 °C to inactivate the enzyme. Real-time PCR analysis was performed using TaqMan primer/probe mixtures (Applied Biosystems) as recommended by the manufacturer and analyzed on an Applied Biosystems 7900HT system.
Chromatin Immunoprecipitation (ChIP) Assay—The ChIP assay was modified from a previously described protocol (24). The peripheral T cells were purified by the CD4+ T cell purification kit (Miltenyi Biotec Inc., Auburn, CA). IL-4 PCR primers were: 1 forward GCCAATCAGCACCTCTCTTC, 1 reverse, TAAAGCCTCATTCCATGGTC; 2 forward, CATCGCTACACCTCCCAC, 2 reverse, CCTTGGTTTCAGCAACTTTAAC.
Statistics—Chi-square tests were used for genotype distribution analysis. T tests were used for statistical analysis of mouse bodyweight, different type of T cell analysis, and T cell proliferation assay. Wilcoxon rank test was used for survival analysis.
RESULTS
Generation of the Mta2 Mutant Mice—Although the composition and biochemical properties of the mammalian NuRD complex have been extensively characterized (4), its biological function is not well understood. To gain insight into its function, we generated a mouse model in which a component of the NuRD complex is deleted by gene targeting. We chose to inactivate Mta2 because the protein encoded by this gene appears to be present only in the NuRD complex. We made a conditional gene targeting vector in which Mta2 exons 4–11 were flanked by two loxP sites (Fig. 1A). Cre-mediated recombination at these two loxP sites resulted in deletion of exons 4–11 and a frameshift in exon 12. As a result, the Mta2 mutant allele would only encode the N-terminal 63 amino acids of the Mta2 protein (Fig. 1A). To investigate the function of Mta2 in whole animal, the Mta22lox/+ mice were crossed with EIIa-Cre transgenic mice. The Mta21lox mice carrying one completely recombined allele (equal to conventional heterozygous knock-out mice) were selected for breeding. The Mta21lox mice will be referred to as Mta2 heterozygous mice and the Mta21lox/1lox mice will be referred to as Mta2 null or Mta2 conventional null mice in this report. As shown in Fig. 1B, the Mta22lox, Mta21lox, and Mta2 wild-type allele can be distinguished by PCR. Western blot analysis of protein extracts derived from Mta2 knockout mice confirmed the lack of Mta2 protein (Fig. 1C).
Mta2 Knock-out Mice Exhibit Multiple Phenotypes Including Lupus-like Autoimmune Diseases—Inactivation of the Mta2 gene caused embryonic and perinatal lethality in about half of Mta2 null mice. Fewer than 50% of Mta2 null mice (in 129/C57/B6 mixed genetic background) survived to adulthood (Table 1). Backcross of the Mta2 null mice onto a C57/B6 background caused more severe embryonic lethality. The Mta2 knock-out embryos exhibited defects in axial skeleton, skin, and craniofacial structure with different penetrance (data not shown). The Mta2 null mice that survived postnatally exhibited smaller body size and female infertility.
TABLE 1.
Inactivation of the Mta2 gene causes partial embryonic lethality Numbers and percentages of newborn mice with three possible Mta2 genotypes (+/+, +/–, and –/–) from Mta2+/– breeding pairs or male Mta2–/– and female Mta2+/– breeding pairs. The percentages of newborn Mta2–/– mice are significantly lower than Mendelian distribution (p < 0.01), indicating some Mta2–/– mice died at embryonic stage.
|
Mta2 genotype
|
|||
|---|---|---|---|
| +/+ | +/– | –/– | |
| Mta2+/– × Mta2+/– | |||
| Number | 53 | 171 | 26 |
| Percentage | 21.2% | 68.4% | 10.4% |
| Mta2–/– × Mta2+/– | |||
| Number | 90 | 26 | |
| Percentage | 77.6% | 22.4% | |
Adult Mta2 mice also developed erosive skin lesions at multiple locations with high penetrance. More than 90% of the Mta2 null mice showed periorbital erosion by 1 month of age (Fig. 2, A and B). From 4 months of age, ∼75% of Mta2 null mice developed additional skin lesions in areas surrounding the mouth and nose, and about 30% of Mta2 null mice showed skin lesions in the neck region (Fig. 2A, upper panels). Histological sections of skin from the neck lesions of the Mta2 mutant mice revealed hyperkeratosis and acanthosis of epidermal cells, as well as a chronic inflammatory response (Fig. 2A, compare two lower panels). The periorbital skin from null mice also showed hyperkeratosis and inflammation. In some regions there were immature hair follicles and a reduced number of hair follicles although frank alopecia was not evident at these early stages. Often there was evidence of dermal and subcutaneous inflammation (panniculitis) and the overall changes could be characterized as a chronic spongiotic dermatitis. The similarity between the skin lesions of Mta2 null mice and those of mice with lupus-like autoimmune disease (30, 31) implies possible defects in the immune system of the Mta2 null mice.
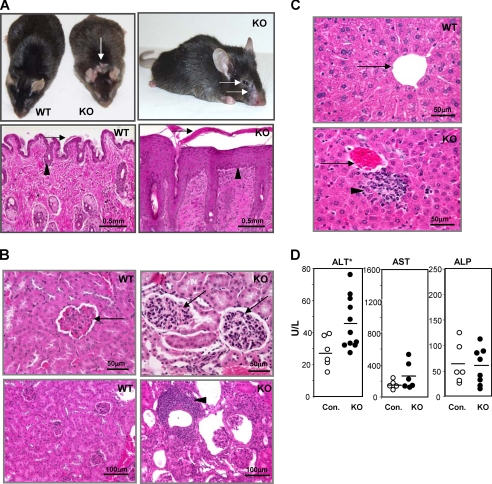
FIGURE 2.
Adult Mta2 knock-out (KO) mice exhibit autoimmunity-related phenotypes in multiple organs. A, upper panels, Mta2 KO mice show skin lesions at eyelid, mouth, nose, and neck area as indicated by arrows; lower panels, hemotoxylene and eosin (H&E) staining of skin sections from wild-type and Mta2 null mice show significant hyperkerotosis (arrow) and epidermal cell hyperplasia (acanthosis) (arrowheads)in Mta2 null mice. B, upper panels, H&E-stained kidney sections of Mta2 null mice reveal mesangial cell proliferation (arrowhead); lower panels, Mta2 null mice also show lymphocyte infiltration (arrow) in kidney. C, Mta2 null mice develop liver inflammation. H&E-stained liver sections of Mta2 null mice reveal lymphocyte infiltration (lower panel, arrowhead). Portal veins are indicated by arrows. D, comparison of the liver enzyme activity levels in serum measured by ELISA. Mta2 null mice had a significantly increased alanine aminotransferase (ALT) level (p < 0.01), indicating liver cell damage in these mice. The mutant mice showed no significant change in the levels of aspartate aminotransferase (AST) and alkaline phosphatase (ALP) in the serum of Mta2 null mice when compared with the wild-type mice. Asterisk indicates the difference is statistically significant.
A histological examination of internal organs revealed that most Mta2 null mice developed global mesangial cell proliferation in kidney (Fig. 2B, compare upper panels) and some of them also showed a significant lymphocyte-rich chronic inflammatory cell infiltration (Fig. 2B, compare lower panels). These renal abnormalities are consistent with histopathological changes occurring with glomerulonephritis seen in murine lupus models. Consistent with above symptoms observed in Mta2 null kidneys, about 40% of adult Mta2 knock-out mice showed moderate (>30 mg/dL) or high (>100 mg/dL) urine protein levels (n = 20). Control mice showed only trace or negative urine protein levels. This phenotype is more severe in female null mice than in the male null mice. In addition, liver inflammation was also found in more than half of the adult Mta2 null mice. The lymphocyte infiltration was generally limited to the portal regions (Fig. 2C).
Consistent with injury to hepatocytes in the Mta2 null mice, ELISA results showed that the serum alanine aminotransferase activity level is increased when compared with that of wild-type and heterozygous littermates (Fig. 2D). However, the activity levels of serum aspartate aminotransferase and alkaline phosphatase did not exhibit a significant difference between wild-type and Mta2 null mice (Fig. 2D).
To further characterize the autoimmune disease observed in Mta2 null mice, we collected serums from mutant and control mice at different ages and examined them for the different autoantibodies. The Crithidia Luciliae assay demonstrated the presence of anti-dsDNA antibody in the Mta2 null serum (Fig. 3A). ELISA analysis showed significantly higher levels of anti-dsDNA, anti-Sm, anti-SSA, and anti-SSB antibodies (Fig. 3B). No difference in autoantibody level had been observed between Mta2 heterozygous mice and wild-type mice. Anti-dsDNA and anti-Sm antibodies are markers of human and murine systemic lupus erythematosus (SLE), and anti-SSA and anti-SSB antibodies are often evident with Sjogren disease or SLE.
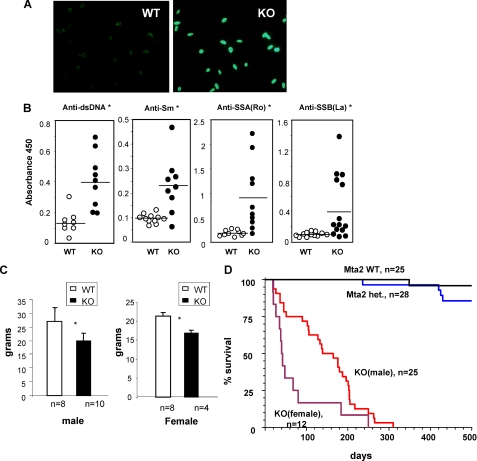
FIGURE 3.
Mta2 null mice develop lupus specific autoantibodies. A, production of anti-dsDNA antibody is detected by the Crithidia Luciliae assay that showed positive anti-dsDNA antibody in the Mta2 null serum but not wild-type serum. B, ELISA analysis demonstrates significantly elevated levels of different autoantibodies, including anti-dsDNA, anti-Sm, anti-SSA, and anti-SSB, in Mta2 null mice when compared with those of wild-type mice (p < 0.02). C, bodyweight of 2-month-old wild type and Mta2 null mice are plotted according to their genders. The average bodyweight of null mice is about 75% of that of their wild-type litter mates. D, Kaplan-Meier survival analysis of Mta2 null and control mice. Mta2 null mice have much shorter lifespans when compared with wild-type or heterozygous mice. Female null mice have a shorter lifespan than the male null mice that is similar to what have been observed in lupus patients and murine lupus models. Asterisk indicates the difference is statistically significant (p < 0.05).
Furthermore, Mta2 null mice are smaller than their littermates. The extent of difference depends on mouse development stages. From perinatal to weaning stage, Mta2 null mice are about half to 2/3 size of the heterozygous or wild-type littermates. When they reach mature age (after 2 months old), both male and female null mice are about 75% of the size of their wild-type littermates (Fig. 3C). Mta2 null mice also have shortened lifespans when compared with their wild-type or heterozygous littermates (Fig. 3D). Interestingly, female knock-out mice exhibit a shorter lifespan than male knock-out mice. Given that 90% of SLE patients are female (32), and some lupus mouse models have shown more severe phenotype in females than in males (33, 34), it is very likely that autoimmune-related symptoms are important factors causing early death in Mta2 null mice. However, our results do not exclude other factors in causing premature death. In summary, our observations strongly suggest that Mta2 null mice develop an autoimmune disease that resembles systemic lupus erythematosus in human.
Bone Marrow Transplantation Recipient Mice Partially Recapitulate the Autoimmune Phenotypes— To test if the autoimmune phenotypes observed in the Mta2 null mice is caused by bone marrow derived from Mta2 null cells; we transferred Mta2 null bone marrow progenitor cells into SCID mice and monitored hematopoietic reconstitution over time. No significant defects were observed in lymphoid and myeloid reconstitution with Mta2 mutant progenitor cells (data not shown). However, recipient mice transplanted with Mta2 null hematopoietic stem and progenitor cell developed skin lesions and reduced body weight (Fig. 4, A and B), similar to Mta2 null mice. When mice were analyzed 8–12 weeks post bone marrow transplantation, normal T cells were present in the thymus and peripheral lymphoid organs (Fig. 4C). However, these Mta2 null T cells were hyperproliferative when stimulated with T cell mitogens (Fig. 4D). The phenotypes are, however, less severe compared with Mta2 null mice, and no obvious liver inflammation or glomerulonephritis was detected in the transplanted mice. No increased level of autoantibodies was observed either (data not shown). These results indicate that hematopoietic cells derived from Mta2 null bone marrow stem cells contribute to but are not sufficient to cause the autoimmune diseases. Therefore, nonhematopoietic cells must have also contributed to the autoimmune phenotypes of Mta2 null mice.
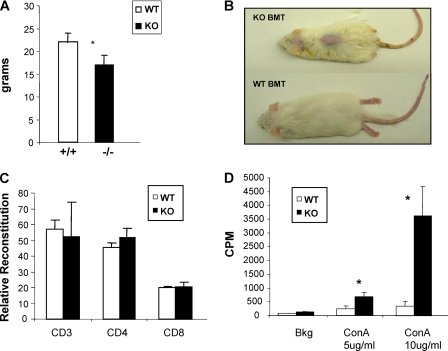
FIGURE 4.
Mta2-deficient BM cells cause decreased bodyweight, skin lesion, and T cell hyperproliferation in recipient SCID mice. A, the transfer of BM cells from Mta2 knock-out (KO) mice cause 25% decreased bodyweight in recipient SCID mice 8 weeks after bone marrow transplantation (p < 0.03). B, a representative SCID mouse received Mta2 KO BMT showed skin lesion (upper mouse), whereas the representative SCID mouse received BMT from wild-type mice exhibited normal skin (lower mouse) 8 weeks after transplantation. In this experiment, all three SCID mice that received Mta2 KO BM cells developed skin lesion and none of the four SCID mice received wild-type BM cells developed skin lesion. C, FACS analysis indicated that transplanted Mta2 KO BM cells successfully reconstituted in T cells in SCID mice. The relative reconstitution of T cells from Mta2 KO BMT SCID mice and WT BMT SCID mice were plotted (n = 3 for each genotype). D, the LN cells from Mta2 KO BMT SCID mice showed hyperproliferation upon different concentrations of concanavalin A (ConA) stimulation. Asterisk indicates the difference is statistically significant (p < 0.05).
T Cell Hyperproliferation in Mta2 Null Mice—The autoimmune phenotypes described above and previous reports on epigenetic regulation in T cell development and differentiation by some NuRD components prompted us to investigate possible T cell defects in Mta2 null mice. We found that Mta2 null mice exhibited normal T cell developments in thymus and peripheral lymphoid organs (Fig. 5A). The development of FoxP3+CD4+CD25+ Treg cells and their functions are also not affected by loss of Mta2 (data not shown). Our data also indicated that the ratio of naïve T cells and memory T cells in Mta2-deficient peripheral lymphoid organs was normal (Fig. 5A). However, when stimulated with anti-CD3, or anti-CD3 in combination with anti-CD28 mAbs, Mta2-deficient T cells from LN were hyperproliferative compared with the LN T cells from control mice (Fig. 5B) (n = 7 for each genotype). Furthermore, purified CD4+CD25- peripheral T cells from Mta2 null mice also showed hyperproliferation in response to TCR stimulation (Fig. 5C). To evaluate whether the observed hyperproliferation is T cell autonomous, we have generated LckCre, Mta22lox/1lox, and LckCre, Mta22lox/2lox mice that exhibit Mta2 gene deletion in T cells. Similar to that observed in the conventional Mta2 null mice, T cells from T cell-specific Mta2 null (TKO) mice also exhibited hyperproliferation upon T cell activation (Fig. 5D). However, no other autoimmune phenotypes were observed in these mice (data not shown), suggesting that non-T cells must have contributed to the autoimmune disease in the Mta2 conventional null mice.
FIGURE 5.
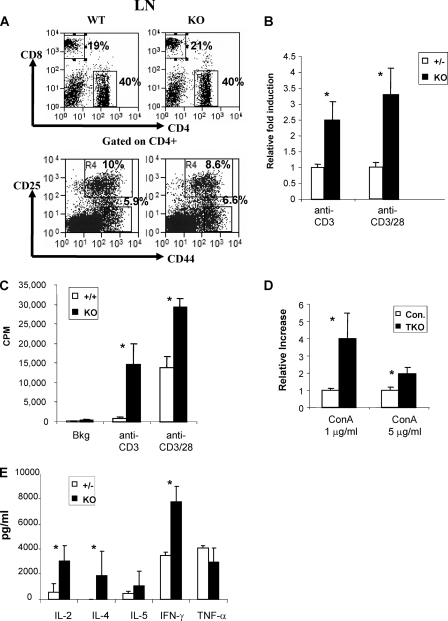
Inactivation of Mta2 gene causes intrinsic T cell hyperproliferation. A, Mta2 null mice showed normal peripheral T cell development. Representative plots of FACS analysis for CD4/CD8 expression on LN cells (upper) and CD44/CD25 (lower) expression on CD4+ LN cells. B, Mta2 null LN cells are hyperproliferative in response to anti-CD3, or anti-CD3 and anti-CD28 mAb stimulation (p < 0.01). Heterozygous littermates were used as controls because no significant difference between heterozygous and wild-type mice has been observed. Data were collected from 5 independent experiments (n = 7 for each genotype). The average value obtained from heterozygous LN cells in each experiment is normalized to 1. C, peripheral CD4+CD25- T cells were purified from Mta2 null and wild-type mice, and then incubated and stimulated with anti-CD3 or anti-CD3 and anti-CD28 mAb antibodies. Mta2 null CD4+CD25- T cells were hyperproliferative compared with wild-type CD4+CD25- T cells. D, T cells from T cell-specific knock-out (KO) mice are hyperproliferative. LN cells from LckCre,Mta22lox/lox mice and LckCre,Mta22lox/2lox mice and TKO are hyperproliferative when compared with LN cells from control mice (Con.) under concanavalin A (ConA) stimulation (n = 4 for each genotype, p < 0.05). Control mice include LckCre, Mta22lox/+, and Mta22lox/+ mice. E, cytokine analysis showed increased secretion of IL-2, IL-4, and IFN-γ by the Mta2 null T cells upon anti-CD3/anti-CD28 mAb stimulation, but no significant difference has been observed in terms of the level of IL-5 or TNF-α (n = 2 for each genotype). Asterisk indicates the difference is statistically significant (p < 0.05).
To understand the molecular basis of T cell hyperproliferation in response to Mta2 inactivation, we analyzed the effects of Mta2 inactivation on the levels of various cytokines induced by the LN-derived lymphocytes. Results shown in Fig. 5E indicate that Mta2 null T cells produced more IL-2, IL-4, and IFN-γ than control cells when stimulated with anti-CD3 and anti-CD28 mAbs, consistent with hyperproliferation of Mta2 null T cells.
Mta2 Regulates IL-4 and IFN-γ Production under Th2 Conditions— In response to Mta2 inactivation, both IFN-γ and IL-4 were hyperinduced in TCR-activated T cells (Fig. 5E). Given that epigenetic mechanisms are involved in silencing IL-4 and IFN-γ during T cell activation under Th1 or Th2 conditions, respectively (21–23), we asked whether Mta2 is involved in silencing of the IL-4 and IFN-γ genes under Th1 or Th2 conditions. Results shown in Fig. 6A indicate Mta2 inactivation did not affect Th1 polarization, suggesting that Mta2 is not involved in modulating IL-4 or IFN-γ expression during Th1 responses. However, expression of both IL-4 and IFN-γ in Mta2 null T cells was elevated under Th2 conditions when compared with that of wild-type T cells (Fig. 6B). These results suggest that Mta2 is involved in suppressing expression of both IL-4 and IFN-γ during Th2 responses.
FIGURE 6.
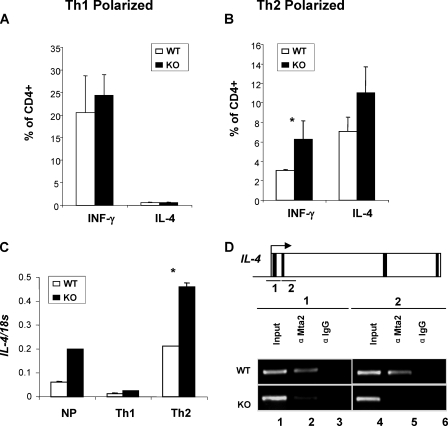
IL-4 is a direct target of Mta2. A, normal Th1 polarization in Mta2 null cells. B, loss of Mta2 function results in increased IFN-γ and IL-4 under Th2 conditions. Results of FACS analysis are shown on A and B as bar graphs representing percentage of IFN-γ+ and IL-4+ gated on CD4+ cells. Data presented are an average of 3 mice per genotype from two independent experiments. Standard deviation is shown as error bars. C, real-time reverse transcriptase-PCR results of IL-4 expression levels in cells activated under non-polarizing, Th1 or Th2 conditions. Results are expressed as the ratio of IL-4 over 18 S to control for amounts of cDNA analyzed (p < 0.01). D, ChIP assays demonstrate that Mta2 is localized to the IL-4 gene in the wild-type, but not in the Mta2 null mice. Exons are indicated by black strips. Asterisk indicates the difference is statistically significant (p < 0.05).
IL-4 Is a Direct Target of Mta2—Considering the fact that Mta2/NuRD is a co-repressor complex (8, 14), one likely explanation for the increased production of cytokines by Mta2 null T cells is increased transcription of these genes caused by inactivation of the co-repressor complex. To explore this possibility, we analyzed the effects of Mta2 inactivation on the expression of IL-4 under stimulation by real-time PCR. Consistent with the increase in the protein levels (Figs. 4E and 6B), inactivation of Mta2 also resulted in an increase in the IL-4 mRNA level (Fig. 6C). To determine whether the IL-4 gene is a direct target of Mta2/NuRD, ChIP assays were performed using a polyclonal antibody against Mta2 in activated T cells. As a control for antibody specificity, rabbit IgG was also included in a parallel experiment. Data shown in Fig. 6D demonstrate that Mta2 binds to the promoter and downstream of the transcription start site (lanes 2 and 5). The ChIP signal is specific because a parallel ChIP assay using Mta2 null cells did not enrich binding of Mta2. Based on these results, we conclude that the IL-4 gene is a direct target of the Mta2-NuRD complex. The elevated expression of IFN-γ under Th2 conditions suggests that Mta2/NuRD likely suppress IFN-γ gene expression. However, we failed to detect direct binding of Mta2 to the IFN-γ promoter region (data not shown).
DISCUSSION
NuRD is a multisubunit protein complex that includes: Mta2, Mbd3, and Mi-2β, HDAC1/2 and RbAp46/48 (4, 9). NuRD is unique because it possesses both histone deacetylase and chromatin remodeling activities. To understand the in vivo function of this interesting complex, mice deficient in Mbd3 and Mi-2β, respectively, have been generated and characterized. Loss of Mbd3 function results in embryonic lethality and loss of ES cell pluripotency, suggesting a key function of NuRD in maintaining ES cell pluripotency and early embryogenesis (15, 16, 35). The phenotypes of T cell-specific Mi-2β knock-out revealed a critical role for Mi-2β in regulating CD4 gene expression. However, instead of being a component of the NuRD co-repressor complex, Mi-2β appeared to activate CD4 expression in T cells by association with p300 and HeLa E-box-binding protein (HEB) at the CD4 enhancer (36). Therefore, the Mta2 knock-out mouse model presented here is the first model for understanding the function of the NuRD complex beyond embryonic development.
Based on production of autoantibodies, glomerulonephritis, skin lesions, and liver symptoms (Figs. 2 and 3), we conclude that Mta2 null mice develop lupus-like autoimmune disease. Systemic lupus erythematosus is a major autoantibody-mediated autoimmune disease. Until now, the etiology of SLE remains unclear. Many knock-out and transgenic mouse models have provided important insights into the cellular and molecular mechanisms underlying lupus, and can be used to test potential lupus drugs. Genes that play important roles in SLE can be largely classified into several functional groups including: 1) apoptosis related, e.g. Fas, Fas-L, and Bcl-2;2) T cell activation related, e.g. CTLA4, PD1, and gadd45; 3) B cell activation related, e.g. Blys, Fyn, and Btk; and 4) phagocytosis and debris clearance related, e.g. c-Mer and DNase I, and transcription factors such as Aiolos. The Mta2 knock-out model described here is the first model to show that defects in chromatin remodeling and histone modification also cause lupus-like disease.
Inactivation of Mta2, although not affecting T cell development, causes T cell hyperproliferation upon TCR-mediated stimulation. Because the percentages of the naïve T cells, regulatory T cells, and memory T cells in peripheral are not affected by Mta2 inactivation (Fig. 5A and data not shown), it is likely that Mta2 may be involved in controlling the threshold of T cell proliferation upon stimulation. This T cell phenotype has been observed in purified native CD4 T cells, in T cell-specific Mta2 null mice, and in T cells from BMT SCID mice, suggesting a cell autonomous hyperproliferation. Consistent with this possibility, Mta2 null mice exhibited autoimmune phenotypes. It is worth noting that Mta2 null-BMT mice and TKO mice only recapitulate some of the Mta2 null mice phenotypes, indicating that non-hematopoietic cells or non-T cells also contribute to the observed autoimmune disease in Mta2 null mice. In a different study, we have found that Mta2 null B cells exhibit severe developmental and differentiation defects (data not shown). However, those defects seem not to contribute to the autoimmune phenotypes observed in Mta2 null mice as B cell-specific knock-out mice do not exhibit any of the autoimmune phenotypes observed in Mta2 conventional null mice.
In this study, we provide evidence supporting a role for Mta2 in modulating cytokine gene expression during T cell activation. It inhibits IL-4 expression in Th2 cells, as a potential mechanism to prevent overproduction of IL-4 and Th2-related autoimmunity. In addition, we also demonstrate that IL-4 is a direct target of Mta2 in T cells. Because Mta2 facilitates deacetylase activity of the NuRD complex (37), our observation that the IL-4 expression level is increased in Mta2 null T cells is consistent with the role of NuRD as a co-repressor. Future studies will address: (a) whether histone acetylation level at IL-4 promoter is affected; (b) whether other components of NuRD are localized at this locus; and (c) whether Mta2 deficiency affects chromatin remodeling activity of NuRD. Our data suggest that an Mta2-independent mechanism must be responsible for silencing the IL-4 gene in Th1 cells. Our studies also indicate that Mta2/NuRD has a role in suppressing IFN-γ expression in Th2 cells. In Th2 cells, elevated IFN-γ is expressed in the presence of higher levels of IL-4, although direct binding of Mta2 to the IFN-γ gene is not detected. Thus, Mta2 may participate in suppressing IFN-γ gene expression via indirect mechanisms. Further characterization of other immune cell types in the Mta2 null mice, identification of more Mta2/NuRD target genes, and epigenetic change occurred at the regulatory regions of these genes will give us a better picture of the role of Mta2/NuRD in the mammalian immune system.
Acknowledgments
We thank Dr. Hyung Kim and Dr. Virginia Godfrey for veterinary help and Manda Edwards for technical assistance.
This work was supported, in whole or in part, by National Institutes of Health Grants GM63067 (to Y. Z.) and AI48407 and HL72240 (to L. S.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: HDAC, histone deacetylase; KO, knockout; WT, wild type; TKO, T cell-specific knockout; BMT, bone marrow transplantation; SCID, severe combined immunodeficiency; ChIP, chromatin immunoprecipitation; SLE, systemic lupus erythematosus; IL, interleukin; IFN, interferon; TCR, T cell receptor; ELISA, enzyme-linked immunosorbent assay; LN, lymph node; mAb, monoclonal antibody; FACS, fluorescence-activated cell sorter; dsDNA, double stranded DNA; ES cell, embryonic stem cell.
References
- 1.Margueron, R., Trojer, P., and Reinberg, D. (2005) Curr. Opin. Genet. Dev. 15 163-176 [DOI] [PubMed] [Google Scholar]
- 2.Martin, C., and Zhang, Y. (2005) Nat. Rev. Mol. Cell. Biol. 6 838-849 [DOI] [PubMed] [Google Scholar]
- 3.Cairns, B. R. (2005) Curr. Opin. Genet. Dev. 15 185-190 [DOI] [PubMed] [Google Scholar]
- 4.Feng, Q., and Zhang, Y. (2003) Curr. Top. Microbiol. Immunol. 274 269-290 [DOI] [PubMed] [Google Scholar]
- 5.Xue, Y., Wong, J., Moreno, G. T., Young, M. K., Cote, J., and Wang, W. (1998) Mol. Cell 2 851-861 [DOI] [PubMed] [Google Scholar]
- 6.Zhang, Y., LeRoy, G., Seelig, H. P., Lane, W. S., and Reinberg, D. (1998) Cell 95 279-289 [DOI] [PubMed] [Google Scholar]
- 7.Zhang, Y., Iratni, R., Erdjument-Bromage, H., Tempst, P., and Reinberg, D. (1997) Cell 89 357-364 [DOI] [PubMed] [Google Scholar]
- 8.Avitahl, N., Winandy, S., Friedrich, C., Jones, B., Ge, Y., and Georgopoulos, K. (1999) Immunity 10 333-343 [DOI] [PubMed] [Google Scholar]
- 9.Bowen, N. J., Fujita, N., Kajita, M., and Wade, P. A. (2004) Biochim. Biophys. Acta 1677 52-57 [DOI] [PubMed] [Google Scholar]
- 10.Wang, H., Cao, R., Xia, L., Erdjument-Bromage, H., Borchers, C., Tempst, P., and Zhang, Y. (2001) Mol. Cell 8 1207-1217 [DOI] [PubMed] [Google Scholar]
- 11.Kehle, J., Beuchle, D., Treuheit, S., Christen, B., Kennison, J. A., Bienz, M., and Muller, J. (1998) Science 282 1897-1900 [DOI] [PubMed] [Google Scholar]
- 12.Unhavaithaya, Y., Shin, T. H., Miliaras, N., Lee, J., Oyama, T., and Mello, C. C. (2002) Cell 111 991-1002 [DOI] [PubMed] [Google Scholar]
- 13.Hollander, M. C., Sheikh, M. S., Bulavin, D. V., Lundgren, K., Augeri-Henmueller, L., Shehee, R., Molinaro, T. A., Kim, K. E., Tolosa, E., Ashwell, J. D., Rosenberg, M. P., Zhan, Q., Fernandez-Salguero, P. M., Morgan, W. F., Deng, C. X., and Fornace, A. J., Jr. (1999) Nat. Genet. 23 176-184 [DOI] [PubMed] [Google Scholar]
- 14.Feng, Q., and Zhang, Y. (2001) Genes Dev. 15 827-832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaji, K., Caballero, I. M., Macleod, R., Nichols, J., Wilson, V. A., and Hendrich, B. (2006) Nat. Cell. Biol. 8 285-292 [DOI] [PubMed] [Google Scholar]
- 16.Kaji, K., Nichols, J., and Hendrich, B. (2007) Development 134 1123-1132 [DOI] [PubMed] [Google Scholar]
- 17.Solari, F., and Ahringer, J. (2000) Curr. Biol. 10 223-226 [DOI] [PubMed] [Google Scholar]
- 18.Fujita, N., Jaye, D. L., Kajita, M., Geigerman, C., Moreno, C. S., and Wade, P. A. (2003) Cell 113 207-219 [DOI] [PubMed] [Google Scholar]
- 19.Toh, Y., Oki, E., Oda, S., Tokunaga, E., Ohno, S., Maehara, Y., Nicolson, G. L., and Sugimachi, K. (1997) Int. J. Cancer 74 459-463 [DOI] [PubMed] [Google Scholar]
- 20.Toh, Y., Kuwano, H., Mori, M., Nicolson, G. L., and Sugimachi, K. (1999) Br. J. Cancer 79 1723-1726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smale, S. T., and Fisher, A. G. (2002) Annu. Rev. Immunol. 20 427-462 [DOI] [PubMed] [Google Scholar]
- 22.Ansel, K. M., Lee, D. U., and Rao, A. (2003) Nat. Immunol. 4 616-623 [DOI] [PubMed] [Google Scholar]
- 23.Lee, G. R., Kim, S. T., Spilianakis, C. G., Fields, P. E., and Flavell, R. A. (2006) Immunity 24 369-379 [DOI] [PubMed] [Google Scholar]
- 24.Avni, O., Lee, D., Macian, F., Szabo, S. J., Glimcher, L. H., and Rao, A. (2002) Nat. Immunol. 3 643-651 [DOI] [PubMed] [Google Scholar]
- 25.Messi, M., Giacchetto, I., Nagata, K., Lanzavecchia, A., Natoli, G., and Sallusto, F. (2003) Nat. Immunol. 4 78-86 [DOI] [PubMed] [Google Scholar]
- 26.Fields, P. E., Lee, G. R., Kim, S. T., Bartsevich, V. V., and Flavell, R. A. (2004) Immunity 21 865-876 [DOI] [PubMed] [Google Scholar]
- 27.Hutchins, A. S., Mullen, A. C., Lee, H. W., Sykes, K. J., High, F. A., Hendrich, B. D., Bird, A. P., and Reiner, S. L. (2002) Mol. Cell 10 81-91 [DOI] [PubMed] [Google Scholar]
- 28.Lakso, M., Pichel, J. G., Gorman, J. R., Sauer, B., Okamoto, Y., Lee, E., Alt, F. W., and Westphal, H. (1996) Proc. Natl. Acad. Sci. U. S. A. 93 5860-5865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kovalev, G. I., Franklin, D. S., Coffield, V. M., Xiong, Y., and Su, L. (2001) J. Immunol. 167 3285-3292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kanauchi, H., Furukawa, F., and Imamura, S. (1991) J. Investig. Dermatol. 96 478-483 [DOI] [PubMed] [Google Scholar]
- 31.Lomvardas, S., and Thanos, D. (2002) Cell 110 261-271 [DOI] [PubMed] [Google Scholar]
- 32.Whitacre, C. C. (2001) Nat. Immunol. 2 777-780 [DOI] [PubMed] [Google Scholar]
- 33.Roubinian, J. R., Talal, N., Greenspan, J. S., Goodman, J. R., and Siiteri, P. K. (1978) J. Exp. Med. 147 1568-1583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Salvador, J. M., Hollander, M. C., Nguyen, A. T., Kopp, J. B., Barisoni, L., Moore, J. K., Ashwell, J. D., and Fornace, A. J., Jr. (2002) Immunity 16 499-508 [DOI] [PubMed] [Google Scholar]
- 35.Hendrich, B., Guy, J., Ramsahoye, B., Wilson, V. A., and Bird, A. (2001) Genes Dev. 15 710-723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Williams, C. J., Naito, T., Arco, P. G., Seavitt, J. R., Cashman, S. M., De Souza, B., Qi, X., Keables, P., Von Andrian, U. H., and Georgopoulos, K. (2004) Immunity 20 719-733 [DOI] [PubMed] [Google Scholar]
- 37.Zhang, Y., Ng, H. H., Erdjument-Bromage, H., Tempst, P., Bird, A., and Reinberg, D. (1999) Genes Dev. 13 1924-1935 [DOI] [PMC free article] [PubMed] [Google Scholar]