Abstract
This study investigated the anatomical relationship between covert and overt shifts of attention. Previous studies have found that the areas of the brain activated by covert and overt shifts of attention are very similar. However, despite a general agreement between studies there are a few issues that merit closer inspection. Primarily, the tasks aiming to produce covert or overt shifts of attention were not always comparable. Secondly, the previous studies disagreed on whether greater neural activity is elicited by covert shifts or overt shifts of attention. Thirdly, the previous studies differed on whether the shifts of attention are exogenously or endogenously driven. Finally, the statistical analyses used by all previous studies failed to account for between subject variability. The present study was designed to address all these issues. Twelve healthy subjects performed either covert or overt shifts of attention while the functional magnetic resonance imaging (fMRI) signal was continuously measured. In line with the previous studies, the present study showed a virtually complete overlap between areas of the brain recruited during covert shifts of attention and areas of the brain recruited during overt shifts of attention. Furthermore, using endogenously driven shifts, of attention this study found that overt shifts of attention resulted in higher levels of activation in the brain than covert shifts of attention. The results of this study provide support for the premotor theory of attention that posits that the attentional and motor systems are the result of neural activation in the same areas of the brain.
Keywords: Attentional orienting, Functional MRI, Saccades, Covert attention shifts, Parietal cortex, Frontal cortex, Premotor theory of attention
1. Introduction
Visuospatial attention refers to the ability to selectively process the relevant events in the visual surroundings and ignore the irrelevant events. Spatial attention can be directed to peripheral visual events in two ways. First, head and eye movements can be employed to gaze directly at an item. This is often referred to as an overt shift of attention. Alternatively, spatial attention can be directed towards the relevant event without movement of the eyes, often referred to as a covert shift of attention. Covert spatial attention allows an observer to ‘look out of the corner of his eye’, independent of eye movements. In addition to the distinction between covert and overt shifts of attention, the literature typically dissociates between reflexive externally driven (exogenous) shifts of attention and strategic (endogenous) shifts of attention. An example of an exogenous overt shift of attention would be a reflexive eye movement in response to a rapidly moving object that appeared in the periphery of the visual field. On the other hand, the voluntary decision to look both directions before crossing a street would reflect an endogenous overt shift of attention. Both covert and overt shifts of attention can be either exogenous or endogenous. This study investigates the relationship between covert and overt shifts of attention.
Logically, the relationship between covert and overt shifts of attention must lie somewhere on a continuum from complete neural independence to identical neural circuitry. Scientists have advocated each of the three possible relationships; (1) Posner and Petersen (1990) suggest covert shifts of attention and overt shifts of attention are completely independent of one another, (2) Corbetta (1998) argues that covert shifts of attention and overt shifts of attention are interdependent and (3) Rizzolatti and colleagues (1987) hypothesize that covert shifts of attention and overt shifts of attention use the same neural circuitry. The pattern of brain activity associated with overt and covert shifts of attention can dissociate between these possibilities. On one extreme end of the debate, Posner & Petersen (1990) argue that covert and overt shifts of attention are mediated by independent systems. This theory would predict that covert shifts of attention activate a different set of brain regions than overt shifts of attention. This theory is known as the modular theory of attention. On the other extreme end of the debate Rizzolatti and colleagues (1987) suggest that covert and overt shifts of attention are governed by the same set of brain areas. In this view, a covert shift of attention is the result of neural activation in the areas of the brain responsible for eliciting eye movement. Accordingly, the only difference between a covert and overt shift of attention is whether an oculomotoric response is executed; the eye movement planning is identical. This theory would predict that covert and overt shifts of attention elicit activation in the same areas of the brain. In essence, it suggests that covert shifts of attention are implemented by commandeering the targeting system used to guide eye movements. This model is known as the premotor theory of attention. The intermediate view put forward by Corbetta (1998) suggests that covert and overt attention will elicit some common and some different regions of brain activation.
Over the years, a number of studies have investigated the relationship between covert and overt shifts of attention by comparing neural activation elicited by covert shifts of attention to neural activation evoked by overt shifts of attention (Beauchamp et al., 2001; Nobre et al., 2000; Perry and Zeki, 2000; Corbetta et al., 1998). These studies have all found that the areas of the brain activated by covert and overt shifts of attention are very similar. This provides support for the premotor theory of attention. Specifically, a fronto-parietal network seems to be reliably activated by both covert and overt shifts of attention (Beauchamp et al., 2001; Nobre et al., 2000; Kanwisher and Wojciulik, 2000; Perry and Zeki, 2000; Corbetta et al., 1998). Despite this apparent agreement between these studies, there are a number of issues that merit closer attention.
Firstly, in order to directly compare neural activation elicited by covert and overt shifts of attention, the tasks aiming to produce covert or overt shifts of attention should be comparable. In this respect neither the study by Nobre and colleagues (2000) nor the study by Corbetta and colleagues (1998) is optimal. In the study by Nobre and colleagues (2000) the visual stimulation differs between the task that requires subjects to make covert shifts of attention and the task that requires subjects to make overt shifts of attention. In the study by Corbetta and colleagues (1998) the visual stimulation between the two tasks is equated, but the amplitude of the shift distance from fixation in the task requiring subjects to make covert shifts of attention is much larger than the amplitude of the shift distance from fixation in the task requiring subjects to make overt shifts of attention. These inequalities between the tasks aiming to produce covert and overt shifts of attention could result in differences in task related neural activity that does not reflect a difference between the neural substrate of covert and overt shifts of attention, but simply a difference in the task demands.
Secondly, the studies disagree on whether greater neural activity is elicited by covert shifts or overt shifts of attention. While some studies argue that greater neural activity is evoked by covert shifts of attention than by overt shifts of attention (Nobre et al., 2000; Perry and Zeki, 2000; Corbetta et al., 1998), another study argues that overt shifts of attention produce greater neural activation than covert shifts of attention (Beauchamp et al., 2001). In both the study by Corbetta and colleagues (1998) and the study by Nobre and colleagues (2000) the larger amount of neural activity during covert shifts of attention could be explained by the differences in task demands between the task that requires subjects to make covert shifts of attention and the task that requires subjects to make overt shifts of attention. However, in both the study by Perry and Zeki (2000) and the study by Beauchamp and colleagues (2001), these task demands are largely equated. The question therefore remains why the study by Perry and Zeki finds greater neural activation during covert shifts of attention while the study by Beauchamp and colleagues finds more neural activation during overt shifts of attention.
Thirdly, the studies differ on whether the shifts of attention are exogenously or endogenously driven. Interestingly, all the studies mentioned above that find more neural activation during covert than during overt shifts of attention also use endogenously driven shifts of attention (Nobre et al., 2000; Perry and Zeki, 2000; Corbetta et al., 1998). The single study that finds more neural activation during overt than during covert shifts of attention uses exogenously driven shifts of attention (Beauchamp et al., 2001). This raises the question of whether the difference between the results of Beauchamp and colleagues (2001) on one hand and Perry and Zeki (2000) on the other hand can be explained by a dissimilarity in the mechanisms underlying exogenously and endogenously driven shifts in covert and overt attention. More specifically, it could be that endogenously driven covert shifts of attention lead to higher amounts of neural activation than endogenously driven overt shifts of attention while exogenously driven covert shifts of attention lead to lower amounts of neural activation than exogenously driven overt shifts of attention. While the results of some studies suggest that exogenously and endogenously driven shifts of attention are governed by the same neural mechanisms (Peelen et al., 2004; Rosen et al., 1999), the results of other studies suggest that exogenously and endogenously driven shifts of attention rely on distinct neural mechanisms (Natale et al., 2006; Hopfinger and West, 2006; Mayer et al., 2004; Mort et al., 2003; Corbetta and Shulman, 2002; Briand, 1998). However, it seems that regardless of whether covert or overt shifts of attention are required, endogenously driven shifts of attention are associated with either similar or higher amounts of neural activation compared to exogenously driven shifts of attention (Kincade et al., 2005; Peelen et al., 2004; Mayer et al., 2004; Mort et al., 2003; Rosen et al., 1999), consistent with the general idea that endogenously driven shifts of attention are more effortful than exogenously driven shifts of attention (Shiffrin and Schneider, 1984; Schneider and Shiffrin, 1984). Nevertheless, to this date no studies exist that directly compare endogenously and exogenously driven shifts in covert and overt attention. This means that the difference between the results from Perry and Zeki (2000) and the results from Beauchamp and colleagues (2001) regarding the type of attentional shift that elicits the largest amount of neural activation could theoretically be attributed to a difference in the mechanisms governing exogenously and endogenously driven shifts of attention in covert and overt attention.
Finally, the statistical analyses of all four studies presented above only perform socalled first level fixed effects fMRI analyses and no second level random effects fMRI analyses. In other words, these studies only take within subject variability into account and not between subject variability. This means that strictly speaking, the results from these studies cannot be generalized to the wider population (Holmes and Friston, 1998). The demonstration of overlap in the areas activated during covert and overt shifts of attention would be more convincing if both within and between subject variability were to be taken into account.
The present study was designed to address all these issues. Subjects were presented with two attentional tasks while the fMRI signal was continuously measured. In one task they were instructed to perform covert shifts of attention while in the other task they were instructed to perform overt shifts of attention. Both the covert and overt shifts of attention tasks consisted of a condition involving shifting attention peripherally and a condition involving maintaining attention centrally. The covert and overt shifts of attention tasks were run in different fMRI runs. Virtually identical visual stimuli were used in both the covert attention shift task and the overt attention shift task and great care was taken to keep the task demands similar. Furthermore, like the shifts of attention in the study performed by Perry and Zeki (200), the shifts of attention in this study were all endogenously driven. If this study, like the study performed by Beauchamp and colleagues (2001) and in contrast to the study performed by Perry and Zeki (2000) finds more neural activation during overt shifts of attention than during covert shifts of attention, then this would not support the suggestion that the deviating findings by Beauchamp and colleagues are somehow due to the fact that their study is the only one that uses exogenously driven shifts of attention. Finally, the present study will employ statistical analyses that control for both within and between subject variability.
2. Results
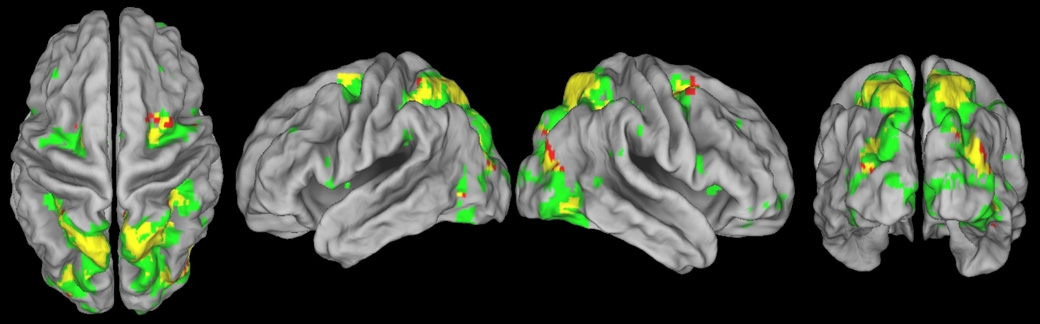
To obtain the areas activated during either covert or overt shifts on a subject-specific level, the fMRI activation in the central attention condition was subtracted from the fMRI activation in the peripheral attention condition for both the covert shift of attention task and the overt shift of attention task for each subject separately. The result of this subtraction was used to perform a number of subsequent group analyses. Firstly, two one-sample t-tests were performed to highlight the areas of the brain activated during either covert or overt shifts of attention over all subjects. These one-sample t-tests showed that both covert and overt shifts of attention resulted in a large amount of activation in the fronto-parietal system (see Figure 1 and Table 1). Secondly, to assess the areas of the brain activated during both covert and overt shifts of attention, an intersection map was calculated between the map showing the areas of the brain significantly activated during covert shift of attention and the map showing the areas of the brain significantly activated during overt shifts of attention. This intersection map showed an extensive degree of overlap in brain areas involved during the covert and overt shifts of attention tasks (see Figure 1). Areas activated during both the covert and the overt shifts of attention tasks included the frontal eye fields, the superior parietal lobes, the intraparietal sulci and the medial occipital gyri. All neural activation was observed bilaterally with a slight increase in activation for the right hemisphere.
Figure 1.
Areas of the brain that are involved in the covert shift of attention task and/or the overt shift of attention task. The areas of the brain significantly activated in the covert shift of attention task are shown in red. The areas of the brain significantly activated in the overt shift of attention task are shown in green. The areas of the brain activated in both the overt and the covert shift of attention task are shown in yellow. All images are in neurological orientation and a significance threshold of 0.05 FDR corrected for multiple comparisons was used.
Table 1.
Brain areas activated by the covert and overt shift of attention tasks.
| Brain area | Covert attention task | Overt attention task |
|---|---|---|
| R superior frontal | 24 −3 60 (4.41) | |
| 24 9 63 (4.10) | ||
| L superior frontal | −21 0 66 (4.07) | −21 −9 60 (4.54) |
| −21 −9 66 (3.51) | ||
| R medial frontal | 36 3 60 (3.82) | 36 −3 60 (4.79) |
| 45 3 54 (4.34) | ||
| 42 33 42 (3.20) | ||
| 33 39 39 (2.97) | ||
| L medial frontal | −42 36 36 (3.36) | |
| R precentral | 30 −3 51 (4.31) | |
| L precentral | −39 0 57 (4.36) | |
| L suppl. motor area | −15 0 66 (3.54) | |
| R medial orbitofrontal | 30 57 −12 (3.17) | |
| 21 39 −18 (2.99) | ||
| R inf. frontal operculum | 54 12 12 (3.38) | |
| L inf. frontal operculum | −60 12 15 (2.81) | |
| −51 12 15 (2.80) | ||
| L rolandic operculum | −51 6 3 (3.12) | |
| L insula | −42 0 3 (2.64) | |
| R anterior cingulate | 12 21 21 (3.10) | |
| R superior parietal | 21 −60 60 (5.15) | |
| 18 −66 66 (5.05) | ||
| 18 −75 57 (4.61) | ||
| L superior parietal | −18 −66 57 (5.45) | −15 −63 69 (4.56) |
| −18 −63 66 (4.73) | −18 −66 57 (4.52) | |
| −15 −75 60 (4.63) | ||
| R inferior parietal | 42 −36 51 (4.84) | |
| L supramarginal gyrus | −63 −39 30 (3.15) | |
| −57 −30 30 (2.90) | ||
| −51 −39 27 (2.84) | ||
| R superior temporal | 54 −42 21 (3.65) | |
| 69 −30 18 (2.94) | ||
| 66 −39 24 (2.73) | ||
| R medial temporal | 57 −51 3 (3.28) | |
| R inferior temporal | 48 −72 −9 (3.92) | |
| 42 −63 −9 (3.88) | ||
| 48 −54 −9 (3.57) | ||
| L fusiform gyrus | −30 −75 −12 (3.89) | |
| R medial occipital | 36 −87 24 (4.66) | |
| 42 −78 15 (3.90) | ||
| L medial occipital | −30 81 27 (4.31) | |
| −27 −72 30 (3.81) | ||
| −33 −90 12 (3.74) | ||
| R precuneus | 6 −51 51 (3.52) | |
| R putamen | 30 18 6 (3.39) | |
| R caudate nucleus | 21 3 18 (3.95) | |
| L caudate nucleus | −21 12 21 (4.08) | |
| −21 3 21 (3.79) | ||
| R extranuclear WM | 18 −15 −3 (3.24) | |
| L thalamus | −9 −21 3 (2.82) | |
Locations of the maxima are given in X Y Z mm (Z-score). Only local maxima more than 8mm apart inclusters larger than 5 voxels are reported. Coordinates are MNI coordinates.
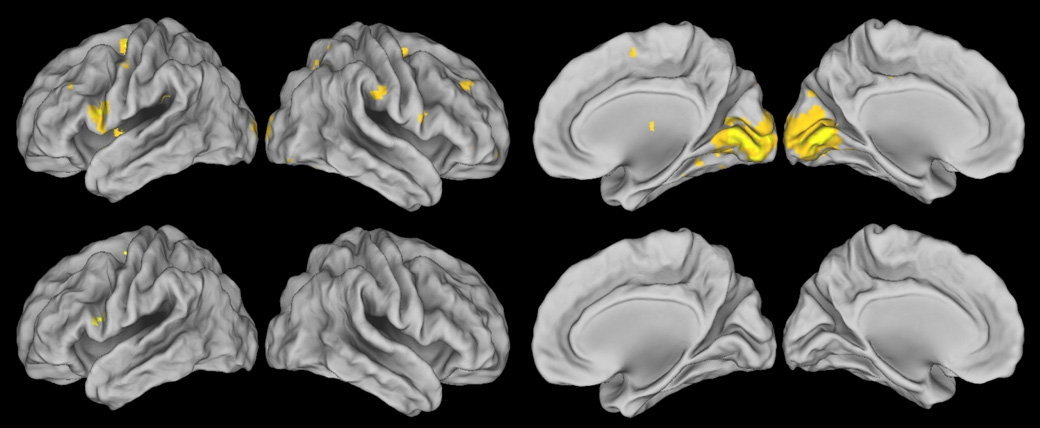
Thirdly, visual inspection of Figure 1 suggests that the extent of activity in areas activated during both the covert and the overt shifts of attention tasks is more widespread during the overt shifts of attention task than during the covert shifts of attention task. A two-sample t-test was performed on the subject-specific results of the subtraction of the fMRI activation in the central attention conditions from the fMRI activation in the peripheral attention conditions to assess whether there were any significant differences between the neural activation associated with the covert shifts of attention task and the neural activation associated with the overt shifts of attention task on the group level. This two-sample t-test showed that areas significantly more activated during the overt shifts of attention task than during the covert shifts of attention task included predominantly the calcarine and lingual gyri bilaterally. Additionally, this comparison revealed several clusters of significant activation in the frontal and parietal lobes (see Figure 2 top row and Table 2). To check whether these additional clusters of significant activation in the frontal and parietal lobes were an artifactual consequence of the high levels of neural activation in the occipital areas, the two-sample t-test comparing the neural activation in the covert shifts of attention task to the neural activation in the overt shifts of attention was also run with the occipital areas excluded from the analysis. This analysis showed that after exclusion of the visual areas, the only area of the brain significantly more activated during overt shifts of attention than during covert shifts of attention was the inferior frontal gyrus of the left hemisphere (see Figure 2 bottom row and Table 2). There were no areas that were significantly more activated by the covert shift of attention task than by the overt shift of attention task.
Figure 2.
Areas of the brain significantly more activated by the overt shift of attention task than by the covert shift of attention task. The top row displays the result of the whole brain analysis, the bottom row displays the result of the analysis without the primary visual areas. Images are displayed in neurological orientation and a significance threshold of 0.05 FDR corrected for multiple comparisons was used.
Table 2.
Areas of the brain significantly more activated by the overt shift of attention task than by the covert shift of attention task with visual areas either included (whole brain) or excluded (masked).
| Brain area | Overt vs. covert attention task whole brain | Overt vs. covert attention task masked |
|---|---|---|
| R anterior cingulum | 18 27 24 (3.94) | |
| R supplementary motor area | 15 0 63 (3.01) | |
| 3 0 63 (3.09) | ||
| R superior frontal | 21 −6 60 (3.65) | |
| R medial frontal | 21 36 27 (3.68) | |
| 33 33 18 (3.78) | ||
| 30 39 36 (3.46) | ||
| L medial frontal | −42 36 36 (3.12) | |
| R inferior frontal operculum | 60 12 15 (3.19) | |
| L inferior frontal operculum | −57 12 18 (4.89) | −57 12 18 (4.89) |
| L inferior frontal triangularis | −45 21 6 (4.07) | |
| R orbital frontal | 30 57 −12 (3.20) | |
| L orbital frontal | −21 48 −9 (3.43) | |
| L rolandic operculum | −48 −24 21 (3.24) | |
| L precentral | −18 −12 63 (4.50) | |
| −36 −3 66 (3.15) | ||
| L insula | −42 −3 0 (3.59) | |
| L postcentral | −54 −18 15 (2.97) | |
| R superior parietal | 24 −60 51 (3.90) | |
| 24 −57 60 (3.66) | ||
| 39 −54 57 (3.48) | ||
| 42 −42 57 (3.19) | ||
| L superior parietal | −15 −66 48 (3.14) | |
| R inferior parietal | 45 −36 48 (3.30) | |
| R supramarginal | 63 −24 30 (3.40) | |
| R lingual gyrus | 12 −87 −12 (4.68) | |
| 3 −72 3 (4.64) | ||
| L lingual | −9 −87 −9 (4.96) | |
| R caudate nucleus | 21 3 18 (4.05) | |
| L caudate nucleus | −24 −3 21 (4.19) | |
| R putamen | 24 3 9 (3.50) | |
| L putamen | −21 3 6 (3.63) | |
| L cerebellum 6 | −30 −63 −24 (3.43) |
Locations of the maxima are given in X Y Z mm (Z-score). Only local maxima more than 8mm apart in clusters larger than 5 voxels are reported. Coordinates are MNI coordinates.
Fourthly, to check for possible differences between the central attention conditions of the overt and covert shifts of attention tasks which could confound the results of the previous comparison of the amount of neural activation in the overt shifts of attention task to the amount of neural activation in the covert shifts of attention task, two 2-sample t-tests were performed. The results of these statistical tests showed no statistical differences between the amount of neural activation of the central conditions of the overt and covert shifts of attention tasks.
3. Discussion
Previous studies have suggested that both covert and overt shifts of attention elicit activation in a fronto-parietal network of brain areas (Beauchamp et al., 2001; Nobre et al., 2000; Perry and Zeki, 2000; Corbetta et al., 1998). This fronto-parietal network has been widely implicated in shifting visual attention (Wager et al., 2004; Kanwisher and Wojciulik, 2000; Wojciulik and Kanwisher, 1999; Gitelman et al., 1999). These studies have generally been interpreted as providing support for the premotor theory of attention (Rizzolatti et al., 1987) that argues that a covert shift in attention is merely an unexecuted overt shift of attention and that covert and overt shifts of attention use the same neural mechanisms.
In line with these previous studies, this study provides additional support to the finding that covert and overt shifts of attention result in neural activation in the same areas of the brain. This study therefore appears to lend further support to the premotor theory of attention. However, there are alternative explanations for the amount of overlap observed. Firstly, it could be that individual neurons in the fronto-parietal system are capable of performing two different cognitive functions. Another possibility is that the fronto-parietal system actually contains two separate groups of neurons, one for the covert shifts of attention and one for the overt shifts of attention and that a tool like fMRI does not have sufficient spatial resolution to distinguish between these two groups of neurons. One way of resolving these issues would be to look at the behaviour of individual neurons when performing either a covert shift of attention or an overt shift of attention. A few studies have looked at the behaviour of individual neurons as a function of either covert or overt shifts of attention in the frontal eye fields of monkeys and these so far seem to support the idea that the frontal eye fields indeed do contain two separate groups of neurons, one for covert shifts of attention and one for overt shifts of attention (Thompson et al., 2005; Schall, 2004) which undermines the premotor theory of attention. On the other hand, the superior colliculus appears to contain neurons that code for both covert and overt shifts of attention (Kustov & Robinson, 1996; Ignashchenkova et al., 2004) which supports the premotor theory of attention. Finally, the amount of overlap between the areas of the brain active during covert shifts of attention and the areas of the brain active during overt shifts of attention can also be explained by the possibility that both the covert and the overt task recruits the eye movement system. In this view, overt shifts of attention recruit the eye movement system for the programming of explicit eye moments, while covert shifts of attention recruit the eye movement system to actively inhibit eye movements to the relevant peripheral location. In other words, any attempt to employ covert attention may require active suppression of eye movements. This issue can be resolved by using a convergent technique that measures brain disruption rather than brain activation. For example, if the premotor theory of attention is correct, disrupting the frontal eye fields (which have traditionally been linked to overt shifts of attention) with transcranial magnetic stimulation should affect both eye movements as well as covert shifts of attention. This is exactly what has been found (Ro et al., 2003; Smith et al., 2005). To summarize, the majority of studies to date seem to support the suggestion that overt and covert shifts of attention rely on the same neural mechanisms. As a consequence, the results of this study can be interpreted as additional support for the premotor theory of attention (Rizzolatti et al., 1987) that argues that a covert shift in attention is merely an unexecuted overt shift of attention and that covert and overt shifts of attention use the same neural mechanisms.
This study also replicates the finding from Beauchamp and colleagues (2001) that overt shifts of attention result in higher levels of activation than covert shifts of attention in the fronto-parietal system activated by both tasks. Other studies found that covert shifts of attention resulted in greater neural activation than overt shifts of attention (Nobre et al., 2000; Perry and Zeki, 2000; Corbetta et al., 1998). However, these studies used endogenously driven shifts of attention while Beauchamp and colleagues used exogenously driven shifts of attention. This means that it was theoretically possible that endogenously driven covert shifts of attention lead to higher amounts of neural activation than endogenously driven overt shifts of attention while exogenously driven covert shifts of attention lead to lower amounts of neural activation than exogenously driven overt shifts of attention. This study succeeded in replicating the findings from Beauchamp and colleagues (2001), despite using endogenously driven shifts of attention. This therefore seems to suggest that the hitherto unique findings from Beauchamp and colleagues cannot be attributed to a difference between exogenous and endogenous attention mechanisms. A possible explanation for overt shifts being accompanied by higher levels of activation than covert shifts of attention in the fronto-parietal network activated by both tasks could be that the level of activation must cross a certain threshold before it can lead to an overt shift of attention. When the activation fails to cross this threshold the resulting shift of attention is covert.
Theoretically, it is possible that in this study the higher levels of neural activation during overt shifts of attention than during covert shifts of attention are due to a difference in neural activation between the two central attention conditions. In the present study, the covert and overt shifts of attention tasks were run in different fMRI runs. In other words, the covert central and peripheral attention conditions were run in a different fMRI run than the overt central and peripheral attention conditions. Since neural activation associated with covert or overt attention was defined as the subtraction of neuronal activity associated with the central attention condition from the neural activation related to the peripheral attention condition, it is theoretically possible that the observed higher amount of neural activation during overt shifts of attention merely reflects a larger engagement of the fronto-parietal system during the covert central condition than during the overt central condition. However, a statistical comparison of the two central attention conditions showed no significant differences in neural activation. Furthermore, the only aspect in which the two central attention conditions differed was that size discrimination was required during the covert central attention condition and not during the overt central attention condition. Therefore, for the observed higher amount of neural activation during overt shifts of attention to reflect a larger engagement of the fronto-parietal system during the covert central condition than during the overt central condition, size discrimination would have to be associated with neural activation in the fronto-parietal system. However, studies in both humans and monkeys suggest that size discrimination is a function of neural activation in occipito-temporal areas in the brain (Cavina-Pratesi et al., 2007; Schiller and Lee, 1991). Moreover, damage to occipito-parietal areas can lead to distorted size perception in patients (Kassubek et al., 1999). It therefore seems highly unlikely that the higher amount of neural activation observed during overt shifts of attention than during covert shifts of atttention can be attributed to a larger engagement of the fronto-parietal system during the covert central condition than during the overt central condition.
Despite impressive amount of overlap in neural activation during covert and overt shifts of attention, there seem to be some loci of activation specifically involved during overt shifts of attention. The largest of these loci of activation is located in the calcarine gyrus and extends into the lingual gyrus bilaterally. Previous studies have also found this area of the brain to be specifically activated by overt shifts of attention and not by covert shifts of attention (Nobre et al., 2000; Perry and Zeki, 2000; Corbetta et al., 1998). One possible explanation for this locus of activation is the eye movements made during overt shifts of attention. Several studies report an increase in neural activation in occipital areas during saccades (Bodis-Wollner et al., 1997; Matsuda et al., 2004). However, other studies report a decrease in neural activation in occipital areas during saccades, known as saccadic suppression (Paus et al., 1995; Kleiser et al., 2004; Sylvester et al., 2005). In the present study the increase in neural activation in occipital areas is most likely a consequence of the fact that during the eye movements made in the overt shifts of attention task the image on the retina changed, while during the covert shifts of attention task the image on the retina did not change. Other loci of activation that appeared to be specifically involved during overt shifts of attention were located in the frontal and parietal lobes, however, an additional analysis without the occipital lobes suggested that most of these neural activations were an artifactual result of the high levels of neural activation in the occipital cortex and the false discovery rate correction for multiple comparisons used in this study. The single locus of activation that remained after the occipital lobe was excluded from the analysis was located in the inferior frontal operculum of the left hemisphere. In the study by Nobre and colleagues (2000) the inferior frontal operculum was activated bilaterally during both covert and overt shifts of attention. In the present study, the inferior frontal operculum was activated bilaterally during overt shifts of attention but not during covert shifts of attention with the left inferior frontal operculum reaching significance in a statistical comparison between areas activated during covert and overt shifts of attention. This finding is in line with previous studies that have associated neural activation in the inferior frontal gyrus with both visually guided saccades and memory guided saccades (Özyurt et al., 2006; Kastner et al., 2007). However, another possible explanation for the neural activation in the inferior frontal gyrus in the overt shifts of attention task is that this neural activation like the neural activation in the occipital areas is associated with the fact that during the eye movements in the overt shifts of attention task the image on the retina changes while this is not the case in the covert shifts of attention task.
To conclude, the amount of neural overlap between overt and covert shifts of attention observed in this study can be interpreted as support for the premotor theory of attention (Rizzolatti et al., 1987) that argues that a covert shift in attention is merely an unexecuted overt shift of attention and that covert and overt shifts of attention use the same neural mechanisms. Furthermore, this study replicates the finding from Beauchamp and colleagues (2001) that overt shifts of attention result in higher levels of activation than covert shifts of attention in the fronto-parietal system activated by both tasks, suggesting that the hitherto unique findings from Beauchamp and colleagues cannot be attributed to a difference between exogenous and endogenous attention mechanisms.
4. Experimental Procedure
4.1. Subjects
12 subjects (9 male, 3 female) participated in this study. Subjects were recruited from the local student population. All subjects were healthy with no history of neurological disorders and had normal or corrected to normal vision. Subjects signed an informed consent approved by the local research ethics committee and were paid for participation in this study.
4.2. Task Design and Procedure
Subjects performed two tasks while lying in the bore of the scanner, one involving covert shifts of attention and one involving overt shifts of attention. A LCD projector illuminated a back projected screen on which the stimuli were presented. Subjects could view this screen via a mirror mounted on the head coil. The order of the two tasks was counterbalanced between subjects. The stimuli used for both tasks were identical, with the only difference being the instructions given to the subjects. Both the covert and the overt task used a blocked fMRI design with two experimental conditions; a peripheral attention condition and a central attention condition. Each experimental condition block lasting 12.8s was separated by a 12.8s rest block. The experimental conditions were presented in a fixed ABAB order, which was the same for each subject. Therefore, for both the covert and the overt task the sequence for a typical subject would be; a 12.8s peripheral attention condition block, a 12.8s rest block, a 12.8s central attention condition block, a 12.8s rest block, a 12.8s peripheral attention condition block and so on. Each task consisted of 49 blocks (including rest blocks). This meant that both the peripheral attention condition blocks and the central attention condition blocks were presented 12 times. The covert and overt shifts of attention tasks were run in separate sessions divided by a short break.
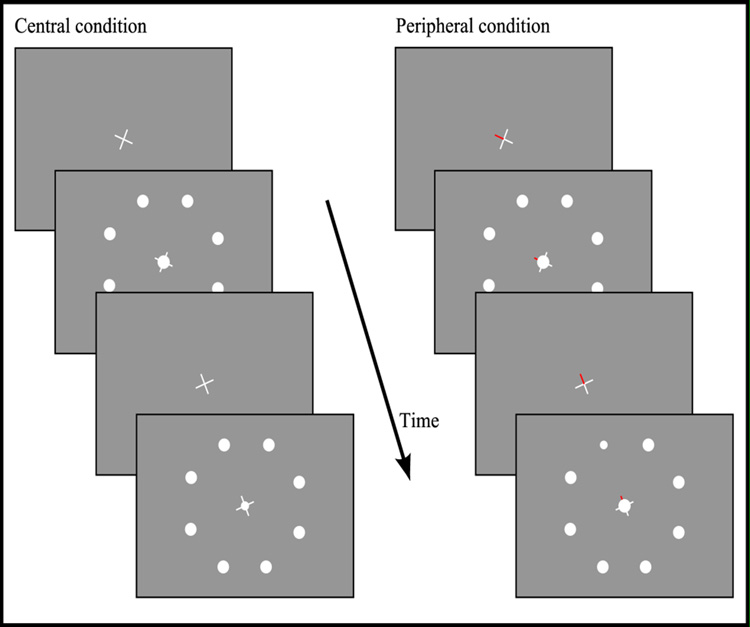
The stimuli and task are adapted from Wojciulik & Kanwisher (1999). In both the covert and the overt shifts of attention task subjects were presented with pictures displaying a central stepwise rotating white cross (subtending approximately 2°) overlaid with a white circle surrounded by 8 peripheral white circles during both peripheral and central attention condition blocks (see Figure 3). The eccentricity of the peripheral white circles was approximately 8° and their size was approximately 1.5°. The central cross was continuously visible.
Figure 3.
Stimuli used for the central and the peripheral attention condition in both the covert shifts of attention task and the overt shifts of attention task.
Stimuli were shown in blocks of 12.8s, with each block composed of 16 trials. The 9 white circles were briefly displayed for each trial, being visible for 400ms and hidden for 400ms. On each trial, a single one of the 9 white circles was randomly chosen to be slightly smaller than normal (from approximately 1.5° to approximately 1°). During blocks, the central cross was rotated 45° every 3.2s.
The visual stimuli were identical between the task requiring covert shifts of attention and the task requiring overt shifts of attention. The only visual difference between the peripheral and central attention condition blocks was that one arm of the central cross was red during the peripheral attention condition blocks. Therefore, the red arm of the white cross pointed to a peripheral target during the peripheral attention condition blocks. In the peripheral attention condition blocks of the overt shifts of attention task, subjects were told to make rapid saccades towards the peripheral circle that the red arm of the central cross was pointing toward. In contrast, during the peripheral attention condition blocks of the covert shifts of attention task, the subject was required to press a button with their right index finger if the red arm pointed to a white circle that was smaller than normal (which occurred on 1/9 of the trials), while keeping their gaze fixated on the central cross. In the central attention condition blocks of the overt shifts of attention task, the instruction was to simply maintain fixation on the central white circle. During the central attention condition blocks of the covert shifts of attention task, the subject was asked to press a button with their right index finger if the centrally presented circle was smaller than normal (an event that occurred in 1/9 of the trials). During the rest blocks between the experimental condition blocks the subjects were told to just fixate on a central fixation cross.
Task demands in the covert and overt shifts of attention tasks were designed to be as comparable as possible to enable a fair comparison between the neural activation associated with covert shifts of attention and the neural activation related to overt shifts of attention. Neural activation associated with covert shifts of attention was defined as the subtraction of neural activation associated with the covert central attention condition from the neural activation related to the covert peripheral attention condition. These conditions were designed to differ only in whether or not a covert attentional shift was required: target detection and a key-press response were required in both the covert central condition and the covert peripheral condition. Therefore, after subtraction only neural activation associated with a covert attentional shift should remain. Likewise, neural activation associated with overt shifts of attention was defined as the subtraction of neural activation related to the overt central attention condition from the neural activation associated with the overt peripheral attention condition. These conditions were designed to differ only in whether or not an overt shift of attention was required: whereas an eye movement was required in the peripheral attention condition, subjects were instructed to maintain central fixation in the central attention condition. Therefore, after subtraction only neural activation associated with an overt attentional shift should remain.
4.3. Imaging and data analysis
Images were collected on a 1.5T Philips Intera at the Queens Medical Centre in Nottingham, UK, using the receive-only quadrature head coil. A high-resolution whole brain T1-weighted anatomical volume was sagittally acquired using an inversion recovery TFE protocol, with a flip angle of 12°, an inversion time of 870ms, a TE of 3.7ms and a TR of 8.0ms. Each slice had a thickness of 1mm with an in-plane resolution of 1×1mm (FOV = 256×256). This anatomical volume was normalized to a standardized template that approximates MNI space using SPM2 (available at www.fil.ion.ucl.ac.uk/spm) to allow for comparisons between subjects (Ashburner and Friston, 2003b; Ashburner and Friston, 2000) with the resulting images resliced to an isotropic 3mm. The initial normalization used a 12 parameter affine transform followed by a nonlinear 3-dimensional discrete cosine transform with a cut-off of 25mm. Reslicing used 4th degree B-spline interpolation.
In addition to the anatomical scan, two sessions of continuous fMRI data were collected (one for each task). Each consisted of 175 whole brain functional T2* EPI volumes collected axially with a flip angle of 90°, a TE of 60ms and a TR of 3.5s. Each functional volume contained 33 slices with a slice thickness of 3mm and an in-plane resolution of 3x3mm (FOV = 192×192). All spatial preprocessing and first-level fixed effects statistical analyses were conducted using SPM2, while the second-level random effects statistical analyses were conducted using SPM5. The volumes were realigned to match the first volume of each session and unwarped to correct for head movement related artifacts (Ashburner and Friston, 2003a; Andersson et al., 2001; Ashburner and Friston, 2000). Reslicing used 4th degree B-spline interpolation. Subjects who showed a movement of an amplitude exceeding 1mm between successive volumes were excluded from further analysis. This led to the exclusion of 2 subjects, leaving 10 subjects for further analysis. Subsequently, the volumes were normalized to a standardized template that approximates MNI space to allow for comparisons between subjects (Ashburner and Friston, 2003b; Ashburner and Friston, 2000) with the resulting images resliced to an isotropic 3mm. The initial normalization used a 12 parameter affine transform followed by a nonlinear 3-dimensional discrete cosine transform with a cut-off of 25mm. Reslicing again used 4th degree B-spline interpolation. Finally, the volumes were smoothed with an isotropic 8mm FWHM Gaussian filter to accommodate between subject differences in brain anatomy, improve the signal to noise ratio and to satisfy the statistical assumptions of SPM2 (Smith, 2001).
The resulting data was processed with a high-pass temporal filter using a 102.4s cutoff (Smith, 2001). Task-related changes in blood-oxygenation level were modelled using the standard SPM2 haemodynamic response (Kiebel and Holmes, 2003; Worsley, 2001; Lange, 2000; Friston et al., 1995). A series of comparisons were conducted designed to determine which brain regions showed task related changes. For both the covert shift of attention task and the overt shift of attention task the fMRI activation in the central attention condition was subtracted from the fMRI activation in the peripheral attention condition for each subject separately (first-level fixed effects analyses). The result of this subtraction was used to perform a number of second-level random effects analyses (Holmes and Friston, 1998). Firstly, two one-sample t-tests were applied to assess the mean level of activation during either the covert or the overt shift of attention tasks across all subjects. Secondly from the results of these one-sample t-tests, two binary maps displaying the areas of the brain significantly activated by either covert or overt shifts of attention were calculated. Subsequently, to assess the areas of the brain activated during both covert and overt shifts of attention, a binary intersection map was calculated from the two maps derived from the onesample t-tests. Thirdly, two random effects two-sample t-tests were performed to compare the fMRI activation during the covert shift of attention tasks to the fMRI activation during the overt shift of attention tasks across all subjects. These two random effects two-sample t-tests were also performed masked with an image of the lower level visual areas BA17 and BA18 obtained from the anatomy toolbox (Eickhoff et al., 2006). Finally, two random effects two-sample t-tests were performed to compare the fMRI activation during central attention condition of the covert shifts of attention task to the fMRI activation during the central attention condition of the overt shifts of attention tasks. For all statistical analyses a threshold of p<0.05 (using false discovery rate to compensate for the large number of comparisons) was applied to determine which voxels were significantly activated at each comparison (Genovese et al., 2002; Benjamini and Hochberg, 1995).
Acknowledgements
We would like to thank the anonymous reviewers for providing us with valuable and constructive feedback on earlier versions of this manuscript. This research was supported by NIH grant R01 NS054266.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersson JLR, Hutton C, Ashburner J, Turner R, Friston K. Modelling geometric deformations in EPI time series. NeuroImage. 2001;13:903–919. doi: 10.1006/nimg.2001.0746. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Image registration. In: Moonen CTW, Bandettini PA, editors. Functional MRI. Heidelberg: Springer-Verlag Berlin; 2000. pp. 285–299. [Google Scholar]
- Ashburner J, Friston KJ. Rigid body registration. In: Frackowiak RSJ, Friston KJ, Frith C, Dolan R, Price CJ, Zeki S, Ashburner J, Penny WD, editors. Human brain function. San Dieg: Academic Press; 2003. pp. 635–654. [Google Scholar]
- Ashburner J, Friston KJ. Spatial normalization using basis functions. In: Frackowiak RSJ, Friston KJ, Frith C, Dolan R, Price CJ, Zeki S, Ashburner J, Penny WD, editors. Human brain function. San Diego: Academic Press; 2003. pp. 655–672. [Google Scholar]
- Beauchamp MS, Petit L, Ellmore TM, Ingeholm J, Haxby JV. A parametric fMRI study of overt and covert shifts of visuospatial attention. NeuroImage. 2001;14:310–321. doi: 10.1006/nimg.2001.0788. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Statist. Soc. B. 1995;57:289–300. [Google Scholar]
- Bodis-Wollner I, Bucher SF, Seelos KC, Paulus W, Reiser M, Oertel WH. Functional MRI mapping of occipital and frontal cortical activity during voluntary and imagined saccades. Neurology. 1997;49:416–420. doi: 10.1212/wnl.49.2.416. [DOI] [PubMed] [Google Scholar]
- Briand KA. Feature integration and spatial attention: More evidence of a dissociation between endogenous and exogenous orienting. J. Exp. Psychol. Hum. Percept. Perform. 1998;24:1243–1256. [Google Scholar]
- Cavina-Pratesi C, Goodale MA, Culham JC. FMRI reveals a dissociation between grasping and perceiving the size of real 3D objects. PLoS. ONE. 2007;2:e424. doi: 10.1371/journal.pone.0000424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M. Frontoparietal cortical networks for directing attention and the eye to visual locations: Identical, independent, or overlapping neural systems? Proc. Natl. Acad. Sci. U. S. A. 1998;95:831–838. doi: 10.1073/pnas.95.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Akbudak E, Conturo TE, Snyder AZ, Ollinger JM, Drury HA, Linenweber MR, Petersen SE, Raichle ME, Van Essen DC, Shulman GL. A common network of functional areas for attention and eye movements. Neuron. 1998;21:761–773. doi: 10.1016/s0896-6273(00)80593-0. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Heim S, Zilles K, Amunts K. Testing anatomically specified hypotheses in functional imaging using cytoarchitectonic maps. NeuroImage. 2006;32:570–582. doi: 10.1016/j.neuroimage.2006.04.204. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline J-P, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: A general linear approach. Hum. Brain Mapp. 1995;2:189–210. [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the False Discovery Rate. NeuroImage. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Gitelman DR, Nobre AC, Parrish TB, LaBar KS, Kim Y-H, Meyer JR, Mesulam MM. A large scale distributed network for covert spatial attention: Further anatomical delineation based on stringent behavioural and cognitive controls. Brain. 1999;122:1093–1106. doi: 10.1093/brain/122.6.1093. [DOI] [PubMed] [Google Scholar]
- Holmes AP, Friston KJ. Generalisability, random effects and population inference. NeuroImage. 1998;7:S754. [Google Scholar]
- Hopfinger JB, West VM. Interactions between endogenous and exogenous attention on cortical visual processing. NeuroImage. 2006;31:774–789. doi: 10.1016/j.neuroimage.2005.12.049. [DOI] [PubMed] [Google Scholar]
- Ignashchenkova A, Dicke PW, Haarmeier T, Thier P. Neuron-specific contribution of the superior colliculus to overt and covert shifts of attention. Nat. Neurosci. 2004;7:56–64. doi: 10.1038/nn1169. [DOI] [PubMed] [Google Scholar]
- Kanwisher N, Wojciulik E. Visual attention: Insights from brain imaging. Nat. Rev. Neurosci. 2000;1:91–100. doi: 10.1038/35039043. [DOI] [PubMed] [Google Scholar]
- Kassubek J, Otte M, Wolter T, Greenlee MW, Mergner T, Lucking CH. Brain imaging in a patient with hemimicropsia. Neuropsychologia. 1999;37:1327–1334. doi: 10.1016/s0028-3932(99)00041-x. [DOI] [PubMed] [Google Scholar]
- Kastner S, DeSimone K, Konen CS, Szczepanski SM, Weiner KS, Schneider KA. Topographic maps in human frontal cortex revealed in memory-guided saccade and spatial working-memory tasks. J. Neurophysiol. 2007;97:3494–3507. doi: 10.1152/jn.00010.2007. [DOI] [PubMed] [Google Scholar]
- Kiebel SJ, Holmes AP. The general linear model. In: Frackowiak RSJ, Friston KJ, Frith C, Dolan R, Price CJ, Zeki S, Ashburner J, Penny WD, editors. Human brain function. San Diego: Academic Press; 2003. pp. 725–760. [Google Scholar]
- Kincade JM, Abrams RA, Astafiev SV, Shulman GL, Corbetta M. An event-related functional magnetic resonance imaging study of voluntary and stimulus-driven orienting of attention. J. Neurosci. 2005;25:4593–4604. doi: 10.1523/JNEUROSCI.0236-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiser R, Seitz RJ, Krekelberg B. Neural correlates of saccadic suppression in humans. Curr. Biol. 2004;14:386–390. doi: 10.1016/j.cub.2004.02.036. [DOI] [PubMed] [Google Scholar]
- Kustov AA, Robinson DL. Shared neural control of attentional shifts and eye movements. Nature. 1996;384:74–77. doi: 10.1038/384074a0. [DOI] [PubMed] [Google Scholar]
- Lange N. Statistical procedures for functional MRI. In: Moonen CTW, Bandettini PA, editors. Functional MRI. Heidelberg: Springer-Verlag Berlin; 2000. pp. 301–335. [Google Scholar]
- Matsuda T, Matsuura M, Ohkubo T, Ohkubo H, Matsushima E, Inoue K, Taira M, Kojima T. Functional MRI mapping of brain activation during visually guided saccades and antisaccades: Cortical and subcortical networks. Psychiatr. Res. 2004;131:147–155. doi: 10.1016/j.pscychresns.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Mayer AR, Dorflinger JM, Rao SM, Seidenberg M. Neural networks underlying endogenous and exogenous visual-spatial orienting. Neuroimage. 2004;23:534–541. doi: 10.1016/j.neuroimage.2004.06.027. [DOI] [PubMed] [Google Scholar]
- Mort DJ, Perry RJ, Mannan SK, Hodgson TL, Anderson E, Quest R, McRobbie D, McBride A, Husain M, Kennard C. Differential cortical activation during voluntary and reflexive saccades in man. Neuroimage. 2003;18:231–246. doi: 10.1016/s1053-8119(02)00028-9. [DOI] [PubMed] [Google Scholar]
- Natale E, Marzi CA, Girelli M, Pavone EF, Pollmann S. ERP and fMRI correlates of endogenous and exogenous focusing of visual-spatial attention. Eur. J. Neurosci. 2006;23:2511–2521. doi: 10.1111/j.1460-9568.2006.04756.x. [DOI] [PubMed] [Google Scholar]
- Nichols T, Brett M, Andersson J, Wager T, Poline J-P. Valid conjunction inference with the minimum statistic. NeuroImage. 2005;25:653–660. doi: 10.1016/j.neuroimage.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Nobre AC, Gitelman DR, Dias EC, Mesulam MM. Covert visual spatial orienting and saccades: Overlapping neural systems. NeuroImage. 2000;11:210–216. doi: 10.1006/nimg.2000.0539. [DOI] [PubMed] [Google Scholar]
- Özyurt J, Rutschmann RM, Greenlee MW. Cortical activation during memory-guided saccades. NeuroReport. 2006;17:1005–1009. doi: 10.1097/01.wnr.0000224765.00078.4e. [DOI] [PubMed] [Google Scholar]
- Paus T, Marrett S, Worsley KJ, Evans AC. Extraretinal modulation of cerebral blood flow in the human visual cortex: Implications for saccadic suppression. J. Neurophysiol. 1995;74:2179–2183. doi: 10.1152/jn.1995.74.5.2179. [DOI] [PubMed] [Google Scholar]
- Peelen MV, Heslenfeld DJ, Theeuwes J. Endogenous and exogenous attention shifts are mediated by the same large-scale neural network. NeuroImage. 2004;22:822–830. doi: 10.1016/j.neuroimage.2004.01.044. [DOI] [PubMed] [Google Scholar]
- Perry RJ, Zeki S. The neurology of saccades and covert shifts in spatial attention: An event-related fMRI study. Brain. 2000;123:2273–2288. doi: 10.1093/brain/123.11.2273. [DOI] [PubMed] [Google Scholar]
- Posner MI, Petersen SE. The Attention System of the Human Brain. Annu. Rev. Neurosci. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Riggio L, Dascola I, Umilta C. Reorienting Attention across the Horizontal and Vertical Meridians - Evidence in Favor of a Premotor Theory of Attention. Neuropsychologia. 1987;25:31–40. doi: 10.1016/0028-3932(87)90041-8. [DOI] [PubMed] [Google Scholar]
- Ro T, Farnè A, Chang E. Inhibition of return and the human frontal eye fields. Exp. Brain Res. 2003;150:290–296. doi: 10.1007/s00221-003-1470-0. [DOI] [PubMed] [Google Scholar]
- Rosen AC, Rao SM, Caffara P, Scaglioni A, Bobholz JA, Woodley SJ, Hammeke TA, Cunningham JM, Prieto TE, Binder JR. Neural basis of endogenous and exogenous spatial orienting: A functional MRI study. J. Cogn. Neurosci. 1999;11:135–152. doi: 10.1162/089892999563283. [DOI] [PubMed] [Google Scholar]
- Schall JD. On the role of frontal eye field in guiding attention and saccades. Vision Res. 2004;44:1453–1467. doi: 10.1016/j.visres.2003.10.025. [DOI] [PubMed] [Google Scholar]
- Schiller PH, Lee K. The role of the primate extrastriate area V4 in vision. Science. 1991;251:1251–1253. doi: 10.1126/science.2006413. [DOI] [PubMed] [Google Scholar]
- Schneider W, Shiffrin RM. Controlled and automatic human information processing: I. Detection, search and attention. Psychol. Rev. 1984;91:1–66. [Google Scholar]
- Shiffrin RM, Schneider W. Automatic and controlled processing revisited. Psychol. Rev. 1984;91:269–276. [PubMed] [Google Scholar]
- Smith DT, Jackson SR, Rorden C. Transcranial magnetic stimulation of the left human frontal eye fields eliminates the cost of invalid endogenous cues. Neuropsychologia. 2005;43:1288–1296. doi: 10.1016/j.neuropsychologia.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Smith SM. Preparing fMRI data for statistical analysis. In: Jezzard P, Matthews PM, Smith SM, editors. Functional MRI: An introduction to methods. New York: Oxford University Press Inc; 2001. pp. 229–241. [Google Scholar]
- Sylvester R, Haynes J-D, Rees G. Saccades differentially modulate human LGN and V1 responses in the presence and absence of visual stimulation. Curr. Biol. 2005;15:37–41. doi: 10.1016/j.cub.2004.12.061. [DOI] [PubMed] [Google Scholar]
- Thompson KG, Biscoe KL, Sato TR. Neuronal basis of covert spatial attention in the frontal eye field. J. Neurosci. 2005;25:9479–9487. doi: 10.1523/JNEUROSCI.0741-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Jonides J, Reading S. Neuroimaging studies of shifting attention: a meta-analysis. NeuroImage. 2004;22:1679–1693. doi: 10.1016/j.neuroimage.2004.03.052. [DOI] [PubMed] [Google Scholar]
- Wojciulik E, Kanwisher N. The generality of parietal involvement in visual attention. Neuron. 1999;23:747–764. doi: 10.1016/s0896-6273(01)80033-7. [DOI] [PubMed] [Google Scholar]
- Worsley KJ. Statistical analysis of activation images. In: Jezzard P, Matthews PM, Smith SM, editors. Functional MRI: An introduction to methods. New York: Oxford University Press Inc; 2001. pp. 251–270. [Google Scholar]