Abstract
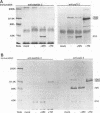
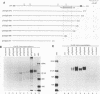
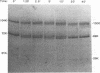
During the synthesis of glycoprotein G-2 (gG-2) of herpes simplex virus type 2, the 104,000-Da gG-2 precursor (104K precursor) is cleaved to generate the 72K and the 31K intermediates. The 72K product is processed to generate the mature gG-2 (molecular mass, 108,000 Da), while the 31K product is additionally processed and secreted into the extracellular medium as the 34K component (H. K. Su, R. Eberle, and R. J. Courtney, J. Virol. 61:1735-1737, 1987). In this study, the orientations of the 31K and 72K products on the 104K precursor were determined by using two antipeptide sera produced in rabbits and a monoclonal antibody, 13 alpha C6, directed against gG-2. The sera prepared against synthetic peptides corresponding to the terminal amino acid residues 67 to 78 and an internal peptide at amino acids 247 to 260 of gG-2 recognized the 104K precursor and the 31K cleavage product but not the 72K intermediate. In contrast, 13 alpha C6 detected the 72K cleavage product and the uncleaved precursor but not the 31K cleavage component. The epitope recognized by 13 alpha C6 was mapped within amino acids 486 to 566. These results suggest that the 31K cleavage product is derived from the amino-terminal portion of the 104K precursor molecule and that the 72K intermediate is derived from the carboxyl terminus. In support of our model described above for the synthesis of gG-2, antibodies recognizing either of the cleavage products reacted with the uncleaved precursor but not with the other cleavage product. By using partial endo-beta-N-acetylglucosaminidase H analysis, two N-linked glycosylation sites were found on each of the cleavage products. The distribution of the N-linked glycosylation sites and the reactivities of the antipeptide sera allowed the cleavage region on the precursor to be mapped to within amino acids 260 to 437.
Full text
PDF

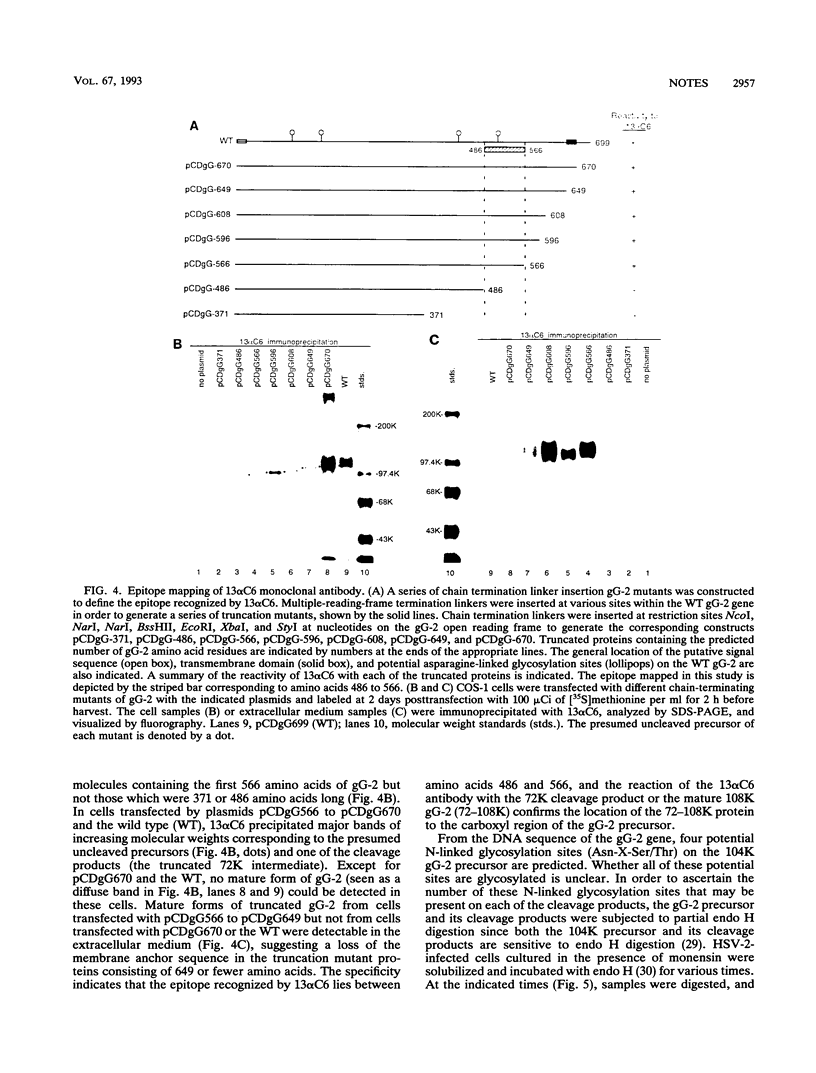
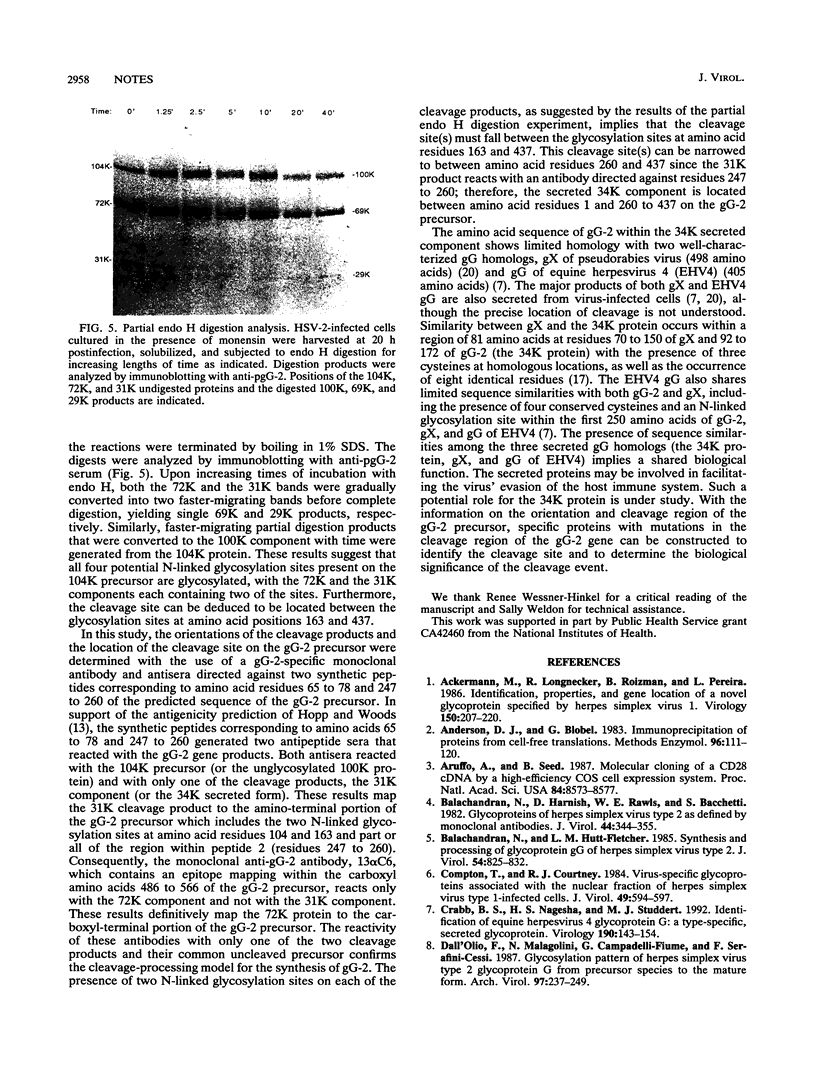

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackermann M., Longnecker R., Roizman B., Pereira L. Identification, properties, and gene location of a novel glycoprotein specified by herpes simplex virus 1. Virology. 1986 Apr 15;150(1):207–220. doi: 10.1016/0042-6822(86)90280-1. [DOI] [PubMed] [Google Scholar]
- Anderson D. J., Blobel G. Immunoprecipitation of proteins from cell-free translations. Methods Enzymol. 1983;96:111–120. doi: 10.1016/s0076-6879(83)96012-3. [DOI] [PubMed] [Google Scholar]
- Aruffo A., Seed B. Molecular cloning of a CD28 cDNA by a high-efficiency COS cell expression system. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8573–8577. doi: 10.1073/pnas.84.23.8573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balachandran N., Harnish D., Rawls W. E., Bacchetti S. Glycoproteins of herpes simplex virus type 2 as defined by monoclonal antibodies. J Virol. 1982 Oct;44(1):344–355. doi: 10.1128/jvi.44.1.344-355.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balachandran N., Hutt-Fletcher L. M. Synthesis and processing of glycoprotein gG of herpes simplex virus type 2. J Virol. 1985 Jun;54(3):825–832. doi: 10.1128/jvi.54.3.825-832.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton T., Courtney R. J. Virus-specific glycoproteins associated with the nuclear fraction of herpes simplex virus type 1-infected cells. J Virol. 1984 Feb;49(2):594–597. doi: 10.1128/jvi.49.2.594-597.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabb B. S., Nagesha H. S., Studdert M. J. Identification of equine herpesvirus 4 glycoprotein G: a type-specific, secreted glycoprotein. Virology. 1992 Sep;190(1):143–154. doi: 10.1016/0042-6822(92)91200-e. [DOI] [PubMed] [Google Scholar]
- Dall'Olio F., Malagolini N., Campadelli-Fiume G., Serafini-Cessi F. Glycosylation pattern of herpes simplex virus type 2 glycoprotein G from precursor species to the mature form. Arch Virol. 1987;97(3-4):237–249. doi: 10.1007/BF01314424. [DOI] [PubMed] [Google Scholar]
- Eberle R., Courtney R. J. Preparation and characterization of specific antisera to individual glycoprotein antigens comprising the major glycoprotein region of herpes simplex virus type 1. J Virol. 1980 Sep;35(3):902–917. doi: 10.1128/jvi.35.3.902-917.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frame M. C., Marsden H. S., McGeoch D. J. Novel herpes simplex virus type 1 glycoproteins identified by antiserum against a synthetic oligopeptide from the predicted product of gene US4. J Gen Virol. 1986 Apr;67(Pt 4):745–751. doi: 10.1099/0022-1317-67-4-745. [DOI] [PubMed] [Google Scholar]
- Hopp T. P., Woods K. R. A computer program for predicting protein antigenic determinants. Mol Immunol. 1983 Apr;20(4):483–489. doi: 10.1016/0161-5890(83)90029-9. [DOI] [PubMed] [Google Scholar]
- Hutchinson L., Browne H., Wargent V., Davis-Poynter N., Primorac S., Goldsmith K., Minson A. C., Johnson D. C. A novel herpes simplex virus glycoprotein, gL, forms a complex with glycoprotein H (gH) and affects normal folding and surface expression of gH. J Virol. 1992 Apr;66(4):2240–2250. doi: 10.1128/jvi.66.4.2240-2250.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden H. S., Buckmaster A., Palfreyman J. W., Hope R. G., Minson A. C. Characterization of the 92,000-dalton glycoprotein induced by herpes simplex virus type 2. J Virol. 1984 May;50(2):547–554. doi: 10.1128/jvi.50.2.547-554.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeoch D. J., Dolan A., Donald S., Rixon F. J. Sequence determination and genetic content of the short unique region in the genome of herpes simplex virus type 1. J Mol Biol. 1985 Jan 5;181(1):1–13. doi: 10.1016/0022-2836(85)90320-1. [DOI] [PubMed] [Google Scholar]
- McGeoch D. J., Moss H. W., McNab D., Frame M. C. DNA sequence and genetic content of the HindIII l region in the short unique component of the herpes simplex virus type 2 genome: identification of the gene encoding glycoprotein G, and evolutionary comparisons. J Gen Virol. 1987 Jan;68(Pt 1):19–38. doi: 10.1099/0022-1317-68-1-19. [DOI] [PubMed] [Google Scholar]
- Olofsson S., Lundström M., Marsden H., Jeansson S., Vahlne A. Characterization of a herpes simplex virus type 2-specified glycoprotein with affinity for N-acetylgalactosamine-specific lectins and its identification as g92K or gG. J Gen Virol. 1986 Apr;67(Pt 4):737–744. doi: 10.1099/0022-1317-67-4-737. [DOI] [PubMed] [Google Scholar]
- Powell K. L., Courtney R. J. Polypeptide synthesized in herpes simplex virus type 2-infected HEp-2 cells. Virology. 1975 Jul;66(1):217–228. doi: 10.1016/0042-6822(75)90192-0. [DOI] [PubMed] [Google Scholar]
- Rea T. J., Timmins J. G., Long G. W., Post L. E. Mapping and sequence of the gene for the pseudorabies virus glycoprotein which accumulates in the medium of infected cells. J Virol. 1985 Apr;54(1):21–29. doi: 10.1128/jvi.54.1.21-29.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richman D. D., Buckmaster A., Bell S., Hodgman C., Minson A. C. Identification of a new glycoprotein of herpes simplex virus type 1 and genetic mapping of the gene that codes for it. J Virol. 1986 Feb;57(2):647–655. doi: 10.1128/jvi.57.2.647-655.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roizman B., Norrild B., Chan C., Pereira L. Identification and preliminary mapping with monoclonal antibodies of a herpes simplex virus 2 glycoprotein lacking a known type 1 counterpart. Virology. 1984 Feb;133(1):242–247. doi: 10.1016/0042-6822(84)90447-1. [DOI] [PubMed] [Google Scholar]
- Su H. K., Courtney R. J. Inducible expression of herpes simplex virus type 2 glycoprotein gene gG-2 in a mammalian cell line. J Virol. 1988 Oct;62(10):3668–3674. doi: 10.1128/jvi.62.10.3668-3674.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su H. K., Eberle R., Courtney R. J. Processing of the herpes simplex virus type 2 glycoprotein gG-2 results in secretion of a 34,000-Mr cleavage product. J Virol. 1987 May;61(5):1735–1737. doi: 10.1128/jvi.61.5.1735-1737.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takatsuki A., Tamura G. Effect of tunicamycin on the synthesis of macromolecules in cultures of chick embryo fibroblasts infected with Newcastle disease virus. J Antibiot (Tokyo) 1971 Nov;24(11):785–794. doi: 10.7164/antibiotics.24.785. [DOI] [PubMed] [Google Scholar]
- Tartakoff A. M. Perturbation of vesicular traffic with the carboxylic ionophore monensin. Cell. 1983 Apr;32(4):1026–1028. doi: 10.1016/0092-8674(83)90286-6. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weldon S. K., Su H. K., Fetherston J. D., Courtney R. J. In vitro synthesis and processing of herpes simplex virus type 2 gG-2, using cell-free transcription and translation systems. J Virol. 1990 Mar;64(3):1357–1359. doi: 10.1128/jvi.64.3.1357-1359.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenske E. A., Bratton M. W., Courtney R. J. Endo-beta-N-acetylglucosaminidase H sensitivity of precursors to herpes simplex virus type 1 glycoproteins gB and gC. J Virol. 1982 Oct;44(1):241–248. doi: 10.1128/jvi.44.1.241-248.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]