Abstract
Wide use of flame retardants can pose an environmental hazard and it is of interest to investigate how they may degrade. We report here that 3,3′,5,5′-tetrabromobisphenol A (TBBPA) is subject to photosensitized oxidation involving singlet molecular oxygen (1O2). By using visible light and Rose Bengal or Methylene Blue as 1O2 photosensitizers, we have found that TBBPA is a 1O2 quencher. The quenching rate constant, kq, depends on TBBPA ionization (pK=7.4). In acetonitrile, where TBBPA is undissociated, the kq value is 6.1×105 M-1s-1 for a TBBPA monomer and decreasing to kq=2.9×104M-1s-1 for TBBPA dimers/aggregates. TBBPA dissociates in aqueous solutions, and its kq value is 1.44×109 M-1s-1 in alkaline solution, decreasing to kq=3.9×108 M-1s-1 at pH 7.2. The strong 1O2 quenching by TBBPA anion initiates an efficient oxidation of TBBPA, which results in oxygen consumption in aqueous micellar (e.g. Triton-X 100) solutions containing photosensitizer. This oxygen consumption is mediated by transient radical species, which we detected by using EPR spectroscopy. We observed two major radicals and one minor radical generated from TBBPA by reaction with 1O2 at pH 10. One was identified as the 2,6-dibromo-p-benzosemiquinone radical (a2H = 2.36 G, g = 2.0056). A second radical (aH = 2.10 G, g = 2.0055) could not be identified but was probably a 2,6-dibromo-p-benzosemiquinone radical containing an EPR silent substituent in the 3-position. Spin trapping with 5,5,-dimethyl-1-pyrroline N-oxide (DPMO) showed that other minor radicals (hydroxyl, carbon centered) are also generated during the reaction of TBBPA with 1O2. The photosensitized production of radicals and oxygen consumption were completely inhibited by the azide anion, an efficient physical 1O2 quencher. Because TBBPA is a stable compound that at neutral pH does not absorb much of the atmosphere-filtered solar radiation, its photosensitized oxidation by 1O2 may be the key reaction initiating/mediating TBBPA degradation in the natural environment.
Introduction
Brominated flame retardants (BFRs) are present in many consumer products ranging from plastics to fabrics (1-3). Of all the BFRs, tetrabromobisphenol A (TBBPA) has the largest production volume (about 50% of the BFRs in current use) (4,5). TBBPA is mainly employed in the preparation of brominated epoxy and polycarbonate resins, which are used in the manufacture of printed circuit boards. This has resulted in a dramatic increase in TBBPA production (6). Fackler has conducted biodegradation studies in several media (soil, river sediment and water) and shown that TBBPA half life approximates 2 months (7-9). In contrast no degradation was observed in sludge under sewage treatment conditions (10). Because TBBPA may bioaccumulate it is a potential environmental health problem (11-14).
A large number of halogen-containing organic compounds have been reported to undergo photochemical transformations, under both experimental and natural conditions (15-17). Photolytic decomposition of TBBPA in the environment occurs in the atmosphere and in surface waters. The rate of TBBPA photodecomposition is highly dependent on the pH as the absorption spectra of the associated/neutral and dissociated/anionic forms are quite different (18). Eriksson et al. (19) have reported that, at pH values below its pKa of ∼7.4, the quantum yield of TBBPA photodecomposition decreases with decreasing pH, while at pH values above its pKa photodecomposition is independent of pH. There are several reported studies on TBBPA decomposition pathways (2,19-22) which suggest that the most important routes involve debromination and scission reactions which yield phenols (2,23,24). Radical reactions are thought to be responsible for the formation of the intermediates in the TBBPA decomposition pathways. However, information on the photochemical properties of TBBPA is limited although this compound is used in large quantities as monomers and additives in plastic materials. Therefore it is of interest to determine its photochemical decomposition pathways and to identify the radical products formed.
In the environment, photochemical transformations proceed by direct photolysis under the action of solar light (25,26). In sensitized redox processes under aerobic conditions, the active reaction intermediates participating in the transformation of the pollutant may be the electronically excited sensitizer molecule or the solvated electron as well as ROS such as singlet oxygen (1O2) or the superoxide anion radical (O2·-) (26-28). We explore here the possibility of photosensitized degradation of TBBPA mediated by singlet oxygen or free radicals. For the generation of singlet oxygen, we used two classical photosensitizers, rose bengal (RB) and methylene blue (MB). To the best of our knowledge, this is the first study to examine experimentally the potential oxidation of TBBPA by singlet oxygen.
Experimental
Materials
TBBPA, rose bengal, methylene blue, sodium azide, perinaphthenone, acetonitrile, Fremy’s salt and 5,5-dimethyl-1-pyrroline N-oxide (DMPO), NaOD, Triton X100 were purchased from Aldrich Chemical Co. (Milwaukee, WI, USA). DMPO was vacuum distilled and stored at -70°C until use. Deuterium oxide was purchased from Cambridge Isotope Laboratories (Andover, MA, USA). Lauryl sulfobetaine (SB-12) was purchased from Sigma Chemical Co. (St. Louis, MO). Catalase and superoxide dismutase were from Boehringer Mannheim (NJ). All other chemicals were reagent grade or better.
All measurements were performed at room temperature. Buffers were prepared from reagent grade or better components, and pH was measured using a glass electrode
Singlet oxygen experiments
Singlet oxygen phosphorescence quenching experiments were performed as described previously (29,30). Briefly, the 1O2 photosensitizer perinaphthenone was excited at 355 nm by a single 6 ns pulse from a Surelite II laser (Continuum, Santa Clara, CA). The 1O2 phosphorescence was detected using a germanium diode (Model 403 HS, Applied Detector Corporation, Fresno, CA) connected to a HP 54111D Digitizing Oscilloscope (Hewlett Packard Colorado Springs, CO) that was interfaced to a PC computer. The decay of 1O2 phosphorescence was measured as a function of TBBPA concentration in MeCN and in MeCN-D2O mixtures, and was used to calculate the quenching rate constants.
The production of 1O2 was measured using a steady-state 1O2 spectrophotometer, as described previously (29,30). The TBBPA samples were irradiated through a broad-band interference filter in a suprasil cell (0.5 cm pathlength) using a 300 watt Hg lamp as a light source. The 1O2 phosphorescence was observed through a monochromator using a germanium diode cooled to 77K. Photobleaching of TBBPA solutions was measured in a steady-state 1O2 spectrophotometer. The absorption spectra were recorded on a Hewlett Packard diode array spectrophotometer Model 8451A (Palo Alto, CA) as a function of irradiation time.
Oxygen consumption
Oxygen consumption was measured with the aid of a Clark electrode. Samples (2.2 ml) were placed in a jacketed cell and irradiated directly with a 150W floodlamp filtered to remove wavelengths below 400 nm. Water at 25° C was circulated through the jacket to prevent sample heating.
EPR study
EPR spectra were recorded using a Bruker EMX spectrometer (Billerica, MA) operating at 10 mW power (9.78 GHz) with 1 G modulation amplitude and 100 kHz modulation frequency. Samples were placed in a quartz flat cell and irradiated directly inside the microwave cavity of the spectrometer using a 1 kW Xe arc lamp. Radiation from the lamp was passed through a filter to remove wavelengths below 400 nm. Hyperfine coupling constants were obtained by accumulating, simulating, and optimizing spectra on an IBM PC computer using software described elsewhere (31). The accuracy of the coupling constant measurements was ± 0.2 G. Fremy’s salt was used as a standard for the determination of the EPR spectral g-values.
Results
Absorption spectra
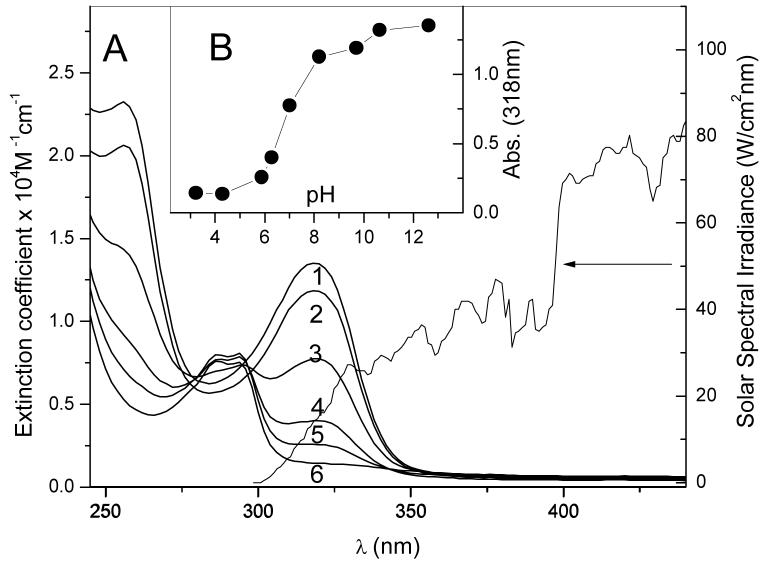
TBBPA is a hydrophobic compound readily soluble in organic solvents. In aqueous solutions, its solubility depends on pH, as its pKa value is about 7.4 (19). We used the surfactants SB-12 and Triton-X 100 to increase TBBPA solubility in water and in water-MeCN mixtures, independent of pH. The pH-dependent absorption spectra of TBBPA are shown in Fig. 1A. The absorption band around 320 nm belongs to the TBBPA anion, and measurement of its intensity confirms that the pKa of TBBPA, in the presence of surfactant, is also around neutral pH (Fig. 1B). When TBBPA is undissociated, e.g. in acidic solutions (Fig. 1A), the 320 nm absorption band has very low intensity so that the main absorption band of TBBPA is below 300 nm.
Figure 1.
Absorption of TBBPA in aqueous micellar solutions. A: Absorption spectra of TBBPA (200 μM) in different UV-transparent buffers (25 mM) containing SB-12 (25 mM). Spectrum 1, pH 12.6; S2, pH 9.7; S3, pH 7.0; S4, pH 6.3; S5, pH 5.8; S6, pH 3.2. Inset B: Peak absorption of TBBPA measured at 318 nm and plotted as a function of pH, revealing pKa value. Cuvette pathlength, 0.5 cm. Also shown is the solar spectral irradiance (modified from (44))
Singlet oxygen
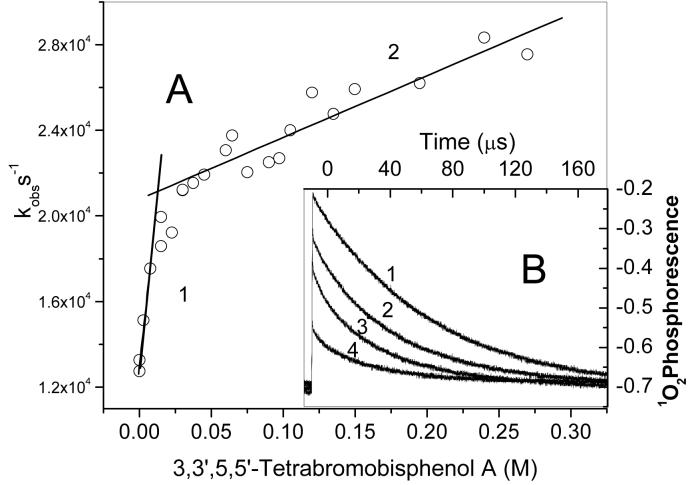
We measured the interaction of TBBPA with 1O2 in MeCN (Fig.2). Time-resolved detection of 1O2 phosphorescence recorded in the presence of increasing concentrations of TBBPA (Fig. 2B) revealed two quenching regions: one of which we assigned to the monomer (Fig. 2A, Line 1), while the other with a lower quenching rate constant may belong to aggregated TBBPA (Fig. 2, line 2). A similarly nonlinear quenching due to aggregation has been observed previously (32). Regardless of which region is considered, the kq values are quite low in MeCN (Fig.2A), indicating that non-dissociated TBBPA interacts weakly with 1O2.
Figure 2.
Quenching of 1O2 phosphorescence by TBBPA in MeCN. A: Observed first order rate constants for 1O2 quenching as a function of TBBPA concentration. The plot was approximated by two linear fittings: Line 1, a TBBPA monomer region yielding kq = (6.1 ± 0.5) × 105 M-1 s-1. Line, 2 a TBBPA dimer/aggregate region yielding kq = (2.9 ± 0.3) × 104 M-1 s-1. Inset B: Examples of 1O2 phosphorescence decay (1-4) in the presence of increasing TBBPA concentrations (0 mM, 15 mM, 45 mM, 135 mM). Perinaphthenone was used as a 1O2 photosensitizer excited at 355 nm.
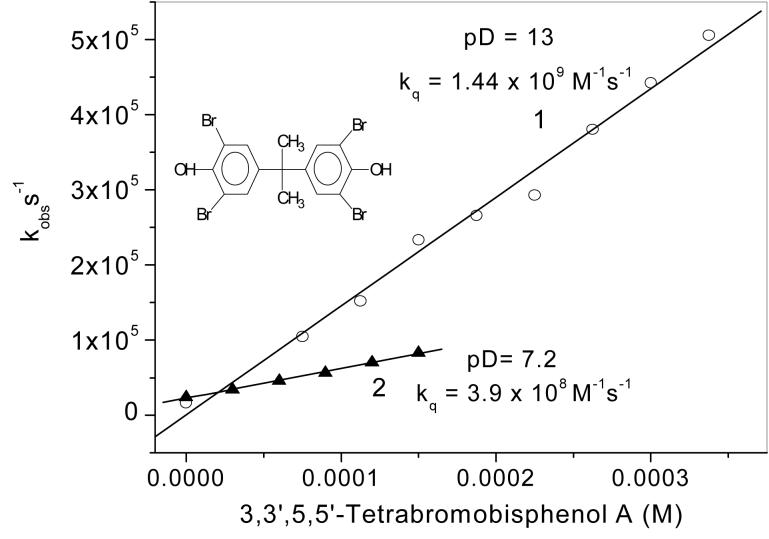
In aqueous alkaline solutions, TBBPA was a very efficient 1O2 quencher (Fig. 3). The quenching is close to the diffusion controlled range in NaOD solution (Fig.3, Line 1). Even at neutral pH, where TBBPA is only partially dissociated, the rate constant is quite high (Fig.3, Line 2), and similar to the value reported for the azide anion in solvent mixtures (33). The fast quenching, resulting in efficient oxygen consumption (vide infra), indicates that the overall quenching has a substantial chemical component, in contrast to the mostly physical quenching by azide. These findings clearly show that the TBBPA anion is easily oxidized by 1O2.
Figure 3.
Quenching of 1O2 phosphorescence by TBBPA in MeCN/D2O mixtures. Plots of observed first order rate constants for 1O2 quenching as a function of TBBPA concentration. Line 1, alkaline solution (pD 13) containing 0.5 M NaOD in MeCN:D2O, 50%:50%, (Vol.:Vol.) mixture; kq = (1.44 ± 0.06) × 109 M-1 s-1. Line 2, neutral solution (pD 7.2) containing SB-12 (50 mM) and phosphate buffer pD 7.2 (10 mM) in MeCN: D2O, 20%:80% (Vol.:Vol.) mixture; kq = (3.9 ± 0.1) × 108 M-1 s-1.
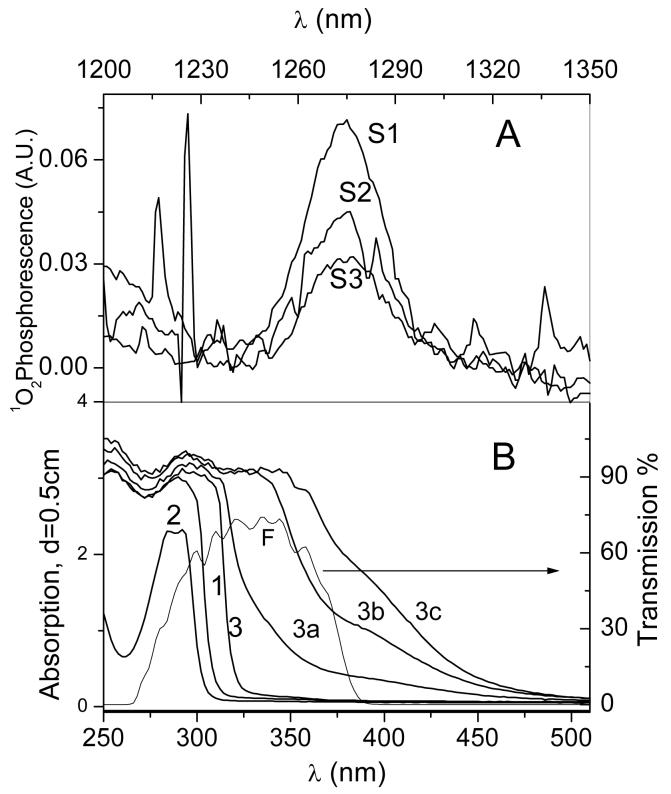
Interestingly, we have found that TBBPA alone can weakly photosensitize 1O2 generation in MeCN when irradiated with intense UV light (Fig. 4A). The phosphorescence spectra of 1O2 are clear and their sequence reflects bi-phasic quenching observed during time-resolved experiments (Fig.2). The strongest spectrum is for 10 mM TBBPA concentration, which permits adequate light absorption (Fig. 4B, S1) with minimal quenching due to aggregation (Fig. 2). The production and quenching of 1O2 by TBBPA in MeCN may have chemical implications because the solution turned yellow during prolonged irradiation, suggesting formation of unknown photoproducts formed either by reaction with singlet oxygen or other photochemical processes (Fig. 4B). However, these products were no better 1O2 photosensitizers than TBBPA itself, because we did not observed any stronger 1O2 production upon photolyte irradiation in the visible region (data not shown). These photochemically-induced changes suggest that, even without addition of external photosensitizers, 1O2 is likely to be involved in TBBPA photochemistry. As singlet oxygen may initiate radical oxidation processes via superoxide and decomposition of hydro(endo)peroxides, we investigated the possible involvement of radicals during TBBPA photo-oxidation using EPR.
Figure 4.
Absorption spectra and photosensitization of 1O2 by TBBPA in MeCN. A: 1O2 phosphorescence observed during the irradiation of TBBPA, 10mM, Spectrum S1; 1mM, S2, and saturated solution, S3. B: The corresponding absorption spectra, 1, 10mM, 2, 1mM, and 3, saturated solutions of TBBPA. The solutions were irradiated by a 300 Watt Hg lamp through water and interference (F) filters. Absorption increase observed during the irradiation of saturated TBBPA solution after 2 min., 3a; 7 min., 3b, and 17 min., 3c, of irradiation.
EPR studies
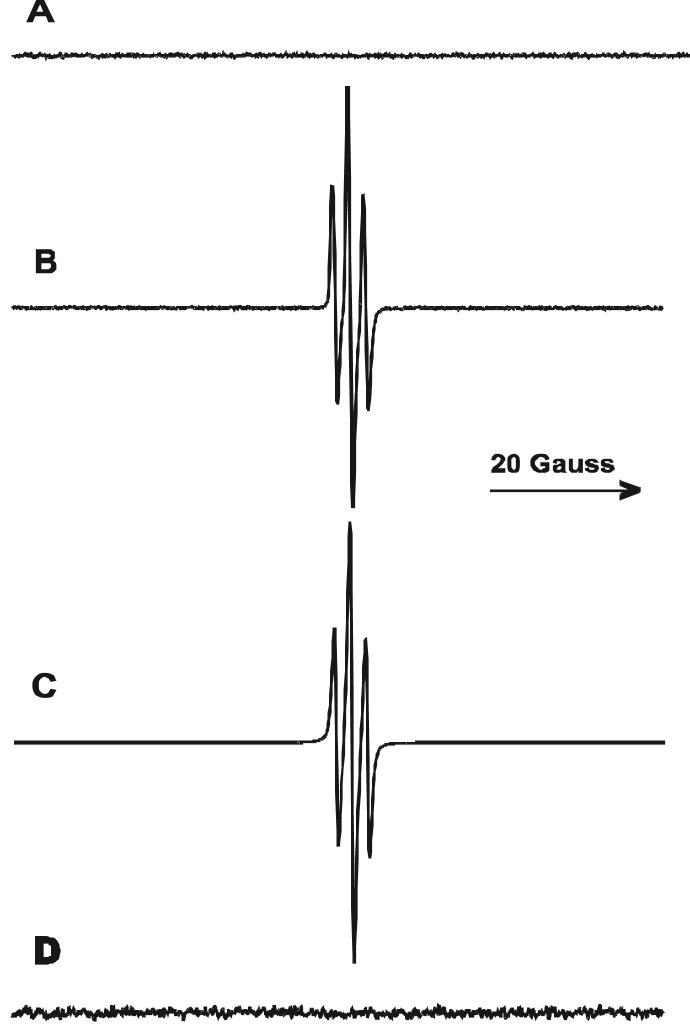
In neutral buffer (pH 7), a strong triplet EPR signal was generated (Fig. 5B) when a solution containing TBBPA and Rose Bengal was irradiated (λ>400 nm). This spectrum was simulated with a coupling constant a2H = 2.36 G (Fig. 5C). Han et al. (34) have reported that 2,6-dibromo-p-benzosemiquinone (Scheme, 1) was generated during ozonation of 2,4,6-tribromophenol under neutral conditions. No EPR signal was detected in the absence of TBBPA (Fig. 5A) or Rose Bengal (data not shown). The EPR parameters of the radical seen in Figure 5B are identical to those previously reported by Han et al. for the 2,6-dibromo-p-benzosemiquinone radical (34) but are quite different from the RB anion radical triplet (a2H = 3.31 G) (35).
Figure 5.
(A) EPR spectrum observed during 5 min of irradiation of RB (20 μM) in the absence of TBBPA in phosphate buffer (pH 7). (B) EPR spectrum observed during the irradiation of RB (20 μM) in the presence of TBBPA (1 mM) in phosphate buffer (pH 7). (C) Simulation spectrum of B with an hfc of a2H = 2.36 G, and g-value of 2.0056. (D) same as B in the presence of sodium azide (20 mM).
Generation of the 2,6-dibromo-p-benzosemiquinone radical was completely inhibited by the addition of sodium azide (Fig. 5D), confirming that its generation was essentially 1O2 dependent. The intensity of the 2,6-dibromo-p-benzosemiquinone radical was linearly dependent on the TBBPA concentration up to 500mM (data not shown). Irradiation of a solution containing TBBPA and methylene blue, another well-known generator of 1O2, gave the same EPR spectrum as seen in Figure 5B (data not shown). These results indicate that the 2,6-dibromo-p-benzosemiquinone radical is one of the byproducts generated from the reaction of 1O2 with the TBBPA present in our buffer solution. The signal intensity of the 2,6-dibromo-p-benzosemiquinone radical increased during irradiation and decayed in a first-order reaction with a rate constant of 1.2 × 102s-1 after the light was turned off (data not shown).
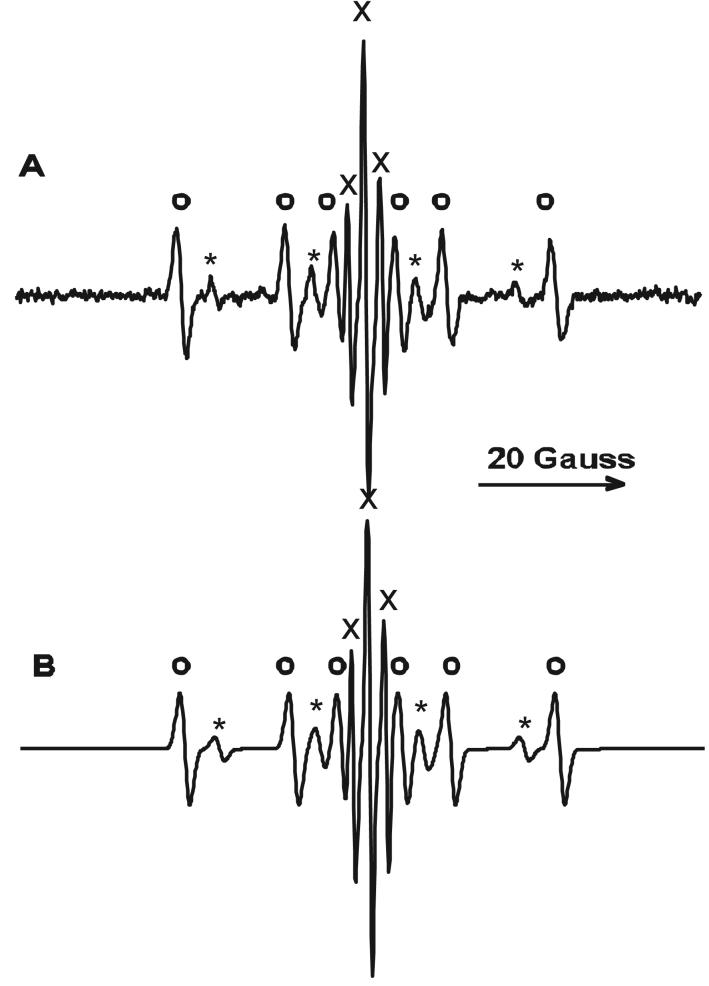
There are several reports suggesting that free radicals such as superoxide anion radical or hydroxyl radical are involved in the decomposition of halogenated phenolic compounds (3,14,24,26,34,36,37). To investigate this possibility we irradiated TBBPA and RB in neutral buffer solution after the addition of the spin trap DMPO. As shown in Fig. 6A, a weak EPR signal consisting of four lines with an intensity ratio of 1:2:2:1 (Fig. 6A, B, asterisks) was observed. This spectrum was identified as DMPO/.OH, the hydroxyl radical adduct of DMPO, suggesting that the .OH radical is generated during the 1O2 induced decomposition of TBBPA. However, DMPO/.OH is also generated by the reaction of 1O2 with DMPO (35) as well as the decomposition of DMPO/.OOH. In addition to DMPO/.OH the same triplet seen in the absence of DMPO (Fig. 6A; cf Fig. 5B,C) was observed although its intensity was reduced. This reduction is due to the faster 1O2 quenching rate constant by DMPO (1.2 × 106 M-1s-1), compared to that of TBBPA (35). Also, another signal consisting of six lines (Fig. 6A, open circles) was observed. The hfsc indicate that it is a carbon centered DMPO adduct (38). Unfortunately the hfsc of carbon centered adducts of DMPO are quite similar and so cannot be used with any certainty to identify the parent radical.
Figure 6.
(A) EPR spectrum observed during 1min irradiation of RB (20 μM), TBBPA (1 mM), and DMPO (100 mM) in phosphate buffer (pH 7). The spectrum contains a triplet 2,6-dibromo-p-semiquinone radical (x, a2H = 2.4 G, 38%) and a contribution from DMPO/.OH (*, aN = 15.0 G, aH = 14.4 G, 19%). The DMPO adduct of an unknown carbon-centered radical is marked with an open circle (○, aN = 16.0 G, aH = 22.9 G, 43%). (B) Computer simulated spectrum of A.
(A) EPR spectrum observed during 1min irradiation of RB (20 μM), TBBPA (1 mM), and DMPO (100 mM) in phosphate buffer (pH 7). The spectrum contains a triplet 2,6-dibromo-p-semiquinone radical (x, a2H = 2.4 G, 27%) and a contribution from DMPO/.OH (*, aN = 15.0 G, aH = 14.4 G, 15%). The DMPO adduct of an unknown carbon-centered radical is marked with an open circle (○, aN = 15.9 G, aH = 22.9 G, 58%). (B) Computer simulated spectrum of A).
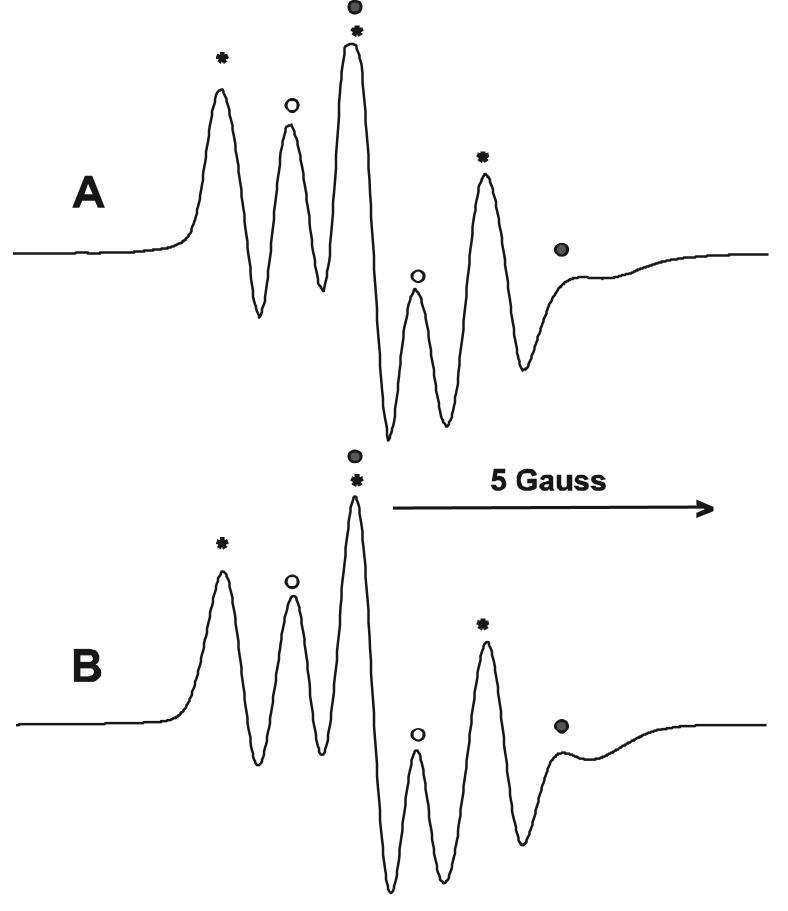
Irradiation (λ>400 nm) of TBBPA and RB in alkaline buffer (pH 10) generated a strong EPR spectrum with contributions from three radicals (Fig. 7A). A simulated spectrum and the values of the splitting constants are shown in Fig. 7B. The species marked with an asterisk consists of a triplet with an intensity ratio of 1:2:1, hfcs of a2H = 2.36 G, and a g-value of 2.0056 (31% of the total EPR intensity). These parameters are in accord with those of the 2,6-dibromo-p-semiquinone radical observed at pH 7 buffer (vide supra). In addition there is a doublet signal with hfcs aH = 3.90 G, and a g-value of 2.0050 (51%). Finally there is a weak doublet (18%) with, aH = 2.10 G and a g-value of 2.0055 (Figure 7A).
Figure 7.
(A) EPR spectrum observed during 5 min of irradiation of RB (20 μM) and TBBPA (1 mM) in phosphate buffer (pH 10). (B) Simulation of spectrum A; the triplet signal is marked with asterisks (*, a2H = 2.36 G, g-value = 2.0056, 31%). The major doublet signal is marked with open circles (○, aH = 2.10 G, g-value = 2.0055, 51%) and a minor doublet signal is marked with closed circles (●, aH = 3.90 G, g-value = 2.0050, 18%).
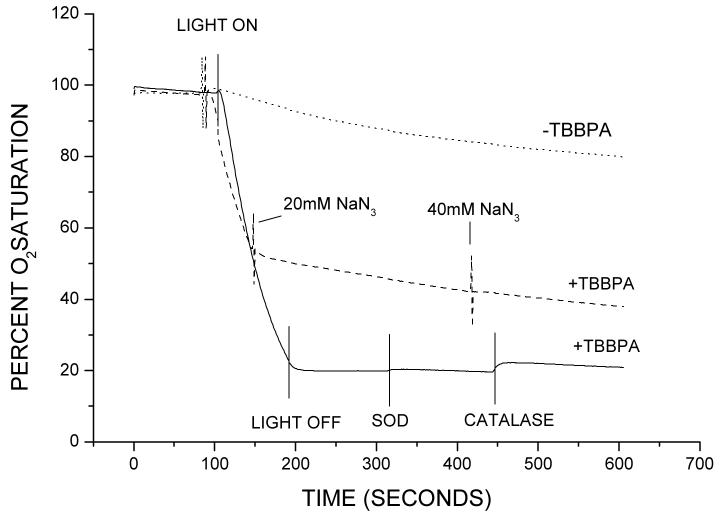
Oxygen photo-consumption
When a micellar solution of TBBPA containing RB was irradiated by visible light (λ>400 nm) oxygen was rapidly consumed (Fig. 8). No significant amount oxygen was recovered after the addition of either SOD or catalase indicating that neither superoxide nor hydrogen peroxide was generated. Oxygen consumption was inhibited by azide anion, a physical quencher of 1O2 (Fig. 8). This result clearly proves that 1O2 is indeed a major oxidant reacting with the TBBPA anion.
Figure 8.
Oxygen photo-consumption observed during the irradiation of RB (50 μM) in the presence of TBBPA (1 mM) in Triton X100 (10 μl/ml) micellar solution pH 10. Where indicated NaN3 (20 or 40 mM), SOD (50 ug/ml) or catalase (6500 units) were added.
Discussion
At neutral pH the absorption spectrum of TBBPA shows a maximum at ∼320 nm which increases as the pH is raised (Figure 1). However, sunlight has very weak intensity at 320 nm suggesting that direct photo-degradation of TBBPA may be environmentally negligible, and that photosensitized degradation, possibly involving 1O2, may be an alternative pathway.
We have found that TBBPA is a very weak 1O2 photosensitizer and a weak 1O2 quencher in an aprotic environment, such as MeCN, where the TBBPA phenol groups are undissociated. At high concentrations in MeCN singlet oxygen quenching by TBBPA decreases even further probably due to aggregation. In contrast, in an aqueous environment at pH values near or above its pKa≈7, TBBPA becomes a very strong 1O2 quencher with kq=1.4×109 M-1s-1. This quenching initiates TBBPA oxidation and results in oxygen consumption (Figure 8) and the generation of radicals (Figures 5-7). A possible mechanism that would explain our findings is shown in the Scheme. Reaction of 1O2 initially generates a hydroperoxide (39) that is oxidized to a peroxyl radical followed by conversion to an alkoxyl radical. The latter undergoes β-scission (40) to give 2,6-dibromobenzoquinone and a carbon-centered radical (Scheme, 2) trapped by DMPO. The alkoxyl radical can also decompose to give the 2,6-dibromo-p-benzosemiquinone radical (Scheme, 1) which was detected by direct EPR. and 4-cyclopropenyl-2,6-dibromophenol, a photoproduct of TBBPA previously identified by Ericksson et al. (19).
No EPR signal was detected in the absence of TBBPA (Fig. 5A) or Rose Bengal. Eriksson et al. (19) have identified 4-hydroxy-2,6-dibromophenol and three isomeric dibromophenols as the major decomposition products of TBBPA in a photochemical transformation system. Tai et al. (37) have proposed that hydroquinone and 4-isopropenylphenol could be formed from the decomposition of bisphenol A under visible light irradiation. Also, semiquinone radicals are generated during the autooxidation of polyhydroxyaromatics such as hydroquinone and dichlorohydroquinones (34,41). Katsumata et al. (42) have suggested that the p-isopropylphenol radical could be generated from the photodegradation of bisphenol. Eriksson et al. (19) have reported that 2,6-dibromo-4-isopropylphenol is generated during the photochemical transformation of TBBPA. Thus it is possible that the carbon-centered radical adduct seen in Figure 6A results from the trapping of the 2,6-dibromo-4-isopropenylphenol radical (Scheme, 2).
Irradiation of TBBPA and RB at pH 10 generated three radicals (Figure 7A) one of which was identified as the 2,6-dibromo-p-benzosemiquinone radical. The EPR spectra of the remaining radicals consisted of doublets indicating coupling to a single hydrogen atom. These radicals may arise from substitution at the 3-position of the 2,6-dibromo-p-benzosemiquinone radical by EPR silent groups (Scheme, 3, R) such as hydroxyl (O- at pH above 7.4) or perhaps bromine.
The photodegradation of TBBPA in the environment may result from direct photolysis as well as reaction with sunlight generated reactive oxygen species such as singlet oxygen. Because the quantum yield of self sensitized singlet oxygen generation by TBBPA is low it is unlikely to play a role in degradation of the retardant. However, previous studies have shown that there are environmental sources of singlet oxygen such as the humic acids (27,43) which could contribute to the destruction of TBBPA. We are currently studying the photodegradation of TBBPA in the presence of humic acid and have detected the 2,6-dibromo-p-benzosemiquinone radical during visible light irradiation of TBBPA/humic acid (unpublished data).
Acknowledgements
This research was supported by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences. The authors also wish to thank Dr. Ann Motten, NIEHS, for critical reading of the manuscript.
Abbreviations
- BFR(s)
brominated fire retardant(s)
- DMPO
5,5,-dimethyl-1-pyrroline N-oxide
- EPR
electron paramagnetic resonance
- MB
methylene blue
- MeCN
acetonitrile
- RB
rose bengal
- ROS
reactive oxygen species
- SB-12
lauryl sulfobetaine
- SOD
superoxide dismutase
- TBBPA
tetrabromobisphenol A
References
- (1).Alaee M, Arias P, Sjodin A, Bergman A. An overview of commercially used brominated flame retardants, their applications, their use patterns in different countries/regions and possible modes of release. Environ. Int. 2003;29:686–689. doi: 10.1016/S0160-4120(03)00121-1. [DOI] [PubMed] [Google Scholar]
- (2).Barontini F, Cozzani V, Marsanich K, Raffa V, Petarca L. An experimental investigation of tetrabromobisphenol A decomposition pathways. J. Anal. Appl. Pyrolysis. 2004;72:41–53. [Google Scholar]
- (3).Reistad T, Mariussen E, Fonnum F. The effect of a brominated flame retardant, tetrabromobisphenol-A, on free radical formation in human neutrophil granulocytes: The involvement of the MAP kinase pathway and protein kinase C. Toxicol. Sci. 2005;56:95–104. doi: 10.1093/toxsci/kfh298. [DOI] [PubMed] [Google Scholar]
- (4).Hornung A, Balabanovich AI, Donner S, Seifert H. Detoxification of brominated pyrolysis oils. J. Anal. Appl. Pyrolysis. 2003;70:723–733. [Google Scholar]
- (5).Bromine Science and Environmental Forum BSEF Major brominated flame retardants volume estimates. 2003 Available from < http:\www.bsef.com/docs/BFR_vols_2001.doc>. Ref Type: Electronic Citation from April 10.
- (6).Bromine Science and Environmental Forum BSEF An introduction to bromine. 2006 Available from < http:\www.bsef.com/docs/BSEF_Bromine_intro.pdf>. Ref Type: Electronic Citation.
- (7).Fackler P. Bioconcentration and elimination of 14C-residues by Eastern oysters (Crassostrea virginica) exposed to tetrabromobisphenol A. Springborn Life Sciences, Inc.; Wareham MA: 1989. [Google Scholar]
- (8).Fackler P. Bioconcentration and elimination of 14C-residues by fathead minnows (Pimephales promelas) exposed to tetrabromobisphenol A. Springborn Life Sciences, Inc; Wareham MA: 1989. [Google Scholar]
- (9).Fackler P. Determination of the biodegradability of tetrabromobisphenol A in soil under aerobic conditions. Springborn Life Sciences, Inc.; Wareham, MA: 1989. [Google Scholar]
- (10).Anonymous . Biodegradation and bioaccumulation data of existing chemicals based on the CSCL. Chemicals Inspection & Testing Institute Toxicology and Information Center; Tokyo, Japan: 1992. [Google Scholar]
- (11).Thomsen C, Lundanes E, Becher G. Brominated flame retardants in plasma samples from three different occupational groups in Norway. J. Environ. Monit. 2001;3:366–370. doi: 10.1039/b104304h. [DOI] [PubMed] [Google Scholar]
- (12).Watanabe I, Sakai SI. Environmental release and behavior of brominated flame retardants. Environ. Int. 2003;29:665–682. doi: 10.1016/s0160-4120(03)00123-5. [DOI] [PubMed] [Google Scholar]
- (13).Berger U, Herzke D, Sandanger TM. Two trace analytical methods for determination of hydroxylated PCBs and other halogenated phenolic compounds in eggs from Norwegian birds of prey. Anal. Chem. 2004;76:441–452. doi: 10.1021/ac0348672. [DOI] [PubMed] [Google Scholar]
- (14).Morris S, Allchin CR, Zegers BN, Haftka JJH, Boon JP, Belpaire C, Leonards PEG, van Leeuwen SPJ, De Boer J. Distribution an fate of HBCD and TBBPA brominated flame retardants in north sea estuaries and aquatic food webs. Environ. Sci. Technol. 2004;38:5497–5504. doi: 10.1021/es049640i. [DOI] [PubMed] [Google Scholar]
- (15).Kulovaara M, Backlund P, Corin N. Light-induced degradation of DDT in humic water. Sci. Total Environ. 1995;170:185–191. [Google Scholar]
- (16).Ohko Y, Ando I, Niwa C, Tatsuma T, Yamammura T, Nakashima T, Kubota Y, Fujishima A. Degradation of bishpenol A in water by TiO2 photocatalyst. Environ. Sci. Technol. 2001;35:2365–2368. doi: 10.1021/es001757t. [DOI] [PubMed] [Google Scholar]
- (17).Yao Y, Kakimoto K, Ogawa I, Kato Y, Hanada Y, Shinohara R, Yoshino E. Photodechlororination pathways of non-ortho substituted PCBs by ultraviolet irradiation in alkaline 2-propanol. Bull. Environ. Contam. Toxicol. 1997;59:238–245. doi: 10.1007/s001289900470. [DOI] [PubMed] [Google Scholar]
- (18).Richard C, Grabner G. Environmental Photochemistry. Vol. 2. Springer Verlag; Berlin, Heidelberg, Germany: 1999. Mechanism of phototransformation of phenol and derivatives in aqueous solution; pp. 139–179. part 1. [Google Scholar]
- (19).Eriksson J, Rahm S, Green N, Bergman A, Jakobsson E. Photochemical transformations of tetrabromobisphenol A and related phenols in water. Chemosphere. 2004;54:117–126. doi: 10.1016/S0045-6535(03)00704-5. [DOI] [PubMed] [Google Scholar]
- (20).Barontini F, Marsanich K, Petarca L, Cozzani V. Thermal degradation and decomposition products of electronic boards containing BFRs. Ind. Engineer. Chem. Res. 2005;44:4186–4199. [Google Scholar]
- (21).Luda MP, Balabanovich AI, Hornung A, Camino G. Thermal degradation of a brominated bisphenol a derivative . Polymers Adv. Technol. 2003;14:741–748. [Google Scholar]
- (22).Marsanich K, Zanelli S, Barontini F, Cozzani V. Evaporation and thermal degradation of tetrabromobisphenol A above the melting point. Thermochim. Acta. 2004;421:95–103. [Google Scholar]
- (23).Factor A. Thermal decomposition of 4,4 prime-isopropylidene bis-2-6-dibromophenol (Tetrabromobisphenol-A) J. Polym. Sci. Part A-1 Polym. Chem. 1973;11:1691–1701. [Google Scholar]
- (24).Shi H, Wang X, Luo Y, Su Y. Electron paramagnetic resonance evidence of hydroxyl radical generation and oxidative damage induced by tetrabromobisphenol A in Carassius auratus. Aquat. Toxicol. 2005;74:365–371. doi: 10.1016/j.aquatox.2005.06.009. [DOI] [PubMed] [Google Scholar]
- (25).Zepp RG, Schlotzhauer RF, Sink RM. Photosensitized transformations involving electronic energy transfer in natural waters. Environ. Sci. Technol. 1985;19:74–81. [Google Scholar]
- (26).Skurlatov YI, Ernestova LS, Vichutinskaya EV, Samsonov DP, Semenova IV. Photochemical transformation of polychlorinated phenols. J. Photochem. Photobiol. A: Chem. 1997;107:207–213. [Google Scholar]
- (27).Haag WR, Hoigne J. Degradation of compounds in water by singlet oxygen. Wat. Chlorin.: Environ. Inpact and Health Effects. 1986;5:1011–1020. [Google Scholar]
- (28).Baxter RM, Carey JH. Evidence for photochemical generation of superoxide ion in humic waters. Nature. 1983;306:575–576. [Google Scholar]
- (29).Bilski P, Chignell CF. Optimization of a pulse laser spectrometer for the measurement of the kinetics of singlet oxygen 1O2 decay in solution. J. Biochem. Biophys. Methods. 1996;33:73–80. doi: 10.1016/s0165-022x(96)00012-7. [DOI] [PubMed] [Google Scholar]
- (30).Hall RD, Buettner GR, Motten AG, Chignell CF. Near-infrared detection of singlet molecular oxygen produced by photosensitization with promazine and chlorpromazine. Photochem Photobiol. 1987;46:295–300. doi: 10.1111/j.1751-1097.1987.tb04769.x. [DOI] [PubMed] [Google Scholar]
- (31).Duling DR. Simulation of multiple isotropic spin-trap EPR spectra. J. Magn. Reson. B. 1994;104:105–110. doi: 10.1006/jmrb.1994.1062. [DOI] [PubMed] [Google Scholar]
- (32).Bilski P, Hideg K, Kalai T, Bilska MA, Chignell CF. Interaction of singlet molecular oxygen with double fluorescent and spin sensors. Free Radic Biol Med. 2003;34:489–495. doi: 10.1016/s0891-5849(02)01330-8. [DOI] [PubMed] [Google Scholar]
- (33).Li MY, Cline CS, Koker EB, Carmichael HH, Chignell CF, Bilski P. Quenching of singlet molecular oxygen (1O2) by azide anion in solvent mixtures. Photochem Photobiol. 2001;74:760–764. doi: 10.1562/0031-8655(2001)074<0760:qosmoo>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- (34).Han S-K, Ichikawa K, Utsumi H. Generation of p-semiquinone radicals from chlorophenols in water during ozonation. Wat. Res. 1998;32:1978–1981. [Google Scholar]
- (35).Bilski P, Reszka K, Bilska M, Chignell CF. Oxidation of the spin trap 5,5-dimethyl-1-pyrroline N-oxide by singlet oxygen in aqueous solution. J. Am. Chem. Soc. 1996;118:1330–1338. [Google Scholar]
- (36).Mazellier P, Bolte M. Heterogeneous light-induced transformation of 2,6-dimethylphenol in aqueous suspensions containing goethite. J. Photochem. Photobiol. A: Chem. 2000;132:129–135. [Google Scholar]
- (37).Tai C, Jiang G, Liu J, Zhou Q, Liu J. Rapid degradation of bisphenol A using air as the oxidant catalyzed by polynuclear phthalocyanine complexes under visible light irradiation. J. Photochem. Photobiol. A: Chem. 2005;172:275–282. [Google Scholar]
- (38).Li ASW, Cummings KB, Roethling HP, Buettner GR, Chignell CF. A spin-trapping database implemented on the IBM PC/AT. J. Mag. Res. 1988;79:140–142. [Google Scholar]
- (39).Foote CS. Photosensitized Oxidation and Singlet Oxygen: Consequences in Biological Systems. In: Pryor WA, editor. Free Radicals in Biology. Vol. 2. Academic Press; New York: 1976. pp. 85–100. [Google Scholar]
- (40).Bietti M, Lanzalunga O, Salamone M. Structural effects on the beta-scission reaction of alkoxyl radicals. Direct measurement of the absolute rate constants for ring opening of benzocycloalken-1-oxyl radicals. Journal of Organic Chemistry. 2005;70:1417–1422. doi: 10.1021/jo048026m. [DOI] [PubMed] [Google Scholar]
- (41).Zang LY, Stone K, Pryor WA. Detection of free radicals in aqueous extracts of cigarette tar by electron spin resonance. Free Radic. Biol. Med. 1995;19:161–167. doi: 10.1016/0891-5849(94)00236-d. [DOI] [PubMed] [Google Scholar]
- (42).Katsumata H, Kawabe S, Kaneco S, Suzuki T, Ohta K. Degradation of bisphenol A in water by the photo-fenton reaction. J. Photochem. Photobiol. A: Chem. 2004;162:297–305. [Google Scholar]
- (43).Sandvik SLH, Bilski P, Pakulski JD, Chignell CF, Coffin RB. Photogeneration of singlet oxygen and free radicals in dissolved organic matter isolated from the Mississippi and Atchafalaya River plumes. Marine Chemistry. 2000;69:139–152. [Google Scholar]
- (44).Inbaraj JJ, Kukielczak BM, Bilski P, He YY, Sik RH, Chignell CF. Photochemistry and photocytotoxicity of alkaloids from Goldenseal (Hydrastis canadensis L.). 2. Palmatine, hydrastine, canadine, and hydrastinine. Chem Res Toxicol. 2006;19:739–744. doi: 10.1021/tx050356u. [DOI] [PubMed] [Google Scholar]