Abstract
A common mutation (C677T) in the gene encoding for methylenetetrahydrofolate reductase (MTHFR) (5-methyltetrahydrofolate:(acceptor) oxidoreductase, EC 1.7.99.5), a key regulatory enzyme in one-carbon metabolism, results in a thermolabile variant of the MTHFR enzyme with reduced activity in vitro. In the present study we used a chromatographic method for folate analysis to test the hypothesis that this mutation would be associated with altered distribution of red blood cell (RBC) folates. An alteration was found as manifested by the presence of formylated tetrahydrofolate polyglutamates in addition to methylated derivatives in the RBCs from homozygous mutant individuals. 5-Methyltetrahydrofolate polyglutamates were the only folate form found in RBCs from individuals with the wild-type genotype. Existence of formylated folates in RBCs only from individuals with the thermolabile MTHFR is consistent with the hypothesis that there is in vivo impairment in the activity of the thermolabile variant of MTHFR and that this impairment results in an altered distribution of RBC folates.
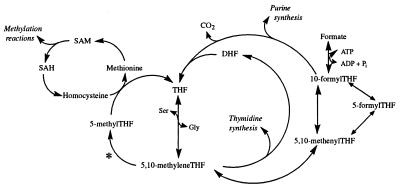
Methylenetetrahydrofolate reductase (MTHFR) (5-methyltetrahydrofolate:(acceptor) oxidoreductase, EC 1.7.99.5) catalyzes the reduction of 5,10-methylenetetrahydrofolate (5,10-methyleneTHF) to 5-methyltetrahydrofolate (5-methylTHF), the methyl donor for methionine synthesis from homocysteine (see Fig. 1). This reaction is important in one-carbon metabolism because methionine, along with other functions, is the precursor of S-adenosylmethionine, the methyl group donor in more than 100 reactions. If not reduced to 5-methylTHF, 5,10-methyleneTHF has two alternate fates. 5,10-MethyleneTHF can transfer its methylene group to dUMP to synthesize dTMP, or 5,10-methyleneTHF can be oxidized to 5,10-methenylTHF, which in turn is converted to 10-formylTHF. This branch of the pathway is necessary for the synthesis of purines. Transfer of the one-carbon group, whether it be in the form of a methyl, methenyl, or formyl group, results in the production of an unsubstituted folate, THF, or dihydrofolate (which is reduced to THF). THF in turn acquires a one-carbon unit, primarily through the acquisition of the serine 3-carbon, to form 5,10-methyleneTHF, thus starting the cycle again. Two features render MTHFR a regulatory enzyme for the folate-dependent flow of one-carbon units into their respective metabolites: the reaction it catalyzes is physiologically irreversible and the enzyme is subject to allosteric product inhibition by S-adenosylmethionine (1). The inhibition by S-adenosylmethionine regulates the amount of 5-methylTHF being synthesized, which then is used to meet the metabolic demands for methionine, leaving the remaining 5,10-methyleneTHF available for the synthesis of thymidylate and purines. Without S-adenosylmethionine inhibition, the irreversibility of the MTHFR reaction renders the availability of nonmethylated folates dependent only on homocysteine methylation.
Figure 1.
Overview of the folate metabolic pathway in humans. The enzymatic reaction catalyzed by MTHFR is indicated by ∗. SAM, S-adenosylmethionine; SAH, S-adenosylhomocysteine.
Rare mutations, which result in severe impairments in MTHFR activity, are associated with hyperhomocysteinemia and clinical manifestations of varying severity (2). Such mutations also have been associated with altered folate form distribution in cultured fibroblasts. This altered distribution was manifested by a lower proportion of 5-methylTHF and a higher proportion of formylated folates than in cultured fibroblasts from controls (3). This alteration in folate form distribution is consistent with our understanding of folate metabolism. Decreased MTHFR activity would result in a lower rate of reduction of 5,10-methyleneTHF to 5-methylTHF, resulting in increased availability of 5,10-methyleneTHF for oxidation to the formylated folate forms. It therefore follows that 5-methylTHF levels would be proportionately lower, whereas nonmethylated folates, in this case formylated folates, would be proportionately higher.
The C677T MTHFR mutation, with an allele frequency of about 35% and a homozygous prevalence of about 12% in the North American Caucasian population (4), is exceedingly more common than the rare MTHFR mutations that produce profound impairments in enzyme activity. When present in a homozygous state, this polymorphism results in a variant of the MTHFR enzyme referred to as the thermolabile MTHFR (5). To date, all data concerning enzyme activity and heat sensitivity of this variant have been obtained in vitro, by using cell-free extracts from lymphocytes or fibroblasts. These in vitro data indicate that the thermolabile MTHFR variant is less stable, as indicated by its sensitivity to heat, and that it has 50–60% lower activity than the thermostable wild-type enzyme (4). Data from a number of studies indicate that individuals with the homozygous mutant T/T genotype have mildly elevated plasma total homocysteine levels compared with individuals with either the heterozygous C/T or homozygous wild-type C/C genotype (4). However, direct in vivo evidence of impaired 5-methylTHF synthesis in individuals with the T/T genotype is lacking. Even the association of the MTHFR genotype with homocysteine is not well understood because it is contingent on folate status. Elevated plasma levels of homocysteine are seen in individuals with the T/T genotype only under conditions of inadequate folate status (6). When folate status is adequate, plasma homocysteine levels are low and independent of genotype.
In this study, we used a chromatographic method to test the hypothesis that the C677T mutation in MTHFR is associated with impaired activity of the MTHFR enzyme in vivo and that this impairment is associated with an altered distribution in red blood cell (RBC) folates. RBC folates have been reported to exist exclusively in the form of 5-methylTHF of various polyglutamate chain lengths (7). An impaired ability to synthesize these forms would be manifested by existence of nonmethylated folate forms.
MATERIALS AND METHODS
MTHFR Genotyping.
In the present study we analyzed folate form distribution in RBCs from a total of 18 individuals, which included eight sex- and age-matched pairs of homozygotes for the C/C and T/T genotypes and two unmatched subjects with T/T genotypes. Volunteers were prescreened to select those with the T/T or C/C MTHFR genotype. DNA was isolated from the buffy coat, and MTHFR genotype was determined by using a published method (5). Informed consent was obtained from all subjects, and the study protocol was approved by the New England Medical Center Human Investigation Review Committee. Volunteers included both males and females in the age range of either 25–37 or 65–75 years old.
Extraction and Analysis of Folates.
After an overnight fast, blood was collected into a Vacutainer tube containing EDTA. RBCs were isolated by centrifugation within 1 hr of collection. RBCs were washed with 9 g/liter of sodium chloride, and washed RBCs were stored at −70°C, until folate analysis.
RBC folates were analyzed by using the principle of our affinity/HPLC method (8), which was modified to increase its sensitivity through the use of electrochemical (coulometric) detection, instead of UV detection (8). More details of this method will be published elsewhere. Briefly, frozen RBCs were homogenized in approximately 10 vol of 0.05 M potassium tetraborate, which contained 10 g/liter of sodium ascorbate and 2 g/liter of Triton X-100 (9). Hemoglobin content was measured in an aliquot of the RBC extract by using a kit (Sigma). The remaining homogenate was heat-extracted and centrifuged. Two milliliters of the supernatant fraction was injected onto an affinity column (10 × 4.6 mm) that contained purified milk folate binding protein covalently bound to Affi-Prep 10 support (Bio-Rad). After washing the affinity column sequentially with a 0.05 M potassium phosphate, pH 7 and water, folates on the affinity column were eluted onto the analytical column [Prism RP (4.6 × 150 mm), Keystone Scientific, Bellefonte, PA] with an acid mobile phase (0.028 M dipotassium phosphate and 0.06 M phosphoric acid in water). Folates then were eluted from the analytical column by using the same aqueous mobile phase at a flow rate of 1 ml/min for 6 min followed by a linear gradient over 50 min to the same mobile phase containing 20% acetonitrile (vol/vol). This elution separates folates both on the basis of their pteridine ring structure and number of glutamate residues. Folate forms elute in the following order: THF, 5-methylTHF, 5,10-methyleneTHF, 5- and 10-formylTHF (which are indistinguishable from each other with this technique), dihydrofolate, and pteroylglutamate. For folates with the same pteridine ring structure, retention time of folate increases with increasing glutamate content of the folate molecule. Folate activity is determined by using an ESA Four Channel Coularray Detector (ESA, Chelmsford, MA) with channels 1–4 set at 100, 300, 500, and 700 mV, respectively. With this detector, folates with different pteridine ring structures give a unique, characteristic electrochemical response across the four channels, irrespective of their glutamate content. Quantification and identification of individual folates is done by comparison to external folate standards of known concentrations. Because the individual folates give a response across several channels, the sum of the areas in all channels displaying a response is used for quantification. In the case of overlapping peaks, such as pentaglutamyl-5,10-methenylTHF and octaglutamyl-5-methylTHF, advantage is taken of the characteristic response of the different folate forms across the four channels. The dominant response for 5-methylTHF is in channels 1 and 2, with minor responses in channels 3 and 4 (see Fig. 2). An electrochemical response for 5,10-methenylTHF is seen only in channels 3 and 4. Thus the concentration of octaglutamyl-5-methylTHF is calculated from the peak areas in channels 1 and 2. The contribution of octaglutamyl-5-methylTHF to channels 3 and 4 can be calculated and subtracted from the total respective areas, leaving the areas contributed by pentaglutamyl-5,10-methenylTHF.
Figure 2.
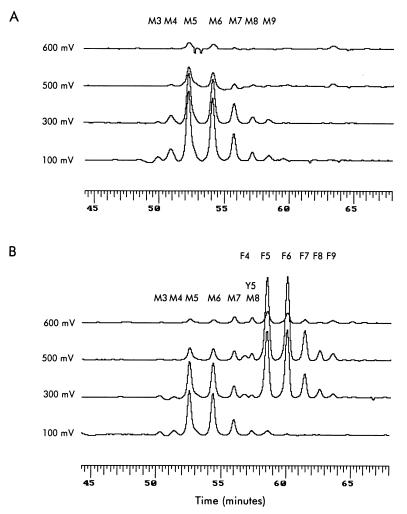
Chromatographic separation of RBC folates. All folate peaks are shown and annotated. Each chromatogram represents the response of channels 1–4, which are set at 100, 300, 500, and 600 mV, respectively. (A) A representative chromatogram of RBC folates from an individual with the C/C MTHFR genotype. (B) A representative chromatogram of RBC folates from an individual with the T/T MTHFR genotype. Identification of the folate derivatives in the various peaks is denoted as: M, 5-methylTHFs; Y, 5,10-methenylTHFs; and F, 10-formylTHFs. Numbers following the letters denote the number of glutamate residues.
In preliminary studies with standards, synthesized as described (8), it was found that there was some conversion of 10-formylTHF to 5,10-methenylTHF during chromatography, presumably because of the acid pH of the mobile phase. Because of the potential for postextraction conversion of 10-formylTHF to 5,10-methenylTHF, peaks corresponding to 5,10-methenylTHF polyglutamates were quantified and their value was added to that of the corresponding 10-formylTHF and hereafter are referred to as formylated THF. Peaks corresponding to 5,10-methenylTHF polyglutamates were seen only in chromatograms with formylated folates and were quantitatively minor (10 ± 5%, mean ± SD) compared with the 10-formylTHF.
RBC folate content, using the same extract used for the HPLC analysis, was measured by a Lactobacillus casei microbial method after treatment with chicken pancreas conjugase (10).
RESULTS
Fig. 2 shows typical chromatograms of RBC folates from individuals with the C/C genotype (Fig. 2A) and with the mutant (T/T) genotype (Fig. 2B). The two chromatograms clearly differ. In the RBCs from the individual with the C/C genotype, folates are comprised entirely of 5-methylTHF with different glutamate chain lengths, whereas the RBCs from the individual with the mutant T/T genotype contain not only the same 5-methylTHF seen in Fig. 1A, but also additional folates characterized by longer retention times and a different electrochemical response. These additional folates are formylated THF polyglutamates. Although possible to measure with our HPLC method, no THF was detected. In the chromatograms shown in Fig. 2, pentaglutamyl-THF would have eluted at about 47 min.
The data obtained from these chromatograms is summarized in Table 1. All RBC folates from individuals with the C/C genotype were in the form of 5-methylTHF polyglutamates. In contrast, RBCs from individuals with the T/T genotype contained both 5-methylTHF and formylated THF polyglutamates, with the proportion of formylated THF ranging from 0% to 59% of total folate. Otherwise, no effect of genotype was seen on total RBC folate content (Table 1) or on the number of glutamate residues (Student’s t test), which averaged 5.69 ± 0.14 and 5.70 ± 0.14 (mean ± SD) per folate molecule for the C/C and T/T genotypes, respectively. There was also no significant difference (Student’s paired t test) in glutamate chain length between the formylated folates (5.75 ± 0.17) and nonformylated folates (5.70 ± 0.11) in RBCs containing both of these folate forms. For comparison, we also measured total folate in the RBCs by microbial assay and found no difference (Student’s t test) between genotypes (2.4 ± 1.1 and 2.3 ± 1.1 nmol folate/g of hemoglobin for the C/C and T/T genotypes, respectively).
Table 1.
Effect of MTHFR genotype on RBC folate content and relative folate form distribution
| MTHFR genotype | Total folate, nmol/g hemoglobin | Number of samples with formylated THF | Formylated THF, percent of total | Methylated THF, percent of total |
|---|---|---|---|---|
| C/C | 3.05 ± 1.01 | 0/8 | 0 | 100 |
| T/T | 2.98 ± 0.95 | 8/10* | 29 ± 22* | 71 ± 22* |
Values represent mean ± SD.
Total folate concentrations represent the sum of the individual folate form concentrations obtained by chromatography.
Statistically significant difference (P < 0.001) between genotypes by using Mann–Whitney U test.
Folate form distribution has been measured in RBCs from a limited number of individuals heterozygous for the C677T mutation (n = 5). So far, only 5-methylTHF polyglutamates have been found in these RBCS (data not shown). However, these findings must be confirmed with a larger sample size.
DISCUSSION
Mature RBCs have a negligible capacity to transport and accumulate folate (11). Thus intracellular RBC folate accumulates during erythropoiesis (11). Previously, RBCs have been reported to contain only 5-methylTHF polyglutamates. Our finding of formylated THF in certain RBCs implies that the process of conversion to methylated folates in the immature RBC was impaired. The presence of formylated folates only in RBCs from individuals homozygous for the T/T mutation is consistent with the hypothesis that the thermolabile MTHFR catalyzes the synthesis of 5-methylTHF less efficiently than the wild-type enzyme.
We found no effect of MTHFR genotype on folate glutamate chain length. This result is consistent with the finding of Foo and coworkers (12), who observed no difference in folate polyglutamate distribution between cultured fibroblasts from individuals with severe MTHFR deficiency and controls. The average glutamate chain length of RBC folates using our method was about 5.7, which was somewhat higher than that measured in human RBCs by Pfeiffer and Gregory (calculated from their data to be 5.2) (13). The difference may have been caused by differences in extraction. RBCs in the present study were extracted at high pH to prevent enzymatic deconjugation of the folates by plasma pteroylpoly-γ-glutamate hydrolase (EC 3.4.19.9) activity. There was no detectable mono-, di-, or triglutamyl-folate forms in the RBC extracts in this study, suggesting total inhibition of the plasma conjugase. Because Pfeiffer and Gregory (13) were assessing the capacity of plasma to deconjugate folates, no attempt was made to prevent deconjugation of the folates and some deconjugation of the folates appears to have occurred, even with no incubation, as demonstrated by the presence of about 5% of the folate as monoglutamyl-5-methylTHF.
Since its discovery, much effort has been directed toward determining the clinical significance of the MTHFR C677T mutation. The original report by Kang and coworkers (14) suggested that the thermolabile MTHFR variant is a risk factor for coronary artery disease. This contention has been supported by some studies (15–17), but refuted by many others (18–20). Studies in the Netherlands, Ireland, and the United States have provided evidence for an association between the T/T mutation and increased risk of neural tube defects (21–23). Other studies, however, have been unable to demonstrate this association (24, 25). Although some of the inconsistencies between studies may be attributable to improper selection of the control populations (26), it is possible that some of the inconsistencies are caused by other factors that affect the activity of the thermolabile enzyme in its natural milieu. In the present study, the wide differences found in the proportion of formylated folate between individuals in the homozygous T/T group are consistent with the view that other factors affect the synthesis of 5-methylTHF by the thermolabile MTHFR. Our previous finding of an interaction between folate status and the homozygous T/T mutation in their effect of homocysteine levels suggests that folate is one of these factors (6). The experimental approach and the data produced in the present study offer a unique opportunity to obtain additional insight into these factors, which could lead to understanding the clinical significance of this mutation.
Important evidence of a clinical effect of the T/T genotype comes from two prospective epidemiological studies that have shown an association between the T/T genotype and a decreased risk of developing colon cancer (27, 28). The reduction of 5,10-methyleneTHF by MTHFR is physiologically irreversible and an excessive accumulation of 5-methylTHF may be detrimental to the de novo synthesis of nucleic acids that use nonmethylated folates as substrates. The observation that the T/T genotype is associated with a lower risk of developing colon cancer could be explained by an impaired activity of the thermolabile variant, resulting in a higher proportion of formylated folates, as shown in the present study. This shift in folate distribution could favor DNA synthesis and repair because of the dependence of these systems on nonmethylated forms of folate.
Acknowledgments
This work was supported with federal funds from the U.S. Department of Agriculture, Agricultural Research Service under Contract No. 53-5K06-5-10.
ABBREVIATIONS
- THF
tetrahydrofolate
- MTHFR
methylenetetrahydrofolate reductase
- 5-methylTHF
5-methyltetrahydrofolate
- RBC
red blood cell
- 5
10-methyleneTHF, 5,10-methylenetetrahydrofolate
References
- 1. Selhub J, Miller J W. Am J Clin Nutr. 1992;55:131–138. doi: 10.1093/ajcn/55.1.131. [DOI] [PubMed] [Google Scholar]
- 2.Goyette P, Sumner J S, Miolos R, Duncan A M, Rosenblatt D S, Matthews R G, Rozen R. Nat Genet. 1994;7:195–200. doi: 10.1038/ng0694-195. [DOI] [PubMed] [Google Scholar]
- 3.Rosenblatt D S, Cooper B A, Lue-Shing S, Wong P W K, Berlow S, Narisawa K, Baumgartner R. J Clin Invest. 1979;63:1019–1025. doi: 10.1172/JCI109370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rozen R. Thromb Haemostasis. 1997;78:523–526. [PubMed] [Google Scholar]
- 5.Frosst P, Blom H J, Milos R, Goyette P, Sheppard C A, Matthews R G, Boers G J H, den Heijer M, Kluijtmans L A J, van den Heuvel L P, Rozen R. Nat Genet. 1995;10:111–113. doi: 10.1038/ng0595-111. [DOI] [PubMed] [Google Scholar]
- 6.Jacques P F, Bostom A G, Williams R R, Ellison R C, Eckfeldt J H, Rosenberg I H, Selhub J, Rozen R. Circulation. 1996;93:7–9. doi: 10.1161/01.cir.93.1.7. [DOI] [PubMed] [Google Scholar]
- 7.Shin Y S, Buehring K U, Stokstad E R L. Arch Biochem Biophys. 1974;163:211–224. doi: 10.1016/0003-9861(74)90471-8. [DOI] [PubMed] [Google Scholar]
- 8.Bagley P J, Selhub J. Methods Enzymol. 1997;281:16–25. doi: 10.1016/s0076-6879(97)81004-x. [DOI] [PubMed] [Google Scholar]
- 9.Bagley P, Weinstock M, Selhub J. FASEB J. 1997;11:A178. , (abstr.). [Google Scholar]
- 10.Tamura T. In: Folic Acid Metabolism in Health and Disease. Picciano M F, Stokstad E R L, Gregory J F III, editors. New York: Wiley; 1995. pp. 122–137. [Google Scholar]
- 11.Shane B. In: Folic Acid Metabolism in Health and Disease. Bailey L B, editor. New York: Dekker; 1995. pp. 1–22. [Google Scholar]
- 12.Foo S K, McSloy R M, Shane B. J Nutr. 1982;122:1600–1608. doi: 10.1093/jn/112.8.1600. [DOI] [PubMed] [Google Scholar]
- 13.Pfeiffer C M, Gregory J F., III Clin Chem. 1996;42:1847–1854. [PubMed] [Google Scholar]
- 14.Kang S-S, Zhou J, Wong P W K, Kowalisyn J, Strokosch G. Am J Hum Genet. 1988;43:414–421. [PMC free article] [PubMed] [Google Scholar]
- 15.Kluijtmans L A J, van den Heuvel L P, Boers G H, Frosst P, Stevens E M, Van Oost B A, den Heijer M, Trijbels F J, Rozen R, Blom H J. Am J Hum Genet. 1996;58:35–41. [PMC free article] [PubMed] [Google Scholar]
- 16.De Franchis R, Mancini F P, D’Angelo A, Sebastio G, Fermo I, de Stefano V, Margaglione M, Mazzola G, di Minno G, Andria G. Am J Hum Genet. 1996;59:262–264. [PMC free article] [PubMed] [Google Scholar]
- 17.Gallagher P M, Meleady R, Shields D C, Tan K S, McMaster D, Rozen R, Evans A, Graham I M, Whitehead A S. Circulation. 1996;94:2154–2158. doi: 10.1161/01.cir.94.9.2154. [DOI] [PubMed] [Google Scholar]
- 18.Brugada R, Marion A J. Atherosclerosis. 1997;128:107–112. doi: 10.1016/s0021-9150(96)05977-1. [DOI] [PubMed] [Google Scholar]
- 19.Verhoef P, Kok F J, Kluijtmans L A, Blom H J, Refsum H, Ueland P M, Kruyssen D A. Atherosclerosis. 1997;132:105–113. doi: 10.1016/s0021-9150(97)00084-1. [DOI] [PubMed] [Google Scholar]
- 20.Wilcken D E, Wang X L, Sim A S, McCredie R M. Arter Thromb Vasc Biol. 1996;16:878–882. doi: 10.1161/01.atv.16.7.878. [DOI] [PubMed] [Google Scholar]
- 21.Van der Put N M J, Steegers-Theunissen R P, Frosst P, Trijbels F J, Eskes T K, van den Heuvel L P, Mariman E C, den Heyer M, Rozen R, Blom H J. Lancet. 1995;346:1070–1071. doi: 10.1016/s0140-6736(95)91743-8. [DOI] [PubMed] [Google Scholar]
- 22.Ou C Y, Stevenson R E, Brown V K, Schwartz C E, Allen W P, Khoury M J, Rozen R, Oakley G P, Jr, Adams M J., Jr Am J Med Genet. 1996;63:610–614. doi: 10.1002/(SICI)1096-8628(19960628)63:4<610::AID-AJMG15>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 23.Whitehead A S, Gallagher P, Mills J L, Kirke P N, Burke H, Molloy A, Weir D G, Shields D C, Scott J M. Q J Med. 1995;88:763–766. [PubMed] [Google Scholar]
- 24.Mornet E, Muller F, Lenvoise-Furet A, Delezoide A L, Col J Y, Simon-Bouy B, Serre J L. Hum Genet. 1997;100:512–514. doi: 10.1007/s004390050544. [DOI] [PubMed] [Google Scholar]
- 25.De Franchis R, Sebastio G, Mandato C, Andria G, Mastroiacovo P. Lancet. 1995;346:1703. doi: 10.1016/s0140-6736(95)92865-0. [DOI] [PubMed] [Google Scholar]
- 26.Posey D L, Khoury M J, Mulinare J, Adams M J, Jr, Ou C-Y. Lancet. 1996;347:686–687. [PubMed] [Google Scholar]
- 27.Ma J, Stampfer M J, Giovannucci E, Artigas C, Hunter D J, Fuchs C, Willett W C, Selhub J, Hennekens C H, Rozen R. Cancer Res. 1997;57:1098–1102. [PubMed] [Google Scholar]
- 28.Chen J, Giovannucci E, Kelsey K, Rimm E B, Stampfer M J, Colditz G A, Speigelman D, Willett W, Hunter D J. Cancer Res. 1996;56:4862–4864. [PubMed] [Google Scholar]