Abstract
The current mechanism proposed for the processing and activation of the 52 kDa lysosomal aspartic protease cathepsin D (cath-D) is a combination of partial auto-activation generating a 51 kDa pseudo-cath-D, followed by enzyme-assisted maturation involving cysteine and/or aspartic proteases and yielding successively a 48 kDa intermediate and then 34 + 14 kDa cath-D mature species. Here we have investigated the in vivo processing of human cath-D in a cath-D-deficient fibroblast cell line in order to determine whether its maturation occurs through already active cath-D and/or other proteases. We demonstrate that cellular cath-D is processed in a manner independent of its catalytic function and that auto-activation is not a required step. Moreover, the cysteine protease inhibitor E-64 partially blocks processing, leading to accumulation of 52-48 kDa cath-D intermediates. Furthermore, two inhibitors, CLICK148 and CA-074Met, specific for the lysosomal cath-L and cath-B cysteine proteases induce accumulation of 48 kDa intermediate cath-D. Finally, maturation of endocytosed pro-cath-D is also independent of its catalytic function and requires cysteine proteases. We therefore conclude that the mechanism of cath-D maturation involves a fully-assisted processing similar to that of pro-renin.
Keywords: Animals; Catalysis; Cathepsin B; physiology; Cathepsin D; metabolism; Cathepsins; physiology; Cell Line, Transformed; Cysteine Endopeptidases; physiology; Enzyme Activation; physiology; Humans; Mice; Protein Processing, Post-Translational; physiology
Keywords: cathepsin, processing, endocytosis, proteolytic activity
INTRODUCTION
Cathepsin D (cath-D) is a major intracellular aspartic protease of endosomes and lysosomes and is related to the other aspartic proteases such a renin, pepsin and yeast protease A [1]. All these enzymes are synthesized as inactive precursors, which are then processed either auto-catalytically (e.g. pepsin) [1] or by other enzymes (e.g. renin) to remove an N-terminal pro-peptide [2]. For cath-D, an intermediate mechanism had been proposed, namely, a partial auto-activation followed by a final processing by other enzymes [3–5].
Cath-D is synthesized on the rough endoplasmic reticulum (RER) as a pre-pro-enzyme that undergoes several proteolytic cleavages during biosynthesis to produce the mature form [3, 6, 7]. Following the initial co-translational removal of the signal peptide to yield pro-cath-D, sugars are attached at two N-linked glycosylation sites and the pro-enzyme is transported to Golgi stacks. The 52 kDa pro-cath-D is marked for mannose-6-phosphate (M6P) receptors and targeted to lysosomes [8, 9], where 44 amino acid residues are ultimately removed from the amino terminus, yielding an active intermediate 48 kDa single-chain molecule. This main proteolytic activation event most likely depends on the action of cysteine lysosomal and/or aspartic proteases [10–12]. Depending on the cell type, cath-D may also be targeted to lysosomes in an M6P-independent manner [12, 13]. The intermediate 48 kDa single-chain species is then cleaved in lysosomes into a mature two-chain enzyme consisting of a light 14 kDa amino-terminal domain and a heavy 34 kDa carboxyl-terminal domain. It is proposed that this proteolytic cleavage is accomplished by cysteine proteases in dense lysosomes, since processing was partially inhibited by leupeptin [14]. Accompanying the conversion to the two-chain species, 7 amino acid residues between the light and heavy chains are removed [15]. Several amino acids are also removed from the carboxyl terminus of heavy chains [16]. Pro-cath-D over-expressed by cancer cells is also secreted in excess and can be endocytosed by both cancer cells and by fibroblasts via M6P-receptors and other unknown receptor(s) [17]. Endocytosed pro-cath-D undergoes maturation successively into 48 kDa intermediate and 34 + 14 kDa mature forms [17, 18]. In addition, secreted pro-cath-D, like pepsinogen, is capable of acid-dependent auto-activation in vitro [19], resulting in a catalytically active pseudo-cath-D, an enzyme species that retains 18 residues (27-44) of the pro-segment.
A major question remains as to whether cath-D requires its proteolytic function and its auto-activation into pseudo-cath-D to be matured in cells by subsequent proteolytic cleavage by cysteine proteases, or pseudo-cath-D is only an in vitro artifact [7, 11]. Certain groups have attempted to elucidate the role of cath-D proteolytic activity and the relevance of pseudo-cath-D for the activation mechanism of pro-cath-D maturation, but no reported investigation has convincingly demonstrated that the activation of pro-cath-D is initiated in the absence of contaminating active cath-D [7, 20]. In the present study, we highlight the fact that the proteolytic activity and the auto-activation of cath-D were not required either for its processing in cells or after endocytosis. We were able to obtain this evidence since we previously engineered a catalytically-inactive D231N mutated cath-D expressed in cath-D-deficient fibroblasts [21]. We have also defined which cysteine proteases were implicated in cath-D processing in cells and after endocytosis.
RESULTS
Catalytic function and auto-activation of human cath-D are not required for its maturation
To determine whether human cath-D requires its catalytic activity for maturation, we investigated the processing of a mutated D231Ncath-D devoid of proteolytic activity expressed in cath-D-deficient CD55−/− fibroblasts [21]. As expected, cellular 52 kDa wild-type pro-cath-D underwent successive maturation into a 48 kDa intermediate and then a 34 kDa mature species in CD55−/−cath-D transfected cells (Figure 1a, left panel). Processing of the D231N mutated pro-cath-D precursor into intermediate and mature species was not prevented by the D231N mutation in CD55−/−D231Ncath-D cells (Figure 1a, right panel). These results indicate that maturation of cath-D in cells occurs in a manner independent of its catalytic activity. However, the precursor, intermediate and mature heavy chain D231Ncath-D all showed an increased electrophoretic mobility, corresponding to a shift of about 1 kDa in the apparent molecular mass, resulting in 51 kDa, 47 kDa and 33 kDa D231Ncath-D species, respectively (Figure 1a, right panel).
Figure 1. Maturation, glycosylation and activation of human wild-type and D231N mutated human cath-D transfected into cath-D-deficient CD55−/− fibroblasts.
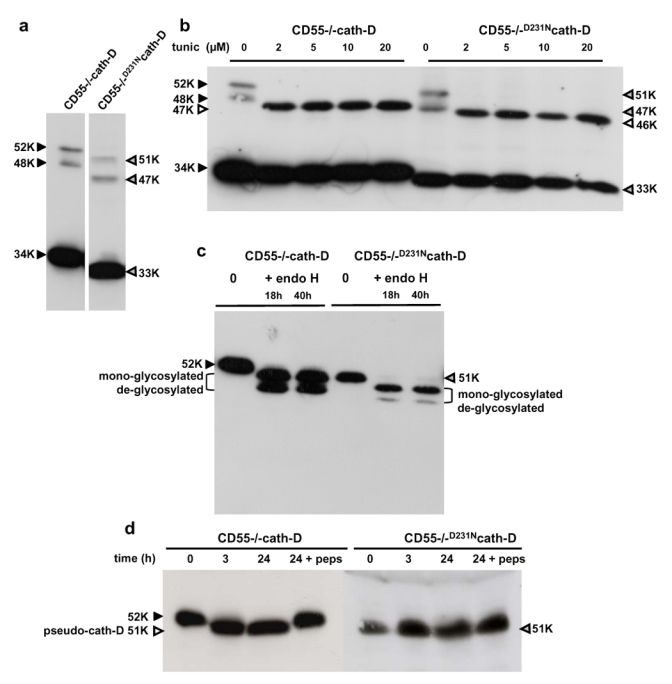
(a) Maturation of wild-type and D231N mutated human cath-D. Cells were cultured in DMEM medium with FCS and cell lysates were analyzed by immunoblotting with an antibody specific for human cath-D.
(b) Effects of tunicamycin on cellular wild-type and D231N mutated cath-D glycosylation. Cells were either untreated or treated with increasing concentrations of tunicamycin for 24 h and cell lysates were analysed for cath-D expression by Western blotting.
(c) Effects of endoglycosidase H on the glycosylation of wild-type and D231N secreted pro-cath-D. Conditioned media from CD55−/−cath-D and CD55−/−D231Ncath-D cells were incubated for different times with or without endoglycosidase H and media were analysed for cath-D expression by Western blotting.
(d) Auto-activation of wild-type and D231N secreted pro-cath-D. Conditioned media from CD55−/−cath-D and CD55−/−D231Ncath-D cells were incubated for different times at pH 3.5 in the absence or presence of pepstatin A, and media were analysed for cath-D expression by Western blotting.
Black arrows indicate the migration of the 3 forms of wild-type cath-D; grey arrows indicate the migration of the 3 forms of D231Ncath-D; white arrows indicate the migration of non-glycosylated cellular cath-D. K= molecular mass in kDa.
As human cath-D carries two-N-linked glycosylation sites at asparagine residues 70 and 199 with about 2 kDa molecular mass, we first examined whether the differences in the electrophoretic mobility between the wild-type and D231N cath-D were due to altered glycosylation. CD55−/−cath-D and CD55−/−D231Ncath-D cells were treated with increasing concentrations of tunicamycin, which inhibits N-linked glycosylation. It has been previously published that tunicamycin treatment of pulse-labelled cells results in a 5 kDa decrease in molecular mass of the cath-D pro-enzyme with no subsequent pro-peptide cleavage [22]. As can be seen in Figure 1b, treatment with tunicamycin induced accumulation of 47 kDa and 46 kDa bands respectively in CD55−/−cath-D and CD55−/−D231Ncath-D cells, which were likely to be the non-glycosylated pro-enzymes. No single-chain enzymes were apparent in CD55−/−cath-D and CD55−/−D231Ncath-D cells as previously described [22]. The 34 kDa and 33 kDa mature forms of wild-type and D231N cath-D detected were apparently synthesized prior to addition of the tunicamycin but were sufficiently stable to be detected by Western blotting. We conclude that the 1 kDa shift in the molecular masses of cellular wild-type and D231N cath-D forms was not due to a difference in glycosylation. We also observed the same shift of 1 kDa in the molecular mass of wild-type and D231N pro-cath-D secreted in the conditioned media by CD55−/−cath-D and CD55−/−D231Ncath-D cells (Figure 1c). Treatment with endoglycosidase H revealed the presence of the mono-glycosylated and de-glycosylated pro-cath-D as previously described [23] (Figure 1c). A similar pattern of de-glycosylation was observed for D231Npro-cath-D, indicating that both wild-type and D231N pro-cath-D carries two N-linked glycosylation sites (Figure 1c). However, de-glycosylation of D231N pro-cath-D revealed predominantly the mono-glycosylated form and only a trace of de-glycosylated protein (Figure 1C). These results suggest that the mutation of cath-D in the D231N catalytic site may result in a conformational change which affects its recognition by endoglycosidase H and therefore its full de-glycosylation. Collectively, these findings demonstrate that the higher electrophoretic mobility of the mutated D231Ncath-D was not due to any alteration of D231Ncath-D glycosylation, since neither tunycamycin (Figure 1b) nor endoglycosidase H (Figure 1c) could restore the same molecular weights of cellular or secreted non-glycosylated D231Ncath-D as compared to non-glycosylated cath-D. Since the shift was observed with both the precursor, intermediate and carboxyl-terminal heavy mature chain of D231Ncath-D, we propose that human D231Ncath-D might have been cleaved after synthesis at its carboxyl-terminus. Our results confirm a previous report that suggested a similar carboxyl-terminal truncation for the 45 kDa single-chain form of D293N mutated mouse cath-D transiently expressed in HEK-293 cells and for precursor, intermediate and mature forms of endogenously expressed mutant cath-D in CONCL sheep fibroblasts [24].
Concerning processing of cellular cath-D, it had been proposed that the 51 kDa pseudo-cath-D detected in vitro in the extracellular medium and generated by auto-activation at an acidic pH might be a necessary intermediate for subsequent maturation [19]. We verified that wild-type pro-cath-D secreted by CD55−/−cath-D cells was capable of acid-dependent auto-activation yielding 51 kDa pseudo-cath-D, and that the aspartic protease inhibitor pepstatin A prevented its auto-activation (Figure 1d). As expected, because of its lack of proteolytic activity, the D231Npro-cath-D 51 kDa precursor secreted by CD55−/−D231Ncath-D cells was not auto-activated into a shorter D231Npseudo-cath-D at acidic pH. Since D231Npro-cath-D underwent maturation in CD55−/−D231Ncath-D cells (Figure 1a), we conclude that pseudo-cath-D generated by auto-activation is not a necessary species for cath-D maturation.
Together, our results demonstrate that maturation of cath-D occurs in cells in a manner independent of its catalytic function and auto-activation. This finding supports a fully-assisted mechanism of maturation mediated by other proteases.
Requirement of cysteine proteases for cath-D processing
To investigate whether cysteine proteases were implicated in cath-D maturation in our model, CD55−/−cath-D and CD55−/−D231Ncath-D cells were first treated with E-64, a broad spectrum irreversible specific inhibitor of cysteine proteases. E-64 blocked processing leading to accumulation of 52-48 kDa cath-D intermediates, with hardly detectable 34 kDa mature form in CD55−/−cath-D cells (Figure 2a, left panel). These processing intermediates were also observed in CD55−/−D231Ncath-D cells (Figure 2a, right panel). Dose-response studies performed with CD55−/−cath-D fibroblasts revealed that treatment with E-64 at a concentration of 100 μM leads to an accumulation of these processing intermediates (Figure 2b). Our various attempts to prevent the appearance of these processing intermediates by the concomitant use of E-64 plus serine, metallo-, or aspartic protease inhibitors were unsuccessful (data not shown). Analysis by 2D gel electrophoresis then highlighted the fact that these 52-48 kDa processing intermediates were not a single species but rather were composed of different processing intermediates (Figure 2c).
Figure 2. Effects of cysteine protease inhibitors on the maturation of wild-type and D231N mutated cath-D in transfected fibroblasts.
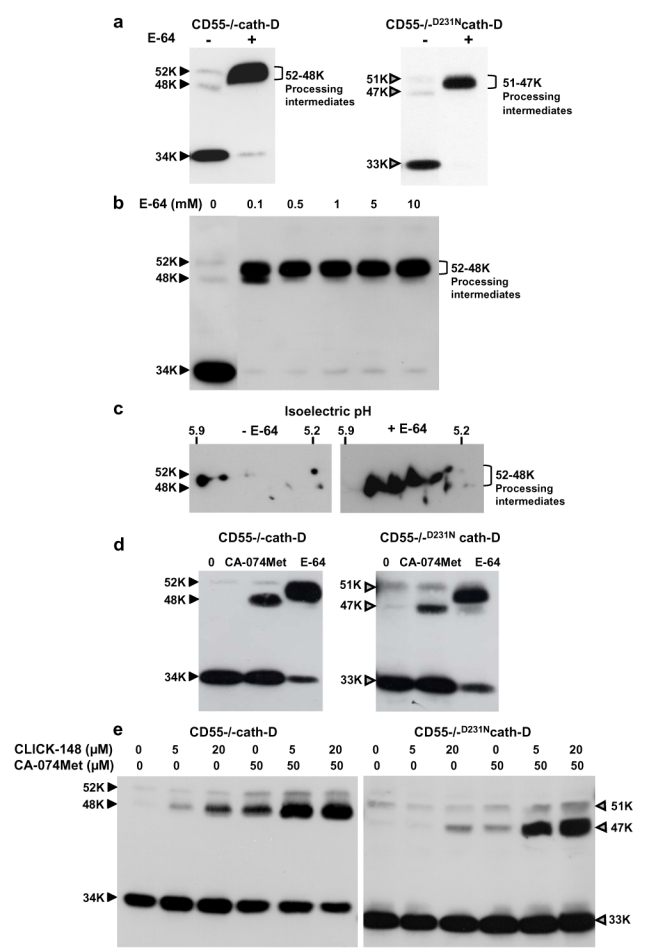
(a) Effects of E-64 in fibroblasts. CD55−/−cath-D and CD55−/−D231Ncath-D cells were either untreated or treated for 48 h with 10 mM E-64 and cellular cath-D was analysed by immunoblotting.
(b) Concentration dependency of the E-64 effects. CD55−/−cath-D cells were either untreated or treated for 48 h with increasing concentrations of E-64 and cellular cath-D was analysed by immunoblotting.
(c) Effects of E-64 using two-dimensional electrophoresis. CD55−/− cath-D cells were either untreated or treated for 48 h with 10 mM E-64 and cellular cath-D was analysed by two-dimensional electrophoresis and immunoblotting.
(d) Effects of CA-074Met. CD55−/−cath-D and CD55−/−D231Ncath-D cells were untreated or treated for 48 h with 50 μM CA-074Met and cellular cath-D was analysed by immunoblotting. The effect of 10 mM E-64 is shown.
(e) Effects of CLICK-148 alone or in combination with CA-074Met. CD55−/−cath-D and CD55−/−D231Ncath-D cells were either untreated or treated for 48 h with 5 or 20 μM CLICK-148 alone or in combination with 50 μM CA-074Met. Cellular cath-D was analysed by immunoblotting.
Brackets indicate the 52-48 kDa processing intermediates detected in CD55−/−cath-D cells and the 51-47 kDa processing intermediates detected in CD55−/−D231Ncath-D cells.
Having shown that both active and inactive cath-D required cysteine proteases for processing, we next attempted to identify which enzymes were involved. The effects of two synthetic cell permeable cysteine protease inhibitors specific for the lysosomal proteases cath-L and cath-B were examined in CD55−/−cath-D transfected fibroblasts. CLICK-148 [26], a cath-L inhibitor and CA-074Met [25], a cath-B inhibitor, caused accumulation of single 48 kDa cath-D in CD55−/−cath-D fibroblasts (Figure 2d–e). Used together, these inhibitors had a cumulative effect, suggesting an additive role of cath-L and -B for cath-D processing (Figure 2e, left panel). Similar qualitative effects of these two inhibitors were observed for D231Ncath-D maturation in CD55−/−D231Ncath-D fibroblasts (Figure 2d–e, right panels). However, CLICK-148 and CA-074Met inhibitors tested alone or in combination were less efficient in preventing D231Ncath-D maturation as compared to wild-type cath-D. These results suggest that the D231N mutation may result in a cath-D conformational change which affects its recognition by specific inhibitors. Our data together suggest that cath-L and cath-B can partially process the 48 kDa intermediate into 34 kDa mature cath-D. Collectively, our results indicate that cath-D is successively matured into various processing intermediates by complex mechanisms involving not only the cysteine proteases but also unidentified protease(s) acting at initial steps. Figure 3 summarizes the identified steps of cath-D processing incorporating our new data.
Figure 3. Schematic representation of human cath-D processing.
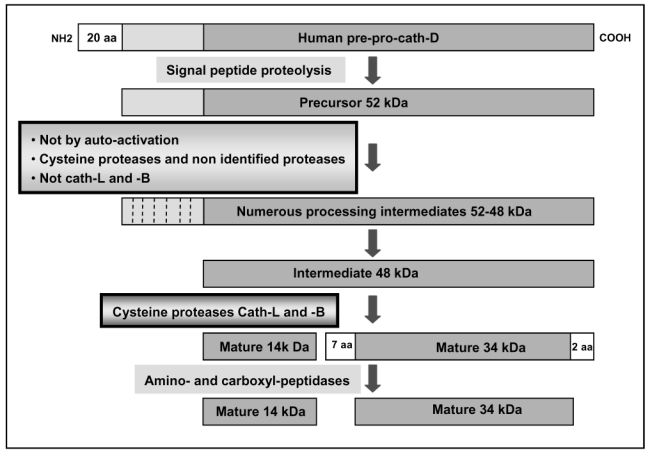
Cath-D is synthesized on the RER as a pre-pro-enzyme that undergoes several proteolytic cleavages during biosynthesis to produce the mature form. Following the initial co-translational removal of the signal peptide to yield 52 kDa pro-cath-D, this pro-enzyme is then processed into numerous 52-48 kDa processing intermediates. These intermediates are not generated by auto-activation, but by the action of unknown proteases whose activities are only partially inhibited by the cysteine protease inhibitor E64, but are not affected by the cath-B or –L inhibitors. The intermediate 48 kDa single-chain species is then cleaved into a mature two-chain enzyme consisting of a light 14 kDa amino-terminal domain and a heavy 34 kDa carboxyl-terminal domain by the cysteine proteases cath-B or cath-L. Accompanying this conversion to the two-chain species, 7 amino acid residues between the 14 KDa light and 34 kDa heavy chains are removed [15]. Several more amino acids are removed from the carboxyl terminus of the 34 kDa heavy chain [16].
Processing of endocytosed pro-cath-D
To examine whether endocytosed pro-cath-D requires its catalytic activity for maturation, we next studied the processing of [35S]methionine-labelled wild-type and D231N secreted pro-cath-D after its endocytosis by unlabelled cath-D-deficient recipient fibroblasts (Figure 4a). SDS-PAGE analysis of 35S-labelled proteins bound and taken up by CD55−/− fibroblasts revealed after anti-cath-D immunoprecipitation that both wild-type and D231N 35S-labelled pro-cath-D were transformed into 35S-labelled mature enzymes (Figure 4a). Our results reveal that endocytosed pro-cath-D is also processed independently of its catalytic activity. To identify which proteases were involved, internalization experiments were performed in the presence of E-64, CLICK-148 or CA-074Met. E-64 blocked processing, leading to accumulation of 52-48 kDa processing intermediates (Figure 4b), whereas CLICK-148 and CA-074Met caused accumulation of 48 kDa intermediate cath-D (Figure 4c–d), as previously observed in cath-D transfected fibroblasts (Figure 2). A synergic effect was observed when cells were treated with both CLICK-148 and CA-074Met inhibitors (Figure 4d). Overall, our data demonstrate that the processing of endocytosed pro-cath-D is independent of its catalytic activity and involved the cysteine proteases with similar mechanisms to those involved in the maturation of cellular cath-D transiting via RER (Figure 3).
Figure 4. Processing of endocytosed wild-type and D231N mutated human cath-D.
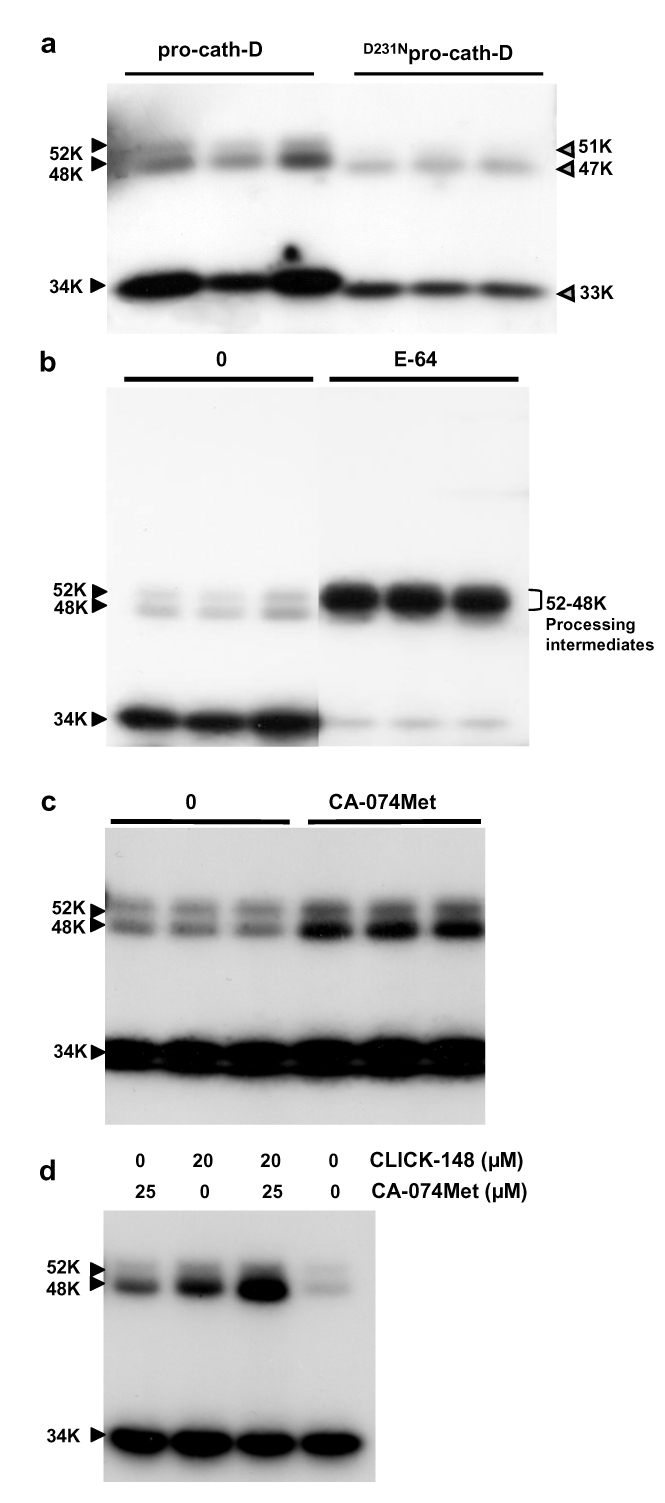
(a) Processing of endocytosed human D231Npro-cath-D. Parental cath-D-deficient CD55−/− fibroblasts were incubated for 18 h with 35S-labelled conditioned medium containing the secreted wild-type or D231N pro-cath-D. After washing, cell lysates containing endocytosed [S35]methionine-labelled cath-D were analyzed by SDS-PAGE after immunoprecipitation. Immunoprecipitation was performed in triplicate.
(b) Effects of E-64. CD55−/− fibroblasts were incubated for 18 h with 35S-labelled conditioned medium containing the wild-type pro-cath-D in the absence or presence of 10 mM E-64. Endocytosed [S35]methionine-labelled cath-D was analyzed by SDS-PAGE, as described in panel a.
(c) Effects of CA-074Met. CD55−/− fibroblasts were incubated for 18 h with 35S-labelled conditioned medium containing pro-cath-D in the absence or presence of 25 μM CA-074Met. Endocytosed [S35]methionine-labelled cath-D was analyzed by SDS-PAGE.
(d) Effects of CLICK-148 alone or in combination with CA-074Met. CD55−/− fibroblasts were incubated for 18 h with the 35S-labelled conditioned medium containing pro-cath-D in the absence or presence of 20 μM CLICK-148 alone or in combination with 25 μM CA-074Met. Endocytosed [S35]methionine-labelled cath-D was analyzed by SDS-PAGE.
DISCUSSION
Mechanisms associated with the processing and activation of lysosomal proteases remain largely unknown [27]. In general, three types have been reported for the processing and activation of aspartic proteinases. The first mechanism, complete auto-activation, has been described for porcine pepsinogen [1]. The second mechanism is represented by fully-assisted activation of pro-renin [2]. The third mechanism, proposed for cath-D, is a combination of partial auto-activation and enzyme-assisted activation yielding mature enzyme [3–5].
The aim of the present work was to investigate the in vivo processing of human cath-D. We used a mouse cath-D-deficient cell line expressing a human catalytically-inactive D231Ncath-D mutant variant to determine whether processing of cath-D zymogen molecules could take place through already active cath-D and/or other proteases [21]. Here, we have demonstrated that the mechanism of cath-D maturation is independent of its catalytic function and involves a fully-assisted processing similar to that of pro-renin.
There has been much controversy over whether processing to pseudo-cath-D occurs in cells and is a required step for subsequent proteolytic cleavage by cysteine proteases, or pseudo-cath-D is only an in vitro artifact [7, 11]. Because aspartic protease family members share a high degree of sequence identity [28, 29], and pepsinogen, the most thoroughly studied aspartic protease, has been shown to undergo activation to pseudo-pepsinogen by an intra-molecular mechanism [30], a similar activation mechanism with pseudo-cath-D as an important intermediate has been proposed for pro-cath-D maturation [4]. However, auto-catalytic removal of the remainder of the pro-peptide has not been demonstrated for cath-D, as it has been for pepsinogen. The inability of pro-cath-D to generate the mature forms of the enzyme by auto-catalysis suggested that either another protease may be required or that autocatalytic activation to pseudo-cath-D does not occur in vivo. Our work reveals that pseudo-cath-D generated by auto-activation in vitro is not a required intermediate for cath-D processing in cells.
Different groups have attempted to elucidate the in vivo relevance of auto-activated pseudo-cath-D and the role of cath-D proteolytic activity for its maturation [7, 20]. Human cath-D mutated at its auto-catalytic site in the pro-peptide was not able to process itself to form pseudo-cath-D in vitro, but it was normally processed to the mature forms of the enzyme when transfected into mouse cells [7]. This study indicates for the first time that auto-activation of pro-cath-D to the pseudo-form was not required for removal of the whole pro-peptide and suggested that pseudo-cath-D was not a normal intermediate of pro-cath-D processing in vivo. However, the possibility remains that the mutant was not cleaved in vivo at an alternative site by the endogenous mouse proteases, such as mouse cath-D, despite the in vitro confirmation of its inability to activate. On the other hand, it was discovered that pro-cath-D mutated either in its auto-catalytic site in the pro-peptide or in its catalytic domain, resulting in a catalytically inactive enzyme, and expressed in a baculovirus system was cleaved at alternative sites in the pro-sequence by insect cath-D and/or additional and, as yet, unknown proteases [20]. In the present study, we have been able to totally exclude any involvement of active cath-D in the maturation process by the use of a cath-D-deficient cell line expressing a proteolytically-inactive D231Ncath-D. Moreover, we have observed that the cysteine protease inhibitor E-64 caused intracellular accumulation of 52-48 kDa processing intermediates in fibroblasts. Accumulation of large amounts of 50 kDa processing intermediate in fibroblasts treated with E-64 had already been suggested earlier [11]. Our diverse attempts to totally prevent cath-D processing by the concomitant use of E-64 and serine, metallo-or aspartic protease inhibitors were unsuccessful (data not shown). Our data therefore argue in favour of the presence of different cleavage sites in the cath-D pro-part, generating in vivo pseudo-cath-D by the action of unidentified proteases, as previously proposed [20], and not by auto-catalysis.
Previous reports have shown that cysteine and aspartic protease inhibitors can partially prevent the processing of pro-cath-D to 48 kDa intermediate form of the enzyme [10–12] and that the proteolytic cleavage of 48 kDa to 34 kDa was accomplished by cysteine proteases in dense lysosomes, since processing was partially inhibited by leupeptin [14]. By the use of two synthetic cell-permeable cysteine protease inhibitors CLICK-148 and CA-074Met, we proposed the participation of cath-L and cath-B, two lysosomal cysteine proteases widely implicated in carcinogenesis for the processing of intermediate 48 kDa to mature 34 kDa cath-D [31]. It is important to note that Mach and colleagues have questioned the selectivity of CA-074Met and suggested its inhibitory effect on both cath-B and cath-L [32]. Since we obtained additive inhibitory effects when both inhibitors were used, we argue that cath-B might also be implicated in cath-D processing. Interestingly, accumulation of intermediate cath-D has been detected in the brains of cath-B/-L double knock-out mice [33] as well as in cath-L-deficient A549 cells and in lung tissue extracts of cath-L (−/−) mice [34]. Our results together with the reports using cath-B/-L and cath-L knock-out cells strongly suggest the involvement of cath-L and cath-B in cath-D processing in addition to other still unidentified proteases [32, 33].
As far as the mechanisms of cath-D maturation following its endocytosis are concerned, nothing has so far been described [35]. We have shown that the processing of endocytosed pro-cath-D is also independent of its catalytic function and requires the cysteine proteases, as expected, since the endocytosed cath-D would reach the same compartment as that synthesized inside the cells.
Taken together, our results indicate that pro-cath-D is successively processed, in a manner independent of its catalytic function and auto-activation, into different intermediate processing species by complex mechanisms involving not only the cysteine proteases but also as yet unidentified proteases. However, since we used a heterogenous expression system, we cannot totally exclude the possibility that human cath-D maturation might somehow slightly differ in human cells. Our main conclusion is that cath-D undergoes maturation by a fully-assisted mechanism similar to that of renin maturation.
MATERIALS AND METHODS
Materials
E-64, CA-074Met, and pepstatin A were purchased from Sigma. Stock solutions of 10 mM CA-074Met and CLICK-148 were prepared in DMSO. DMSO vehicle was used as internal control in the studies with CA-074Met and CLICK-148. A stock solution of 100 mM E-64 was prepared in DMEM.
Cell lines
CD55−/− cath-D-deficient immortalized mouse fibroblasts were kindly provided by Dr Christoph Peters (University of Freiburg, Germany) and Dr Paul Saftig (Christian-Albrechts-University, Germany) [36]. CD55−/−cath-D and CD55−/−D231Ncath-D cell lines were generated by stable transfection of wild-type or D231N mutated human cath-D into CD55−/− fibroblast cells as described earlier [21]. These cells were cultured in DMEM medium with 10% fetal calf serum (FCS, GibcoBRL).
Cath-D immunoblotting
Cells grown to 80% confluency in DMEM medium supplemented with 10% FCS were either untreated or treated with 10 mM E-64, 50 μM CA-074Met or 5–20 μM CLICK-148. Cells remained viable under these conditions and were lysed after 48 h in lysis buffer (20 mM Tris-Hcl [pH 7.4], 137 mM NaCl, 1 mM MgCl2, 1 mM CaCl2, 0.5% Nonidet P-40, 1 mM phenylmethylsulphonyl fluoride, 1 mM dithiothreitol, 1 mM vanadate, 10% glycerol, and 100 kallikrein units/ml Trasylol). After gentle shaking for 20 min at 4°C, cell extracts were obtained by centrifugation in a microfuge at 13,000 rpm for 15 min. Equal amounts of protein (100 μg for CD55−/− cath-D cells and 300 μg for CD55−/−D231Ncath-D cells) from cell extracts, quantitated by the Bradford assay, or equal volumes (80 μl) of conditioned media, were separated on a 12 or 15% gel by SDS-PAGE. Proteins were electro-transferred to Hybond-C extra membranes (Amersham Biosciences) and incubated with 1 μg/ml anti-human cath-D monoclonal antibody (#610800, BD Biosciences) that recognizes 52, 48 and 34 KDa cath-D forms. Proteins were then visualized with horseradish peroxidase-conjugated sheep anti-mouse immunoglobulin (ECL Amersham) followed by the Renaissance chemiluminescence system (Perkin Life Sciences).
Glycosylation and auto-activation of pro-cath-D
To inhibit N-linked glycosylation, cells were grown for 24 h in medium supplemented with 10% FCS containing increasing concentrations of tunicamycin (Sigma; stock solution: 10 mM in DMSO). Cell extracts (100 μg of proteins for CD55−/− cath-D cells and 300 μg for CD55−/−D231Ncath-D cells) were then analysed for cath-D expression by immunoblotting as described above. For digestion of N-linked oligosaccharides, culture medium conditioned for 4 days in the presence of 5% FCS was incubated with 50 mU/ml of endoglycosidase H (Sigma) at 37°C in a 50 mM citrate phosphate buffer [pH 5.4] containing a protease inhibitor cocktail (Boehringer Mannheim). To auto-activate in vitro the secreted pro-cath-D, culture medium conditioned for 48 h with 10% FCS was incubated at 37°C for various times at pH adjusted to 3.5 by the addition of 1 N HCl in the presence or absence of 1 mM pepstatin A. Samples were then neutralized to pH 7.4 with 1 N NaOH. Conditioned media (80 μl) were then analysed for cath-D expression by immunoblotting as described above.
Two-dimensional electrophoresis
First dimension electrofocusing gels contained 9.5 M urea, 2% ampholines, 2% NP-40 and 5% DTT with pH 3–10. Second dimension gels contained 10% acrylamide, 0.1% bis-acrylamide. Cath-D expression was then analyzed by immunoblotting as described above.
Endocytosis of secreted pro-cath-D
Established 3Y1-Ad12 cancer cells stably transfected either with wild-type or D231N mutated cath-D [37] were labelled with 200 μCi/ml [35S]methionine for 24 h in DMEM without methionine and FCS, and the labelled conditioned culture medium was used directly for internalization studies. Parental CD55−/− fibroblasts plated in 6-well plates were incubated in 1 ml of serum-free DMEM medium supplemented with the conditioned medium corresponding to 3 × 106 cpm of total TCA-precipitable proteins with an excess of non-radioactive methionine (10 mM), in the presence or absence of E-64, CA074-Met or CLICK-148. After 18 h incubation, medium was discarded and cells were washed 3 times, then lysed with 700 μl extraction buffer (10 mM NaPO4 [pH 7.4], 10 mM EDTA, 10 mM NaCl, 1% NP-40, 1 M Trasylol, 1 mM PMSF). Cell extracts were immunoprecipited with the M1G8 anti-cath-D-antibody and protein A-Sepharose as described previously [17] and analyzed by 12% SDS-PAGE and fluorography.
Acknowledgments
We thank Dr C. Peters (University of Freiburg, Germany) and Dr P. Saftig (Christian-Albrechts-University, Germany) for the kind gift of the CD55−/− fibroblast cell line, J. Y. Cance for the photographs, and N. Kerdjadj for secretarial assistance. This work was supported by Institut National de la Santé et de la Recherche Médicale, University of Montpellier I, Association pour la Recherche sur le Cancer (grant 3344).
Footnotes
Authors contributed equally to the work.
lnserm, U583, Montpellier, F-34000 France.
References
- 1.Tang J, Wong RN. Evolution in the structure and function of aspartic proteases. J Cell Biochem. 1987;33:53–63. doi: 10.1002/jcb.240330106. [DOI] [PubMed] [Google Scholar]
- 2.Hsueh WA, Baxter JD. Human prorenin. Hypertension. 1991;17:469–477. doi: 10.1161/01.hyp.17.4.469. [DOI] [PubMed] [Google Scholar]
- 3.Richo G, Conner GE. Proteolytic activation of human procathepsin D. Adv Exp Med Biol. 1991;306:289–296. doi: 10.1007/978-1-4684-6012-4_35. [DOI] [PubMed] [Google Scholar]
- 4.Conner GE, Richo G. Isolation and characterization of a stable activation intermediate of the lysosomal aspartyl protease cathepsin D. Biochem. 1992;31:1142–1147. doi: 10.1021/bi00119a024. [DOI] [PubMed] [Google Scholar]
- 5.Larsen LB, Boisen A, Petersen TE. Procathepsin D cannot autoactivate to cathepsin D at acid pH. FEBS Lett. 1993;319:54–58. doi: 10.1016/0014-5793(93)80036-t. [DOI] [PubMed] [Google Scholar]
- 6.Hasilik A, Neufeld EF. Biosynthesis of lysosomal enzymes in fibroblasts. Synthesis as precursors of higher molecular weight. J Biol Chem. 1980;255:4937–4945. [PubMed] [Google Scholar]
- 7.Richo GR, Conner GE. Structural requirements of procathepsin D activation and maturation. J Biol Chem. 1994;269:14806–14812. [PubMed] [Google Scholar]
- 8.Von Figura K, Hasilik A. Lysosomal enzymes and their receptors. Annu Rev Biochem. 1986;55:167–193. doi: 10.1146/annurev.bi.55.070186.001123. [DOI] [PubMed] [Google Scholar]
- 9.Kornfeld S. Lysosomal enzyme targeting. Biochem Soc Trans. 1990;18:367–74. doi: 10.1042/bst0180367. [DOI] [PubMed] [Google Scholar]
- 10.Hentze M, Hasilik A, von Figura K. Enhanced degradation of cathepsin D synthesized in the presence of the threonine analog beta-hydroxynorvaline. Arch Biochem Biophys. 1984;230:375–382. doi: 10.1016/0003-9861(84)90120-6. [DOI] [PubMed] [Google Scholar]
- 11.Samarel AM, Ferguson AG, Decker RS, Lesch M. Effects of cysteine protease inhibitors on rabbit cathepsin D maturation. Am J Physiol. 1989;257:C1069–1079. doi: 10.1152/ajpcell.1989.257.6.C1069. [DOI] [PubMed] [Google Scholar]
- 12.Rijnboutt S, Kal AJ, Geuze HJ, Aerts H, Straus GJ. Mannose 6-phosphate-independent targeting of cathepsin D to lysosomes in HepG2 cells. J Biol Chem. 1991;266:23586–23592. [PubMed] [Google Scholar]
- 13.Capony F, Braulke T, Rougeot C, Roux S, Montcourrier P, Rochefort H. Specific mannose-6-phosphate receptor-independent sorting of pro-cathepsin D in breast cancer cells. Exp Cell Res. 1994;215:154–163. doi: 10.1006/excr.1994.1327. [DOI] [PubMed] [Google Scholar]
- 14.Gieselmann V, Hasilik A, von Figura K. Processing of human cathepsin D in lysosomes in vitro. J Biol Chem. 1985;260:3215–3220. [PubMed] [Google Scholar]
- 15.Kobayashi T, Honke K, Gasa S, Fujii T, Maguchi S, Miyazaki T, Makita A. Proteolytic processing sites producing the mature form of human cathepsin D. Int J Biochem. 1992;24:1487–1491. doi: 10.1016/0020-711x(92)90076-d. [DOI] [PubMed] [Google Scholar]
- 16.Erickson AH, Blobel G. Carboxyl-terminal proteolytic processing during biosynthesis of the lysosomal enzymes beta-glucuronidase and cathepsin D. Biochem. 1983;22:5201–5205. doi: 10.1021/bi00291a021. [DOI] [PubMed] [Google Scholar]
- 17.Laurent-Matha V, Farnoud MR, Lucas A, Rougeot C, Garcia M, Rochefort H. Endocytosis of pro-cathepsin D into breast cancer cells is mostly independent of mannose-6-phosphate receptors. J Cell Sci. 1998;111:2539–2549. doi: 10.1242/jcs.111.17.2539. [DOI] [PubMed] [Google Scholar]
- 18.Vignon F, Capony F, Chambon M, Freiss G, Garcia M, Rochefort H. Autocrine growth stimulation of the MCF 7 breast cancer cells by the estrogen-regulated 52 K protein. Endocrinol. 1986;118:1537–1545. doi: 10.1210/endo-118-4-1537. [DOI] [PubMed] [Google Scholar]
- 19.Hasilik A, von Figura K, Conzelmann E, Nehrkorn H, Sandhoff K. Lysosomal enzyme precursors in human fibroblasts. Activation of cathepsin D precursor in vitro and activity of beta-hexosaminidase A precursor towards ganglioside GM2. Eur J Biochem. 1982;125:317–321. doi: 10.1111/j.1432-1033.1982.tb06685.x. [DOI] [PubMed] [Google Scholar]
- 20.Wittlin S, Rosel J, Hofmann F, Stover DR. Mechanisms and kinetics of procathepsin D activation. Eur J Biochem. 1999;265:384–393. doi: 10.1046/j.1432-1327.1999.00747.x. [DOI] [PubMed] [Google Scholar]
- 21.Laurent-Matha V, Maruani-Herrmann S, Prébois C, Beaujouin M, Glondu M, Noël A, Alvarez-Gonzalez ML, Blacher S, Coopman P, Baghdiguian S, Gilles C, Loncarek J, Freiss G, Vignon V, Liaudet-Coopman E. Catalytically-inactive human cathepsin D triggers fibroblast invasive growth. J Cell Biol. 2005;168:489–499. doi: 10.1083/jcb.200403078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fortenberry SC, Schorey JS, Chirgwin JM. Role of glycosylation in the expression of human procathepsin D. J Cell Sci. 1995;108:2001–2006. doi: 10.1242/jcs.108.5.2001. [DOI] [PubMed] [Google Scholar]
- 23.Touitou I, Garcia M, Westley B, Capony F, Rochefort H. Effect of tunicamycin and endoglycosidase H and F on the estrogen regulated 52000-Mr protein secreted by breast cancer cells. Biochimie. 1985;67:1257–1266. doi: 10.1016/s0300-9084(85)80135-8. [DOI] [PubMed] [Google Scholar]
- 24.Partanen S, Storch S, Loffler HG, Hasilik A, Tyynela J, Braulke TA. Replacement of the active-site aspartic acid residue 293 in mouse cathepsin D affects its intracellular stability, processing and transport in HEK-293 cells. Biochem J. 2003;369:55–62. doi: 10.1042/BJ20021226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Premzl A, Zavasnik-Bergant V, Turk V, Kos J. Intracellular and extracellular cathepsin B facilitate invasion of MCF-10A neoT cells through reconstituted extracellular matrix in vitro. Exp Cell Res. 2003;283:206–214. doi: 10.1016/s0014-4827(02)00055-1. [DOI] [PubMed] [Google Scholar]
- 26.Katunuma N, Murata E, Kakegawa H, Matsui A, Tsuzuki H, Tsuge H, Turk D, Turk V, Fukushima M, Tada Y, Asao T. Structure based development of novel specific inhibitors for cathepsin L and cathepsin S in vitro and in vivo. FEBS Left. 1999;458:6–10. doi: 10.1016/s0014-5793(99)01107-2. [DOI] [PubMed] [Google Scholar]
- 27.Ishidoh K, Kominami E. Processing and activation of lysosomal proteinases. Biol Chem. 2002;383:1827–1831. doi: 10.1515/BC.2002.206. [DOI] [PubMed] [Google Scholar]
- 28.Tang J, James MN, Hsu IN, Jenkins JA, Blundell TL. Structural evidence for gene duplication in the evolution of the acid proteases. Nature. 1978;271:618–621. doi: 10.1038/271618a0. [DOI] [PubMed] [Google Scholar]
- 29.Koelsch G, Mares M, Metcalf P, Fusek M. Multiple functions of pro-parts of aspartic proteinase zymogens. FEBS Lett. 1994;343:6–10. doi: 10.1016/0014-5793(94)80596-2. [DOI] [PubMed] [Google Scholar]
- 30.Bustin M, Conway-Jacobs A. Intramolecular activation of porcine pepsinogen. J Biol Chem. 1971;246:615–620. [PubMed] [Google Scholar]
- 31.Duffy MJ. Proteases as prognostic markers in cancer. Clin Cancer Res. 1996;2:613–618. [PubMed] [Google Scholar]
- 32.Montaser M, Lalmanach G, Mach L. CA-074, but not its methyl ester CA-074Me, is a selective inhibitor of cathepsin B within living cells. Biol Chem. 2002;383:1305–1308. doi: 10.1515/BC.2002.147. [DOI] [PubMed] [Google Scholar]
- 33.Felbor U, Kessler B, Mothes W, Goebel HH, Ploegh HL, Branson RT, Olsen BR. Neuronal loss and brain atrophy in mice lacking cathepsins B and L. Proc Natl Acad Sci U S A. 2002;99:7883–7888. doi: 10.1073/pnas.112632299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wille A, Gerber A, Heimburg A, Reisenauer A, Peters C, Saftig P, Reinheckel T, Welte T, Buhling F. Cathepsin L is involved in cathepsin D processing and regulation of apoptosis in A549 human lung epithelial cells. Biol Chem. 2004;385:665–670. doi: 10.1515/BC.2004.082. [DOI] [PubMed] [Google Scholar]
- 35.Clague MJ. Molecular aspects of the endocytic pathway. Biochem J. 1998;336:271–282. doi: 10.1042/bj3360271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saftig P, Hetman M, Schmahl W, Weber K, Heine L, Mossmann H, Koster A, Hess B, Evers M, Von Figura K, Peters C. Mice deficient for the lysosomal proteinase cathepsin D exibit progressive atrophy of the intestinal mucosa and profound destruction of lymphoid cells. EMBO J. 1995;14:3599–3608. doi: 10.1002/j.1460-2075.1995.tb00029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Glondu M, Coopman P, Laurent-Matha V, Garcia M, Rochefort H, Liaudet-Coopman E. A mutated cathepsin-D devoid of its catalytic activity stimulates the growth of cancer cells. Oncogene. 2001;20:6920–6929. doi: 10.1038/sj.onc.1204843. [DOI] [PubMed] [Google Scholar]


