Purification and crystallization of ginkbilobin-2 and its selenomethionine derivative allowed the collection of complete data to 2.38 Å resolution and multiwavelength anomalous diffraction data sets, respectively.
Keywords: ginkbilobin-2, antifungal proteins, Ginkgo biloba
Abstract
The antifungal protein ginkbilobin-2 (Gnk2) from Ginkgo biloba seeds does not show homology to other pathogenesis-related proteins, but does show homology to the extracellular domain of plant cysteine-rich receptor-like kinases. Native Gnk2 purified from ginkgo nuts and the selenomethionine derivative of recombinant Gnk2 (SeMet-rGnk2) were crystallized by the sitting-drop vapour-diffusion method using different precipitants. X-ray diffraction data were collected from Gnk2 at 2.38 Å resolution and from SeMet-rGnk2 at 2.79 Å resolution using a synchrotron-radiation source. The crystals of both proteins belonged to the primitive cubic space group P213, with unit-cell parameters a = b = c = 143.2 Å.
1. Introduction
Plants have evolved a variety of potent defence mechanisms, including the synthesis of secondary metabolites and antimicrobial proteins (Wallace, 2004 ▶; Selitrennikoff, 2001 ▶), since they have no immune system against pathogenic fungi and bacteria. In the plant life cycle, the seed-germination period is especially vulnerable to pathogen attack because rupture of the seed coat could allow pathogens to invade the seed-storage tissues. Therefore, many plant seeds contain antifungal and antibacterial proteins to protect the nutritious tissues from invaders. Recently, we found that the endosperm of ginkgo (Ginkgo biloba) seeds contains ginkbilobin-2 (Gnk2), a novel antifungal protein (Sawano et al., 2007 ▶). Ginkgo is one of the oldest gymnosperm species and is sometimes called a ‘living fossil’. Gnk2 consists of 108 amino acids with three disulfide bonds and inhibits the growth of plant and human pathogenic fungi such as Fusarium oxysporum and Candida albicans (Sawano et al., 2007 ▶).
Interestingly, Gnk2 did not show any sequence similarity to other antimicrobial proteins such as cyclophilin, defensin, miraculin and thaumatin (Sawano et al., 2007 ▶). However, this protein does have considerable homology (84–85%) to embryo-abundant proteins (EAPs) from two other gymnosperms, Picea abies and P. glauca. Plant EAPs are expressed in the late stage of seed maturation and are involved in protecting the plant against damage from environmental stress, especially drought (Shao et al., 2005 ▶). However, Gnk2 showed no sequence homology to angiosperm EAPs, indicating that these proteins have important functions during seed development across the subdivision barrier between gymnosperms and angiosperms. Furthermore, the deduced sequences of plant receptor-like kinases (RLKs) from the angiosperms Oryza sativa and Arabidopsis thaliana are also 28–31% identical to that of Gnk2 (Sawano et al., 2007 ▶).
The plant RLKs play major roles in cellular processes and stress response, including hormonal response, cell differentiation, plant growth and development, self-incompatibility and pathogen recognition (Morris & Walker, 2003 ▶). Among them, the cysteine-rich RLKs (CRKs) are a newly classified member with a C-X 8-C-X 2-C motif and are involved in plant defence responses, being induced by pathogen infection (Czernic et al., 1999 ▶). The C-X 8-C-X 2-C motif is completely conserved in the sequences of both Gnk2 and the two EAPs, indicating that these cysteine residues are involved in functions such as mediating protein–protein interactions and in a critical step in the activation of many animal receptor kinases upon ligand binding (Hardie, 1999 ▶). Therefore, the crystallization and structure determination of Gnk2 would help us to understand the roles of the conserved cysteine-rich region Cys62–Cys71–Cys74.
Here, we report the purification, crystallization and preliminary X-ray analysis of native Gnk2 purified from ginkgo seeds and the selenomethionine derivative of recombinant Gnk2 (SeMet-rGnk2) overexpressed using an Escherichia coli expression system.
2. Materials and methods
2.1. Protein purification
Gnk2 was extracted from ginkgo nuts and partially purified using a gel-filtration column as described previously (Sawano et al., 2007 ▶). SeMet-rGnk2 was overexpressed in the periplasm of E. coli BL21 (DE3) by a coexpression system using pET-26b vector (Novagen) and pDsbABCD1 vector encoding the four Dsb proteins that catalyze the formation and isomerization of disulfide bonds (Kurokawa et al., 2000 ▶). The DNA sequence encoding Gnk2 (residues Ala1–Phe108) and a stop codon were inserted into the pET-26b vector between NcoI and BamHI sites. The expressed protein has an additional Met residue at the NH2-terminal sequence of Gnk2. The cells were cotransformed with the pET-26b and pDsbABCD1 vectors. The transformed cells were cultured at 310 K in M9 medium containing 0.4%(w/v) glucose, 0.1 mg l−1 thiamine, 1 mM MgSO4, 4.2 mg l−1 FeSO4, 20 mg l−1 kanamycin and 17 mg l−1 chloramphenicol. After 15 min incubation (OD600 = ∼0.3), 50 mg l−1 of Ile, Leu, Val and l-selenomethionine and 100 mg l−1 Lys, Phe and Thr as well as 200 mg l−1 arabinose (for induction of the Dsb proteins) were added to the mixture. The recombinant protein was induced by the addition of 0.5 mM isopropyl β-d-thiogalactopyranoside after 15 min incubation at 310 K. The cells were then cultured for 12 h at 298 K and harvested by centrifugation. SeMet-rGnk2 was extracted from the cells by osmotic shock, purified and concentrated using an SP-Sepharose column (GE Healthcare Bioscience Corp.) equilibrated with 20 mM MES buffer pH 6.0. The recombinant protein was eluted with the MES buffer and 300 mM NaCl. For additional purification, the protein solution containing Gnk2 or SeMet-rGnk2 was applied onto the same column. Each protein solution was dialyzed against MES buffer and applied onto a Mono-S cation-exchange column (GE Healthcare Bioscience Corp.). The column was eluted with a linear gradient of 0–400 mM NaCl in MES buffer. After final purification using a Superdex-75 gel-filtration column (GE Healthcare Bioscience Corp.) equilibrated with MES buffer containing 200 mM NaCl, the protein solution was dialyzed against 10 mM Tris–HCl pH 7.0 and concentrated to 20 mg ml−1 using VivaSpin (Sartorius AG). The protein concentrations of Gnk2 and SeMet-rGnk2 were estimated from the absorbance at 280 nm using an absorption coefficient of 6335 M −1 cm−1 (Pace et al., 1995 ▶).
2.2. Dynamic light-scattering study
Dynamic light-scattering (DLS) measurements were performed using a DynaPro-MSTC instrument (Protein Solutions Inc.). Gnk2 was prepared at 20 mg ml−1 in 10 mM Tris–HCl pH 7.0 and measured at 298 K. DLS data were automatically collected using DYNAMICS v.5.1 (Protein Solutions Inc.) and ten data points with baseline levels of 0.998–1.002 were used to calculate the Stokes radius and the polydispersity of the sample.
2.3. Crystallization and X-ray data collection
Initial crystallization tests were carried out with Crystal Screen HT and Index HT kits (Hampton Research) in 96-well plates using the sitting-drop vapour-diffusion method (Corning). For refinement of the crystallization conditions, 1 µl protein solution was mixed with an equal volume of reservoir solution and equilibrated against 0.5 ml reservoir solution at 293 K in 24-well plates using the sitting-drop vapour-diffusion method (Hampton Research). Crystals of Gnk2 and SeMet-rGnk2 suitable for X-ray analysis were obtained under the following reservoir-solution conditions: 21%(w/v) polyacrylic acid 5100, 20 mM MgCl2 and 0.1 M MES–NaOH pH 6.6 for Gnk2 and 12%(v/v) dioxane, 1.6 M ammonium sulfate and 0.1 M MES–NaOH pH 6.6 for SeMet-rGnk2.
As a cryoprotectant, reservoir solution containing 32%(v/v) glycerol was added to drops containing protein crystals. The crystals were picked up in a nylon loop (Hampton Research) and mounted for flash-cooling at 100 K. X-ray diffraction data were collected with synchrotron radiation using an ADSC Quantum-210 CCD detector system on the AR-NW12 beamline at Photon Factory (Tsukuba, Japan). The total diffraction data for Gnk2 consisted of 124 images, each exposed for 10 s with a 1° oscillation and a wavelength of 1.00000 Å at a crystal-to-detector distance of 235 mm. For multiwavelength anomalous diffraction data collection from the SeMet-rGnk2 crystal, three wavelengths, peak (0.97909 Å), edge (0.97934 Å) and remote (0.96411 Å), were determined from the selenium absorption spectrum. The data set at each wavelength was composed of 180 images and collected using a 0.5° oscillation with a crystal-to-detector distance of 291 mm and an exposure time of 2 s for each image. All diffraction data were indexed, integrated and scaled with the HKL-2000 program suite (Otwinowski & Minor, 1997 ▶).
3. Results
We have established the preparation and crystallization of native and recombinant Gnk2. Although the crystallization conditions for Gnk2 and SeMet-rGnk2 differed, the shapes of their crystals and the time course of the crystallization was quite similar and the crystallographic parameters were identical. The crystals appeared in about 3 d and grew in a cubic form with all sides approximately 0.3 mm within two weeks. Fig. 1 ▶ shows a typical Gnk2 crystal after two or more weeks. Both crystals belonged to the primitive cubic space group P213, with unit-cell parameters a = b = c = 143.2 Å. X-ray diffraction data sets for Gnk2 and SeMet-rGnk2 were processed using data in the resolution ranges 50–2.38 Å and 30–2.79 Å, respectively. Fig. 2 ▶ shows an image of the X-ray diffraction data of Gnk2. The DLS measurements suggested that the Stokes radius of Gnk2 was 1.68 nm, corresponding to a molecular weight of 10.2 kDa with a polydispersity of 10.8%, and revealed that the native protein is present as a monomer in solution. Assuming the presence of four monomers in an asymmetric unit of the cubic crystal, the typical Matthews coefficient and solvent content were estimated as 5.25 Å3 Da−1 and 76.6%, respectively (Matthews, 1968 ▶). These values were also consistent with those of the SeMet-rGnk2 crystal. The crystal parameters and data-collection statistics are summarized in Table 1 ▶. Three-dimensional structure determination will be performed using the multiple-wavelength anomalous dispersion method and the SeMet-rGnk2 data set.
Figure 1.
Typical crystals of Gnk2 grown at 293 K using polyacrylic acid 5100 as the precipitant. The sides of the largest crystal are approximately 0.3 mm. None of the crystals display polarization.
Figure 2.
An X-ray diffraction image (1.0° oscillation) from a Gnk2 crystal. The edge of the detector corresponds to a resolution of 2.0 Å.
Table 1. Crystal parameters and data-collection statistics of native ginkbilobin-2 and the selenomethionine derivative of recombinant ginkbilobin-2.
Values in parentheses are for the highest resolution shell.
| SeMet rGnk2 | ||||
|---|---|---|---|---|
| Gnk2 | Peak | Edge | Remote | |
| Space group | P213 | P213 | ||
| Unit-cell parameters (Å) | a = b = c = 143.2 | a = b = c = 143.2 | ||
| Wavelength (Å) | 1.00000 | 0.97909 | 0.97934 | 0.96411 |
| Resolution range (Å) | 50.0–2.38 (2.47–2.38) | 30.0–2.79 (2.89–2.79) | ||
| Observed reflections | 602150 | 272058 | 270119 | 275233 |
| Unique reflections | 39529 | 24549 | 24638 | 24734 |
| Data completeness (%) | 100 (100) | 99.8 (100) | 99.8 (100) | 99.9 (100) |
| Redundancy | 15.2 (14.5) | 11.1 (11.0) | 11.0 (11.0) | 11.2 (11.1) |
| Rsym† | 0.061 (0.375) | 0.041 (0.155) | 0.051 (0.168) | 0.048 (0.199) |
| 〈I〉/〈σ(I)〉 | 61.2 (8.2) | 71.7 (23.0) | 70.9 (20.9) | 68.2 (16.9) |
R
sym = 
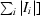 , where I
i is the ith intensity measurement of the reflection hkl, including symmetry-related reflections, and 〈I〉 is its average.
, where I
i is the ith intensity measurement of the reflection hkl, including symmetry-related reflections, and 〈I〉 is its average.
Acknowledgments
We would like to thank the scientists and staff at the Photon Factory. The synchrotron-radiation experiments were performed at AR-NW12 in the Photon Factory, Tsukuba, Japan (Proposal No. 2006S2-006). We also thank the ‘National BioResource Project (NIG, Japan): E. coli’ for the gift of JM109 pDsbABCD1 strain. This research was supported in part by the National Project on Protein Structural and Functional Analyses of the Ministry of Education, Culture, Sports, Science and Technology of Japan.
References
- Czernic, P., Visser, B., Sun, W., Savoure, A., Deslandes, L., Marco, Y., Van Montagu, M. & Verbruggen, N. (1999). Plant J.18, 321–327. [DOI] [PubMed]
- Hardie, D. G. (1999). Annu. Rev. Plant Physiol. Plant Mol. Biol.50, 97–131. [DOI] [PubMed]
- Kurokawa, Y., Yanagi, H. & Yura, T. (2000). Appl. Environ. Microbiol.66, 3960–3965. [DOI] [PMC free article] [PubMed]
- Matthews, B. W. (1968). J. Mol. Biol.33, 491–497. [DOI] [PubMed]
- Morris, E. R. & Walker, J. C. (2003). Curr. Opin. Plant Biol.6, 339–342. [DOI] [PubMed]
- Otwinowski, Z. & Minor, W. (1997). Methods Enzymol.276, 307–326. [DOI] [PubMed]
- Pace, C. N., Vajdos, F., Fee, L., Grimsley, G. & Gray, T. (1995). Protein Sci.4, 2411–2423. [DOI] [PMC free article] [PubMed]
- Sawano, Y., Miyakawa, T., Yamazaki, H., Tanokura, M. & Hatano, K. (2007). Biol. Chem.388, 273–280. [DOI] [PubMed]
- Shao, H. B., Liang, Z. S. & Shao, M. A. (2005). Colloids Surf. B Biointerfaces, 45, 131–135.
- Selitrennikoff, C. P. (2001). Appl. Environ. Microbiol.67, 2883–2894. [DOI] [PMC free article] [PubMed]
- Wallace, R. J. (2004). Proc. Nutr. Soc.63, 621–629. [DOI] [PubMed]




