Abstract
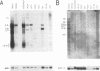
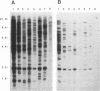
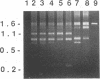
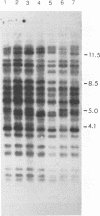
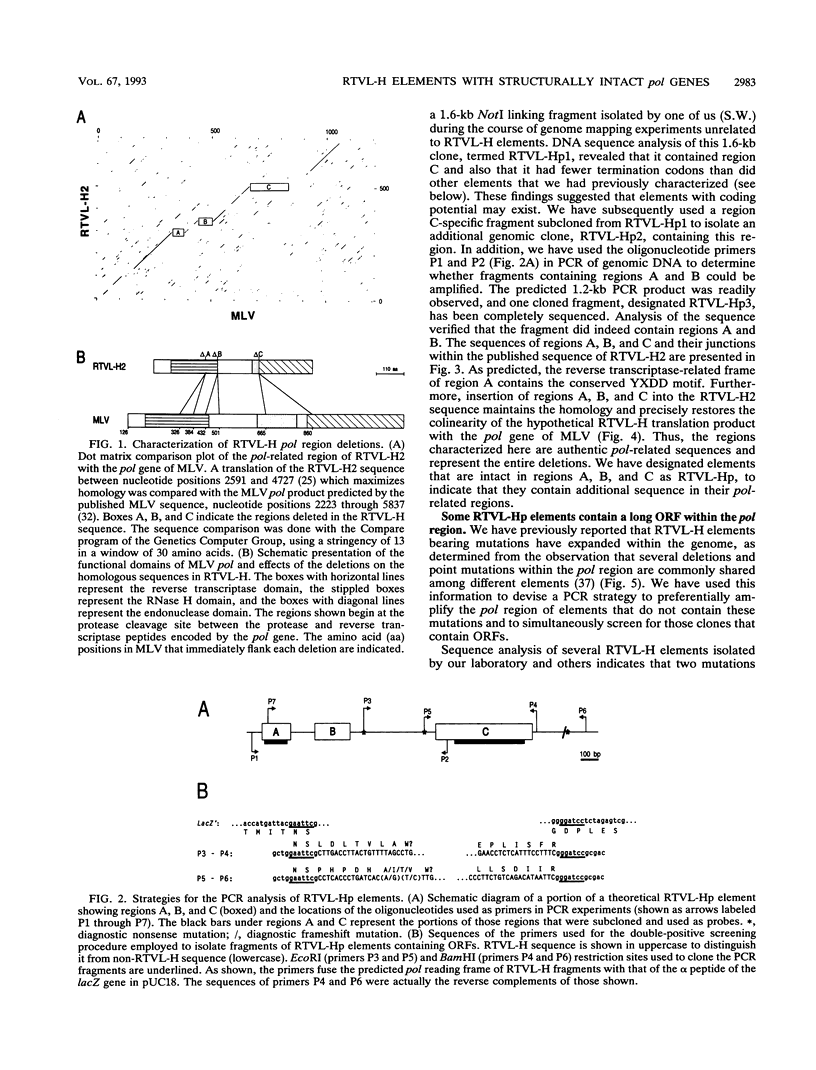
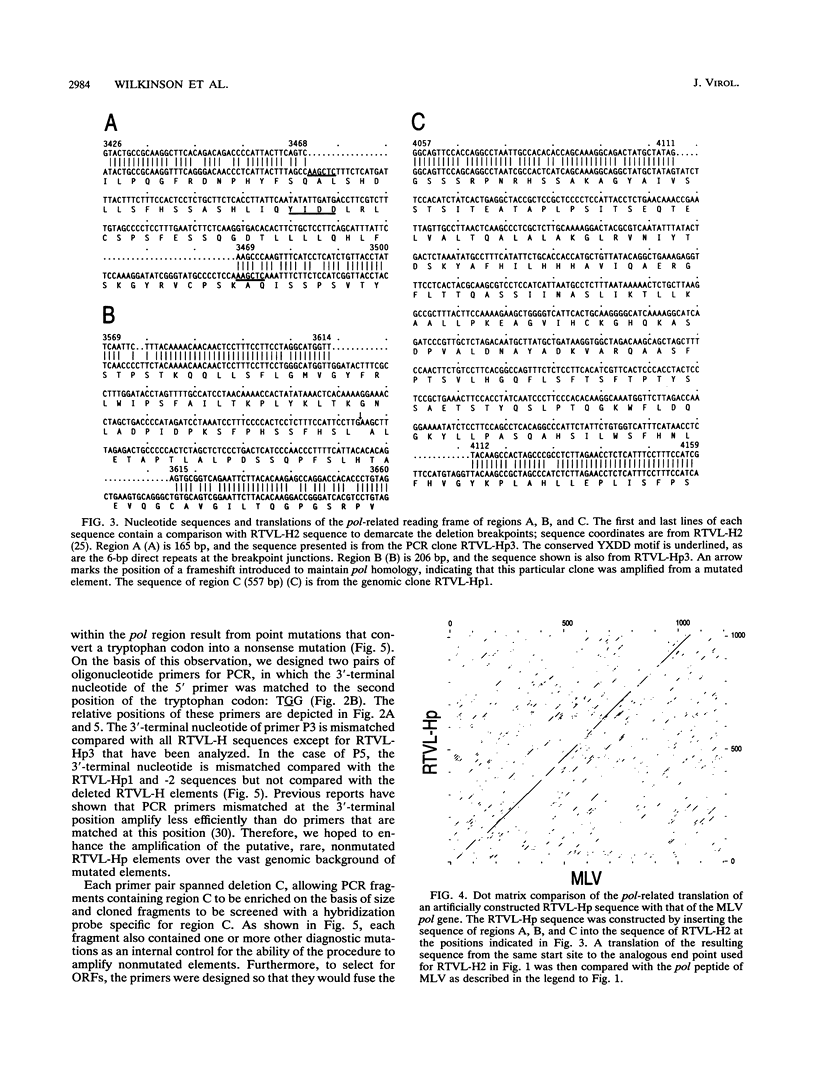
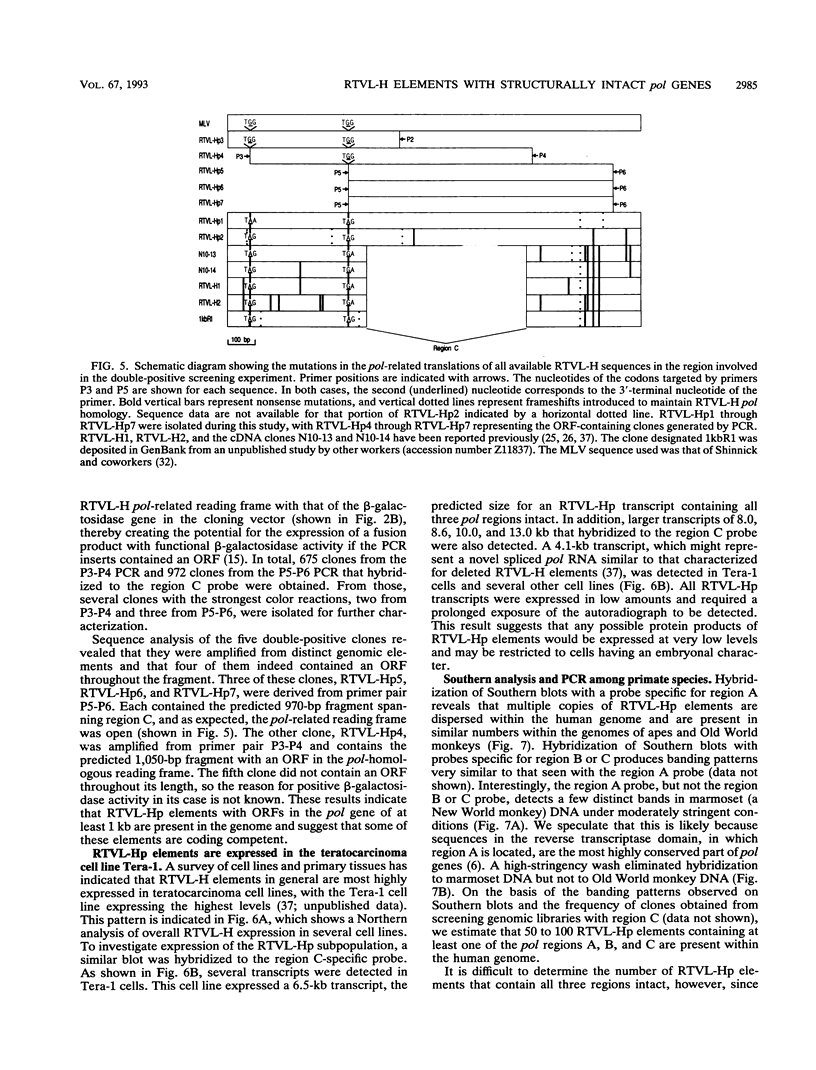
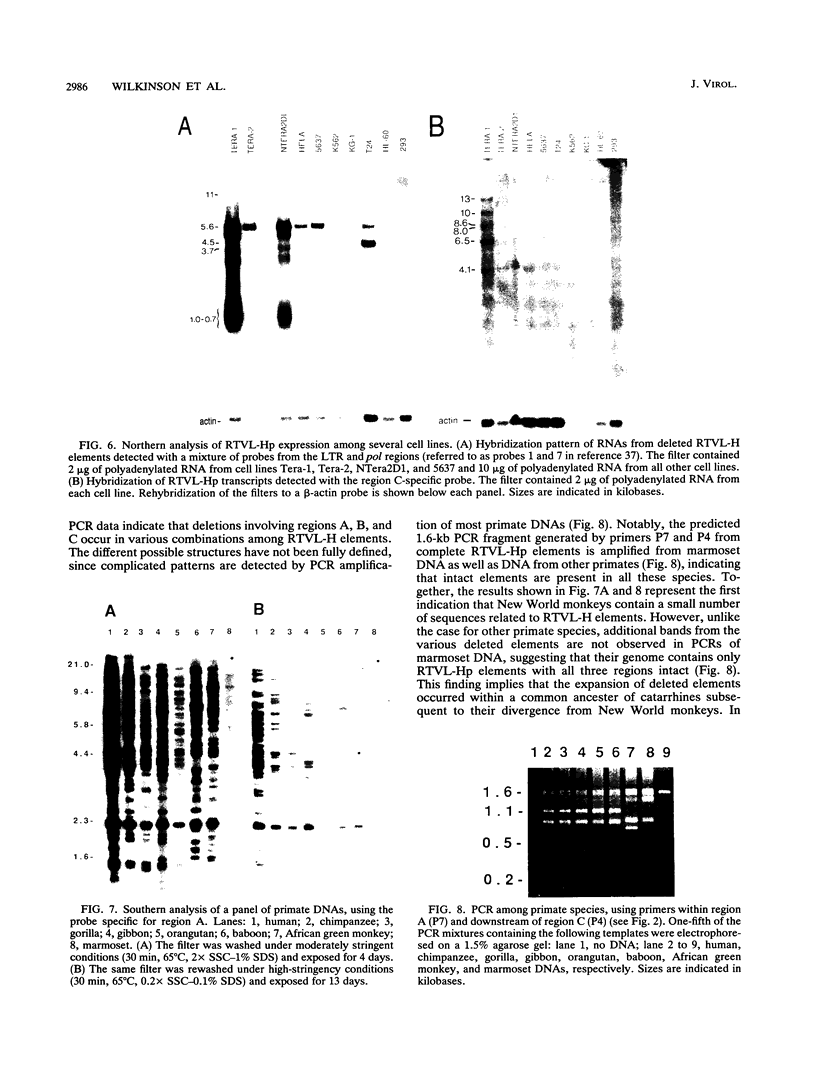
We report the discovery of a subgroup of RTVL-H human endogenous retroviral elements, designated RTVL-Hp, that is intact in the pol region which is deleted in the vast majority of RTVL-H elements. As a consequence, RTVL-Hp elements contain critical functional domains in their pol region that other RTVL-H elements lack. We estimate that the haploid genomes of humans, apes, and Old World monkeys contain 50 to 100 copies of RTVL-Hp elements and 800 to 1,000 deleted sequences. The major amplification of deleted elements appears to have occurred after the divergence of Old World and New World monkeys, since we have obtained evidence that a few intact RTVL-Hp elements, but no deleted forms, are present in marmoset DNA. Using the polymerase chain reaction coupled with a direct screen for open reading frames, we have isolated fragments from four RTVL-Hp elements amplified from human DNA that contain an open reading frame throughout a region of pol that is disrupted by diagnostic mutations in all other RTVL-H sequences that we had previously analyzed. Northern (RNA) hybridization analysis shows that unit-length RTVL-Hp transcripts are expressed in the human teratocarcinoma cell line Tera-1. Together, the results presented here suggest that a small functional subfamily of RTVL-H elements is present in the human genome.
Full text
PDF

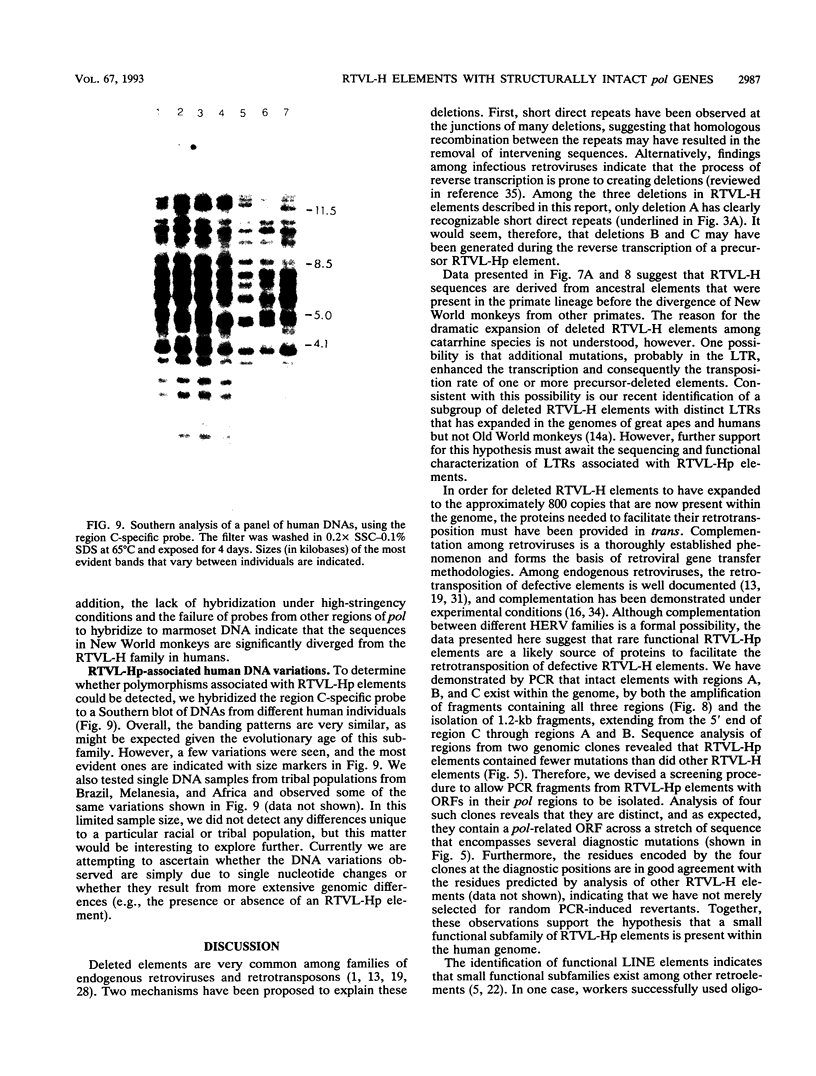


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bronson D. L., Saxinger W. C., Ritzi D. M., Fraley E. E. Production of virions with retrovirus morphology by human embryonal carcinoma cells in vitro. J Gen Virol. 1984 Jun;65(Pt 6):1043–1051. doi: 10.1099/0022-1317-65-6-1043. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombroski B. A., Mathias S. L., Nanthakumar E., Scott A. F., Kazazian H. H., Jr Isolation of an active human transposable element. Science. 1991 Dec 20;254(5039):1805–1808. doi: 10.1126/science.1662412. [DOI] [PubMed] [Google Scholar]
- Doolittle R. F., Feng D. F., Johnson M. S., McClure M. A. Origins and evolutionary relationships of retroviruses. Q Rev Biol. 1989 Mar;64(1):1–30. doi: 10.1086/416128. [DOI] [PubMed] [Google Scholar]
- Faff O., Murray A. B., Schmidt J., Leib-Mösch C., Erfle V., Hehlmann R. Retrovirus-like particles from the human T47D cell line are related to mouse mammary tumour virus and are of human endogenous origin. J Gen Virol. 1992 May;73(Pt 5):1087–1097. doi: 10.1099/0022-1317-73-5-1087. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Feuchter-Murthy A. E., Freeman J. D., Mager D. L. Splicing of a human endogenous retrovirus to a novel phospholipase A2 related gene. Nucleic Acids Res. 1993 Jan 11;21(1):135–143. doi: 10.1093/nar/21.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feuchter A., Mager D. Functional heterogeneity of a large family of human LTR-like promoters and enhancers. Nucleic Acids Res. 1990 Mar 11;18(5):1261–1270. doi: 10.1093/nar/18.5.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser C., Humphries R. K., Mager D. L. Chromosomal distribution of the RTVL-H family of human endogenous retrovirus-like sequences. Genomics. 1988 May;2(4):280–287. doi: 10.1016/0888-7543(88)90015-8. [DOI] [PubMed] [Google Scholar]
- Fredholm M., Policastro P. F., Wilson M. C. The dispersion of defective endogenous murine retroviral elements suggests retrotransposition-mediated amplification. DNA Cell Biol. 1991 Dec;10(10):713–722. doi: 10.1089/dna.1991.10.713. [DOI] [PubMed] [Google Scholar]
- Goodchild N. L., Wilkinson D. A., Mager D. L. A human endogenous long terminal repeat provides a polyadenylation signal to a novel, alternatively spliced transcript in normal placenta. Gene. 1992 Nov 16;121(2):287–294. doi: 10.1016/0378-1119(92)90133-a. [DOI] [PubMed] [Google Scholar]
- Gray M. R., Colot H. V., Guarente L., Rosbash M. Open reading frame cloning: identification, cloning, and expression of open reading frame DNA. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6598–6602. doi: 10.1073/pnas.79.21.6598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidmann O., Heidmann T. Retrotransposition of a mouse IAP sequence tagged with an indicator gene. Cell. 1991 Jan 11;64(1):159–170. doi: 10.1016/0092-8674(91)90217-m. [DOI] [PubMed] [Google Scholar]
- Hirose Y., Takamatsu M., Harada F. Presence of env genes in members of the RTVL-H family of human endogenous retrovirus-like elements. Virology. 1993 Jan;192(1):52–61. doi: 10.1006/viro.1993.1007. [DOI] [PubMed] [Google Scholar]
- Johnson M. S., McClure M. A., Feng D. F., Gray J., Doolittle R. F. Computer analysis of retroviral pol genes: assignment of enzymatic functions to specific sequences and homologies with nonviral enzymes. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7648–7652. doi: 10.1073/pnas.83.20.7648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P. M., Lyden T. W., Mwenda J. M. Endogenous retroviral expression in the human placenta. Am J Reprod Immunol. 1990 Aug;23(4):115–120. doi: 10.1111/j.1600-0897.1990.tb00683.x. [DOI] [PubMed] [Google Scholar]
- Kuff E. L., Lueders K. K. The intracisternal A-particle gene family: structure and functional aspects. Adv Cancer Res. 1988;51:183–276. doi: 10.1016/s0065-230x(08)60223-7. [DOI] [PubMed] [Google Scholar]
- Larsson E., Kato N., Cohen M. Human endogenous proviruses. Curr Top Microbiol Immunol. 1989;148:115–132. doi: 10.1007/978-3-642-74700-7_4. [DOI] [PubMed] [Google Scholar]
- Leib-Mösch C., Brack-Werner R., Werner T., Bachmann M., Faff O., Erfle V., Hehlmann R. Endogenous retroviral elements in human DNA. Cancer Res. 1990 Sep 1;50(17 Suppl):5636S–5642S. [PubMed] [Google Scholar]
- Loeb D. D., Padgett R. W., Hardies S. C., Shehee W. R., Comer M. B., Edgell M. H., Hutchison C. A., 3rd The sequence of a large L1Md element reveals a tandemly repeated 5' end and several features found in retrotransposons. Mol Cell Biol. 1986 Jan;6(1):168–182. doi: 10.1128/mcb.6.1.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löwer J., Wondrak E. M., Kurth R. Genome analysis and reverse transcriptase activity of human teratocarcinoma-derived retroviruses. J Gen Virol. 1987 Nov;68(Pt 11):2807–2815. doi: 10.1099/0022-1317-68-11-2807. [DOI] [PubMed] [Google Scholar]
- Mager D. L., Freeman J. D. Human endogenous retroviruslike genome with type C pol sequences and gag sequences related to human T-cell lymphotropic viruses. J Virol. 1987 Dec;61(12):4060–4066. doi: 10.1128/jvi.61.12.4060-4066.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mager D. L., Henthorn P. S. Identification of a retrovirus-like repetitive element in human DNA. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7510–7514. doi: 10.1073/pnas.81.23.7510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mager D. L. Polyadenylation function and sequence variability of the long terminal repeats of the human endogenous retrovirus-like family RTVL-H. Virology. 1989 Dec;173(2):591–599. doi: 10.1016/0042-6822(89)90570-9. [DOI] [PubMed] [Google Scholar]
- Prachar J., Vrbenská A., Hlubinová K., Simkovic D., Kovarík A. Retrovirus like particles produced by human embryonal cells and cell lines derived from human malignancies. II. Protein structure. Neoplasma. 1987;34(2):129–138. [PubMed] [Google Scholar]
- Reeves R. H., O'Brien S. J. Molecular genetic characterization of the RD-114 gene family of endogenous feline retroviral sequences. J Virol. 1984 Oct;52(1):164–171. doi: 10.1128/jvi.52.1.164-171.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shell B., Szurek P., Dunnick W. Interruption of two immunoglobulin heavy-chain switch regions in murine plasmacytoma P3.26Bu4 by insertion of retroviruslike element ETn. Mol Cell Biol. 1987 Apr;7(4):1364–1370. doi: 10.1128/mcb.7.4.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinnick T. M., Lerner R. A., Sutcliffe J. G. Nucleotide sequence of Moloney murine leukaemia virus. Nature. 1981 Oct 15;293(5833):543–548. doi: 10.1038/293543a0. [DOI] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. DNA sequence analysis with a modified bacteriophage T7 DNA polymerase. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4767–4771. doi: 10.1073/pnas.84.14.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchenio T., Heidmann T. Defective retroviruses can disperse in the human genome by intracellular transposition. J Virol. 1991 Apr;65(4):2113–2118. doi: 10.1128/jvi.65.4.2113-2118.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson D. A., Freeman J. D., Goodchild N. L., Kelleher C. A., Mager D. L. Autonomous expression of RTVL-H endogenous retroviruslike elements in human cells. J Virol. 1990 May;64(5):2157–2167. doi: 10.1128/jvi.64.5.2157-2167.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood S., Schertzer M., Drabkin H., Patterson D., Longmire J. L., Deaven L. L. Characterization of a human chromosome 8 cosmid library constructed from flow-sorted chromosomes. Cytogenet Cell Genet. 1992;59(4):243–247. doi: 10.1159/000133260. [DOI] [PubMed] [Google Scholar]
- Xiong Y., Eickbush T. H. Origin and evolution of retroelements based upon their reverse transcriptase sequences. EMBO J. 1990 Oct;9(10):3353–3362. doi: 10.1002/j.1460-2075.1990.tb07536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]