Abstract
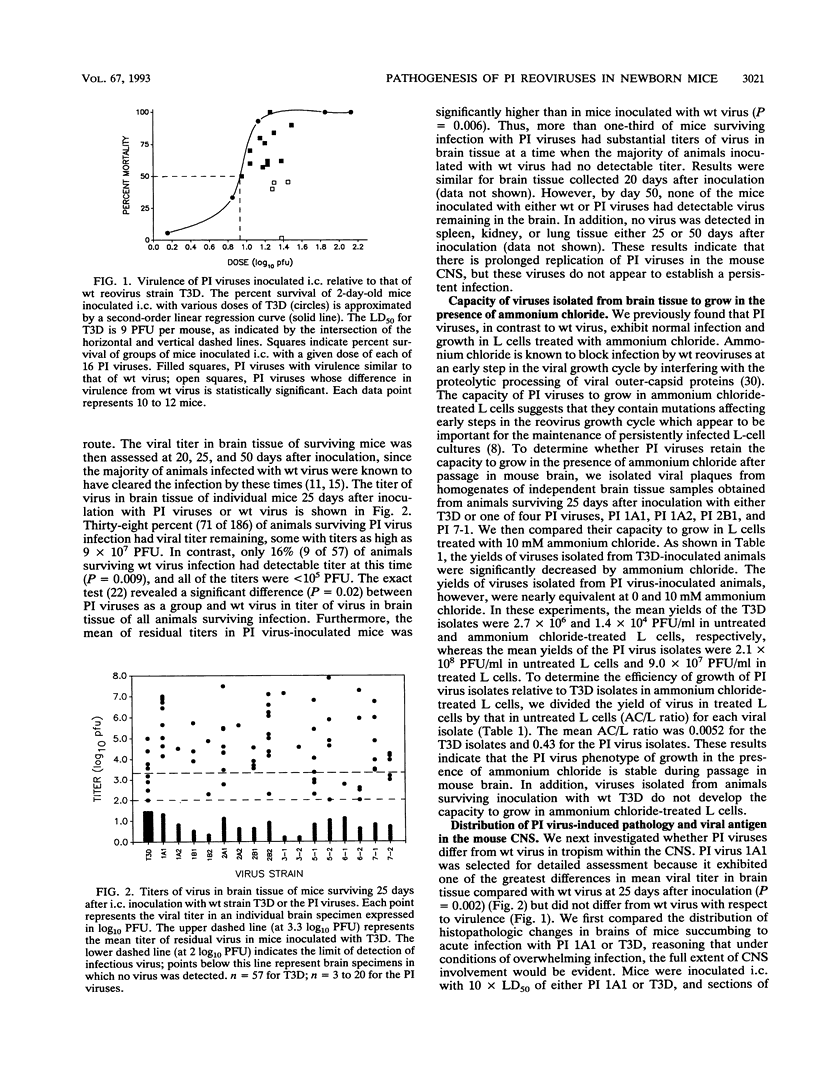
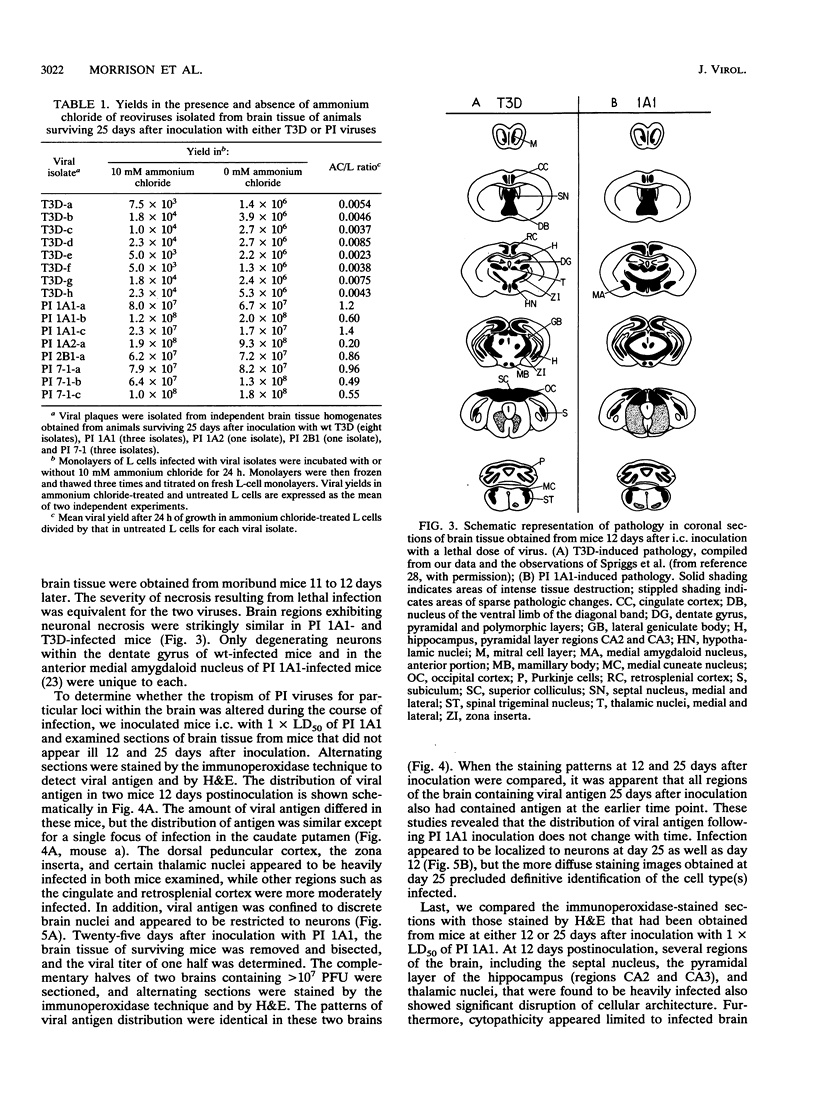
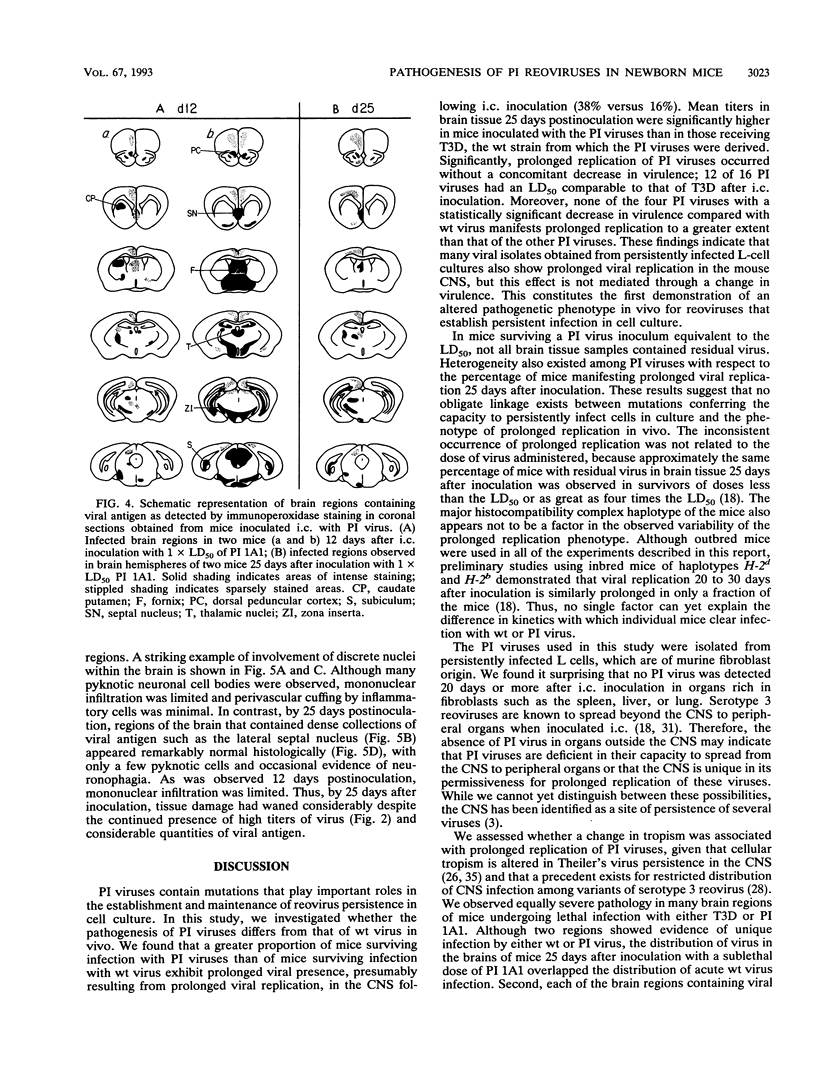
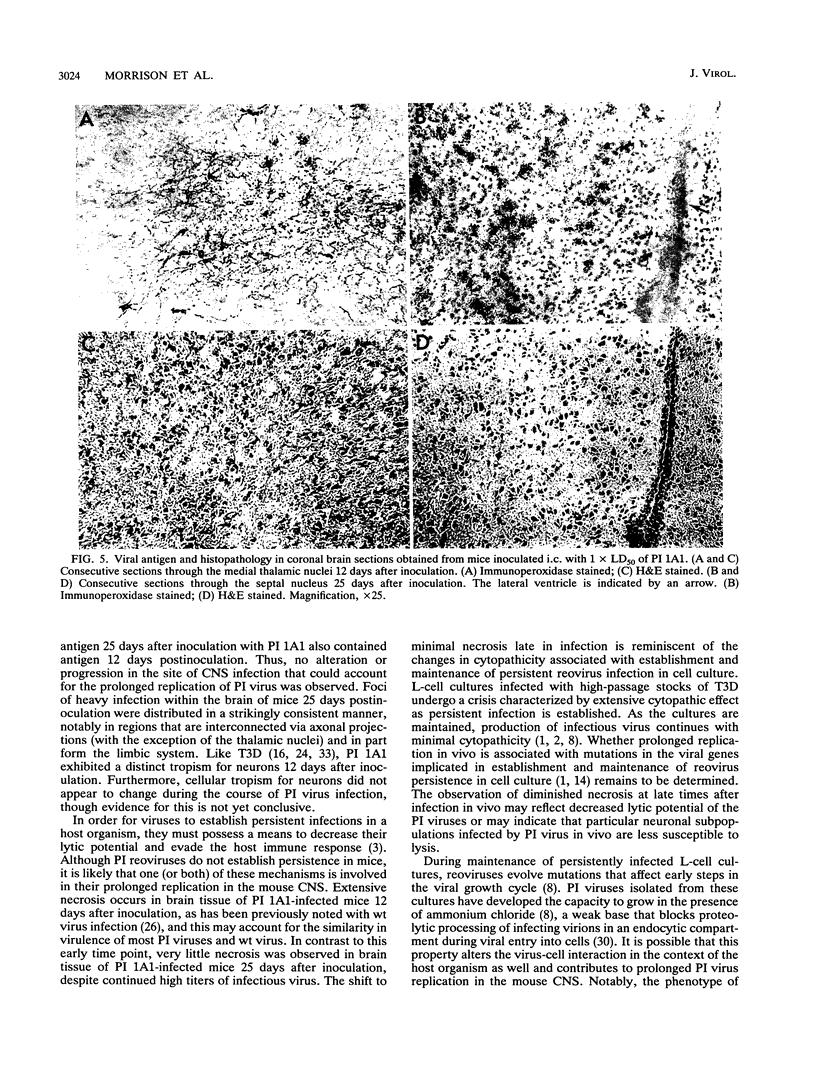
We examined pathogenic characteristics of plaque-purified reoviruses isolated from persistently infected L-cell cultures (PI viruses) after intracranial inoculation into newborn mice. The PI viruses were isolated from independent cultures initiated with high-passage stocks of the wild-type (wt) strain, type 3 Dearing. The virulence of most PI viruses was equivalent to that of the wt strain. However, replication of PI viruses in the central nervous system of infected mice was prolonged to 25 (but not 50) days postinoculation. Thirty-eight percent (n = 186) of mice inoculated with the PI viruses had residual virus detectable in brain tissue 25 days after inoculation, in contrast to only 16% (n = 57) of mice inoculated with wt virus (P = 0.009). Mean residual brain titers were more than 20-fold higher in mice inoculated with PI viruses compared with wt virus (4.3 x 10(4) versus 2.1 x 10(3); P = 0.006). Tropism of PI virus within the brain resembled that of wt virus, and the distribution of PI virus antigen in the brain did not change over time. The extent of necrosis in the brains of mice harboring PI virus 25 days after inoculation was minimal, despite continued presence of high titers of infectious virus. The latter observation resembles the absence of cytopathicity seen in L-cell cultures persistently infected with reovirus. These observations suggest that the interaction of PI viruses with cells can be altered in vivo as well as in cell culture, but virus is eventually cleared from the infected animal.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed R., Fields B. N. Role of the S4 gene in the establishment of persistent reovirus infection in L cells. Cell. 1982 Mar;28(3):605–612. doi: 10.1016/0092-8674(82)90215-x. [DOI] [PubMed] [Google Scholar]
- Ahmed R., Graham A. F. Persistent infections in L cells with temperature-sensitive mutants of reovirus. J Virol. 1977 Aug;23(2):250–262. doi: 10.1128/jvi.23.2.250-262.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bednarik D. P., Folks T. M. Mechanisms of HIV-1 latency. AIDS. 1992 Jan;6(1):3–16. doi: 10.1097/00002030-199201000-00001. [DOI] [PubMed] [Google Scholar]
- Burgert H. G., Maryanski J. L., Kvist S. "E3/19K" protein of adenovirus type 2 inhibits lysis of cytolytic T lymphocytes by blocking cell-surface expression of histocompatibility class I antigens. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1356–1360. doi: 10.1073/pnas.84.5.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dermody T. S., Nibert M. L., Wetzel J. D., Tong X., Fields B. N. Cells and viruses with mutations affecting viral entry are selected during persistent infections of L cells with mammalian reoviruses. J Virol. 1993 Apr;67(4):2055–2063. doi: 10.1128/jvi.67.4.2055-2063.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutko F. J., Oldstone M. B. Cytomegalovirus causes a latent infection in undifferentiated cells and is activated by induction of cell differentiation. J Exp Med. 1981 Nov 1;154(5):1636–1651. doi: 10.1084/jem.154.5.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauci A. S., Schnittman S. M., Poli G., Koenig S., Pantaleo G. NIH conference. Immunopathogenic mechanisms in human immunodeficiency virus (HIV) infection. Ann Intern Med. 1991 Apr 15;114(8):678–693. doi: 10.7326/0003-4819-114-8-678. [DOI] [PubMed] [Google Scholar]
- Furlong D. B., Nibert M. L., Fields B. N. Sigma 1 protein of mammalian reoviruses extends from the surfaces of viral particles. J Virol. 1988 Jan;62(1):246–256. doi: 10.1128/jvi.62.1.246-256.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory C. D., Murray R. J., Edwards C. F., Rickinson A. B. Downregulation of cell adhesion molecules LFA-3 and ICAM-1 in Epstein-Barr virus-positive Burkitt's lymphoma underlies tumor cell escape from virus-specific T cell surveillance. J Exp Med. 1988 Jun 1;167(6):1811–1824. doi: 10.1084/jem.167.6.1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauffman R. S., Ahmed R., Fields B. N. Selection of a mutant S1 gene during reovirus persistent infection of L cells: role in maintenance of the persistent state. Virology. 1983 Nov;131(1):79–87. doi: 10.1016/0042-6822(83)90535-4. [DOI] [PubMed] [Google Scholar]
- Kaye K. M., Spriggs D. R., Bassel-Duby R., Fields B. N., Tyler K. L. Genetic basis for altered pathogenesis of an immune-selected antigenic variant of reovirus type 3 (Dearing). J Virol. 1986 Jul;59(1):90–97. doi: 10.1128/jvi.59.1.90-97.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis G., Kilham L., Gonatas N. K. Reovirus type 3 encephalitis: observations of virus-cell interactions in neural tissues. I. Light microscopy studies. Lab Invest. 1971 Feb;24(2):91–100. [PubMed] [Google Scholar]
- Matloubian M., Somasundaram T., Kolhekar S. R., Selvakumar R., Ahmed R. Genetic basis of viral persistence: single amino acid change in the viral glycoprotein affects ability of lymphocytic choriomeningitis virus to persist in adult mice. J Exp Med. 1990 Oct 1;172(4):1043–1048. doi: 10.1084/jem.172.4.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison L. A., Sidman R. L., Fields B. N. Direct spread of reovirus from the intestinal lumen to the central nervous system through vagal autonomic nerve fibers. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3852–3856. doi: 10.1073/pnas.88.9.3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldstone M. B., Buchmeier M. J. Restricted expression of viral glycoprotein in cells of persistently infected mice. Nature. 1982 Nov 25;300(5890):360–362. doi: 10.1038/300360a0. [DOI] [PubMed] [Google Scholar]
- Oldstone M. B. Molecular anatomy of viral persistence. J Virol. 1991 Dec;65(12):6381–6386. doi: 10.1128/jvi.65.12.6381-6386.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raine C. S., Fields B. N. Reovirus type 3 encephalitis--a virologic and ultrastructural study. J Neuropathol Exp Neurol. 1973 Jan;32(1):19–33. doi: 10.1097/00005072-197301000-00002. [DOI] [PubMed] [Google Scholar]
- Roos R. P., Stein S., Routbort M., Senkowski A., Bodwell T., Wollmann R. Theiler's murine encephalomyelitis virus neutralization escape mutants have a change in disease phenotype. J Virol. 1989 Oct;63(10):4469–4473. doi: 10.1128/jvi.63.10.4469-4473.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvato M., Borrow P., Shimomaye E., Oldstone M. B. Molecular basis of viral persistence: a single amino acid change in the glycoprotein of lymphocytic choriomeningitis virus is associated with suppression of the antiviral cytotoxic T-lymphocyte response and establishment of persistence. J Virol. 1991 Apr;65(4):1863–1869. doi: 10.1128/jvi.65.4.1863-1869.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spriggs D. R., Bronson R. T., Fields B. N. Hemagglutinin variants of reovirus type 3 have altered central nervous system tropism. Science. 1983 Apr 29;220(4596):505–507. doi: 10.1126/science.6301010. [DOI] [PubMed] [Google Scholar]
- Stevens J. G. Human herpesviruses: a consideration of the latent state. Microbiol Rev. 1989 Sep;53(3):318–332. doi: 10.1128/mr.53.3.318-332.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturzenbecker L. J., Nibert M., Furlong D., Fields B. N. Intracellular digestion of reovirus particles requires a low pH and is an essential step in the viral infectious cycle. J Virol. 1987 Aug;61(8):2351–2361. doi: 10.1128/jvi.61.8.2351-2361.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler K. L., Virgin H. W., 4th, Bassel-Duby R., Fields B. N. Antibody inhibits defined stages in the pathogenesis of reovirus serotype 3 infection of the central nervous system. J Exp Med. 1989 Sep 1;170(3):887–900. doi: 10.1084/jem.170.3.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virgin H. W., 4th, Bassel-Duby R., Fields B. N., Tyler K. L. Antibody protects against lethal infection with the neurally spreading reovirus type 3 (Dearing). J Virol. 1988 Dec;62(12):4594–4604. doi: 10.1128/jvi.62.12.4594-4604.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALTERS M. N., JOSKE R. A., LEAK P. J., STANLEY N. F. MURINE INFECTION WITH REOVIRUS: I. PATHOLOGY OF THE ACUTE PHASE. Br J Exp Pathol. 1963 Aug;44:427–436. [PMC free article] [PubMed] [Google Scholar]
- Wechsler S. L., Fields B. N. Differences between the intracellular polypeptides of measles and subacute sclerosing panencephalitis virus. Nature. 1978 Mar 30;272(5652):458–460. doi: 10.1038/272458a0. [DOI] [PubMed] [Google Scholar]
- Zurbriggen A., Fujinami R. S. A neutralization-resistant Theiler's virus variant produces an altered disease pattern in the mouse central nervous system. J Virol. 1989 Apr;63(4):1505–1513. doi: 10.1128/jvi.63.4.1505-1513.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]



