Abstract
Breast self-examination (BSE) is widely recommended for breast cancer prevention. Following recent controversy over the efficacy of mammography, it may be seen as an alternative. We present a meta-analysis of the effect of regular BSE on breast cancer mortality. From a search of the medical literature, 20 observational studies and three clinical trials were identified that reported on breast cancer death rates or rates of advanced breast cancer (a marker of death) according to BSE practice. A lower risk of mortality or advanced breast cancer was only found in studies of women with breast cancer who reported practising BSE before diagnosis (mortality: pooled relative risk 0.64, 95% CI 0.56–0.73; advanced cancer, pooled relative risk 0.60, 95% CI 0.46–0.80). The results are probably due to bias and confounding. There was no difference in death rate in studies on women who detected their cancer during an examination (pooled relative risk 0.90, 95% CI 0.72–1.12). None of the trials of BSE training (in which most women reported practising it regularly) showed lower mortality in the BSE group (pooled relative risk 1.01, 95% CI 0.92–1.12). They did show that BSE is associated with considerably more women seeking medical advice and having biopsies. Regular BSE is not an effective method of reducing breast cancer mortality.
Keywords: breast self-examination, breast cancer, mortality, meta-analysis
For many years, women have been taught methods of breast self-examination (BSE) and it is recommended that they practise this regularly (Boyle et al, 1995; Shapiro et al, 1998), usually every month. There is a belief that among women who practise BSE, those who develop breast cancer are more likely to find it at an earlier stage and this is expected to lead to earlier treatment and hence decrease their risk of dying from the disease. Breast self-examination is appealing as a routine screening method because the examination has no financial cost (apart from the initial instruction sessions) and can be conducted in private. Most studies on the effectiveness of BSE have been observational. They suggest that women who practise BSE are more likely to find their breast tumour themselves, that the tumour tends to be smaller and that these women have an increased survival (Hackshaw, 1996; International Agency for Research on Cancer (IARC), 2002). However, survival time as an outcome measure can be misleading because of lead-time bias, in which BSE only identifies cancers at an earlier stage but has no effect on prognosis. Using mortality rates instead of survival time can overcome much of this bias.
Recently, the International Agency for Research on Cancer (2002) published a review on breast-cancer screening that reported the individual results from observational studies of BSE in relation to survival and stage of cancer, and those from randomised trials and cohort studies in relation to mortality. We here, however, present a meta-analysis of BSE and breast-cancer mortality by reviewing the published evidence from both observational studies and randomised trials, including those based on women with advanced breast cancer (used as a marker of death), and pooling the results. We look at three aspects of BSE; women who practise BSE, women who find their cancer during one of their regular examinations, and women who are taught BSE and advised to practise it regularly.
METHODS
Data sources and study selection
Studies that reported on rates of death from breast cancer or rates of advanced breast cancer (a marker of death) according to BSE practice were identified from Medline, Embase and Cancerlit (1966–2002), and included in the analysis. Keywords used were ‘breast cancer’ with ‘BSE’ or ‘self-examination’. In some studies, women were classified according to whether they practised BSE regularly or not. In other studies, women were classified according to the method of detecting the cancer: during BSE, by chance (e.g. while washing or dressing), mammography or examination by physician. Below we describe the main common features of the studies, but in the interest of brevity we do not provide further details, since these can be obtained directly from the individual published reports.
We included results on mortality or, as a surrogate for death, advanced breast cancer (defined as stage III or IV, regional or distant). Analyses are presented separately for these two outcomes.
The following types of studies were included in the analyses.
Studies on women newly diagnosed with breast cancer
A total of 15 studies were based only on women newly diagnosed with breast cancer (Greenwald et al, 1978; Smith et al, 1980; Feldman et al, 1981; Tamburini et al, 1981; Foster and Costanza, 1984; Owen et al, 1985; Smith and Burns, 1985; Ogawa et al, 1987; Huguley et al, 1988; Kuroishi et al, 1992; Le Geyte et al, 1992; Kurebayashi et al, 1994; Auvinen et al, 1996; McPherson et al, 1997; Koibuchi et al, 1998) and they were divided into four groups based on two different measures of outcome and two different measures of exposure.
Women who reported practising BSE or not and were then followed up for several years (usually about 5 years) to see who later died from breast cancer.
Women who reported whether they found their cancer during self-examination or by chance and were then followed up for several years to see who later died from breast cancer.
Women found to have advanced breast cancer at the time of initial diagnosis who reported retrospectively on whether they practised BSE or not.
Women found to have advanced breast cancer at the time of initial diagnosis who reported retrospectively on whether they had found their cancer during self-examination or by chance (e.g. washing and dressing).
In most studies mammography use was not stated, although it would not have been offered to many women since these studies were based on women diagnosed before the mid-1980s when such screening was not commonplace. In other studies, mammography use was low (2% of cancers detected by mammography, in Feldman et al, 1981) or similar between the BSE and non-BSE groups (Smith and Burns, 1985). In one study (Koibuchi et al, 1998), all women had a clinical examination as part of a mass-screening programme. A difference between the BSE and non-BSE group was reported with respect to mammography use in one study (18% in the BSE group compared to 7% in the non-BSE group, Huguley et al, 1988) and mass screening by clinician examination in another study (37% in the BSE group and 21% in the non-BSE group, Kuroishi et al, 1992); analyses were performed both with and without these two studies.
Cohort studies of women with and without breast cancer
The two cohort studies were from Finland (Gastrin et al, 1994) and the USA (Holmberg et al, 1997). In these, breast cancer death rates according to BSE practice were reported in populations of women followed up for over 13 years. In one study (Gastrin et al, 1994), mammography was used only as a method of further investigation after a woman found a lump by BSE. In the other study, the follow-up period was until 1972 when mammography was not commonplace.
Case–control studies of women with and without breast cancer
There were three case–control studies, two from the USA (Newcomb et al, 1991; Muscat and Huncharek, 1992) and one from Canada that was nested within a randomised trial of mammography (Harvey et al, 1997). In each study, cases (women who had died from breast cancer or had advanced cancer) and age-matched controls (women without breast cancer) were asked about their past BSE practice. One study also further matched for screening centre and enrolment year.
Clinical trials
One trial, from the UK, was nonrandomised (UK Trial of Early Detection of Breast Cancer Group, 1999) and two, from China (Thomas et al, 1997,2002) and Russia (Semiglazov et al, 1992,1996,1999), were randomised. The nonrandomised trial was based on comparing the breast cancer death rates after 16 years follow-up in two centres, in which women aged 45–64 years were invited to attend a BSE session, with the rates in four centres, in which women were not invited for either BSE training or mammography.
The two randomised trials were large. The one from China (Thomas et al, 1997,2002) was based on randomising 520 factories in Shanghai, in which all women in a particular factory were either given three sessions on how to practise BSE or they were not. In total, there were about 267 000 women aged 30–69 years. Recruitment began in 1989 and interim results were reported after 5 years. The trial in Russia involved two cities (Moscow and St Petersburg (formerly Leningrad)), but only the results from St Petersburg have been published; approximately five (Semiglazov et al, 1992), nine (Semiglazov et al, 1996) and 13 (Semiglazov et al, 1999) years after recruitment began in 1985. This trial included about 120 000 women aged 40–64 years and, similar to the one in China, randomisation was undertaken according to the place of work and BSE was taught during several sessions. Information on the following was also extracted from the reports of the two randomised trials; the number of women who sought medical advice after finding a lump, the number who had a biopsy and the number diagnosed with breast cancer. Mammography screening was not available to women in either trial.
Attendance of the BSE training sessions in the UK trial was low; only 31 and 53% of women in the two centers, respectively, accepted the invitation to be taught BSE. Attendance in the trial from China was high; 98% received baseline instruction and in one cohort with complete information on attendance (representing about half the women in the BSE group in the trial) 84% had attended all three training sessions. The reports from the Russian trial were based on women who had received training in BSE.
Definition of BSE practice
In the studies based on only women newly diagnosed with breast cancer, the definition of BSE practice varied. It was monthly (Ogawa et al, 1987; Auvinen et al, 1996), monthly or several times a year (Feldman et al, 1981; Tamburini et al, 1981; Foster and Costanza, 1984; Koibuchi et al, 1998) or at least two (Smith and Burns, 1985) or three (Smith et al, 1980) times per year. In several studies, about half or more of the women in the BSE groups had reported that they checked their breasts monthly (Feldman et al, 1981; Smith and Burns, 1985; Ogawa et al, 1987; Le Geyte et al, 1992). In the two cohort studies (Gastrin et al, 1994; Holmberg et al, 1997) women were classified as BSE practitioners, if they did so monthly. In the Russian trial, 76% of women taught BSE reported practising it at least every 2 months (Semiglazov et al, 1999), and in the Chinese trial women practised BSE at least every 4–5 months during the first 4–5 years of the trial and were strongly encouraged to practise it monthly (Thomas et al, 2002).
Statistical analysis
The relative risks (or odds ratio) and 95% confidence intervals (CI) were estimated from the data in each study. They were pooled on a log scale and weighted by the inverse of the variance, with allowance for any heterogeneity (DerSimonian and Laird, 1986).
RESULTS
Table 1 shows results from the observational studies based only on women with breast cancer.
Table 1. Observational studies of women with breast cancer; the number of deaths or advanced cancers and relative risk of dying from breast cancer in women who practise BSE compared to those who do not and in those who found their cancer during an examination.
|
Women who practise BSE regularly |
Women who never or rarely practise BSE |
|||||
|---|---|---|---|---|---|---|
| Study (first author), country | Age of women (years) | No. of breast cancer deaths or advanced cancers | No. of women with breast cancer | No. of breast cancer deaths or advanced cancers | No. of women with breast cancer | Relative risk of death or advanced cancer (95% CI) |
| Breast cancer death | ||||||
| Foster, 1984, USA | All (20–97) | 61 | 424 | 108 | 411 | 0.55 (0.40–0.75) |
| 22–49 | 18 | 134 | 15 | 58 | 0.52 (0.26–1.03) | |
| 50–97 | 43 | 287 | 92 | 346 | 0.56 (0.39–0.81) | |
| Huguley, 1988, USA | All (unspec.) | 327 | 1398 | 260 | 681 | 0.61 (0.52–0.72) |
| Le Geyte, 1992, UK | 15–59 | 60 | 226 | 130 | 390 | 0.80 (0.59–1.08) |
| Kurebayashi, 1994, Japan | All (unspec.) | 3 | 91 | 10 | 132 | 0.44 (0.12–1.58) |
| Auvinen, 1996, Finland | All (unspec.) | — | 246 | — | 104 | 0.85 (0.53–1.33) |
| Advanced breast cancer | ||||||
| Smith, 1980, USA | 30–80 | 44 | 107 | 24 | 57 | 0.98 (0.59–1.61) |
| Feldman, 1981, USA | All (unspec.) | 137 | 408 | 256 | 588 | 0.77 (0.63–0.95) |
| Tamburini, 1981, Italy | 35–64 | 34 | 170 | 90 | 330 | 0.73 (0.49–1.09) |
| Foster, 1984, USA | All (20–97) | 41 | 422 | 123 | 410 | 0.32 (0.23–0.46) |
| Smith, 1985, USA | 20–54 | 75 | 185 | 67 | 134 | 0.81 (0.58–1.13) |
| Ogawa, 1987, Japan | 25–77 | 3 | 30 | 20 | 116 | 0.58 (0.17–1.95) |
| Huguley, 1988, USA | All (unspec.) | 225 | 1396 | 246 | 680 | 0.45 (0.37–0.53) |
| Kurebayashi, 1994, Japan | All (unspec.) | 7 | 91 | 18 | 132 | 0.56 (0.24–1.35) |
| Koibuchi, 1998, Japan | All (unspec.) | 3 | 68 | 18 | 174 | 0.43 (0.13–1.45) |
|
Cancer found during BSE |
Cancer found by accidenta |
|||||
| Breast cancer death | ||||||
| Greenwald, 1978, USA | All (unspec.) | 16 | 55 | 65 | 182 | 0.81 (0.47–1.41) |
| Kuroishi, 1992, Japan | All (unspec.) | — | 347 | — | 1322 | 0.57 (0.33–0.99) |
| Auvinen, 1996, Finland | All (unspec.) | — | 34 | — | 104 | 1.06 (0.88–1.26) |
| McPherson, 1997, USA | 40–49 | 33 | 200 | 70 | 364 | 0.86 (0.57–1.30) |
| Advanced breast cancer | ||||||
| Greenwald, 1978, USA | All (unspec.) | 11 | 55 | 56 | 182 | 0.65 (0.34–1.24) |
| Owen, 1985, USA | All (unspec.) | 76 | 185 | 539 | 1168 | 0.89 (0.70–1.13) |
| Kuroishi, 1992, Japan | All (unspec.) | 28 | 355 | 224 | 1327 | 0.47 (0.32–0.69) |
In one study (Greenwald et al, 1978), 20% of women in this group practised BSE although found their cancer by chance. Italics indicate that the data were estimated from results presented in the paper.
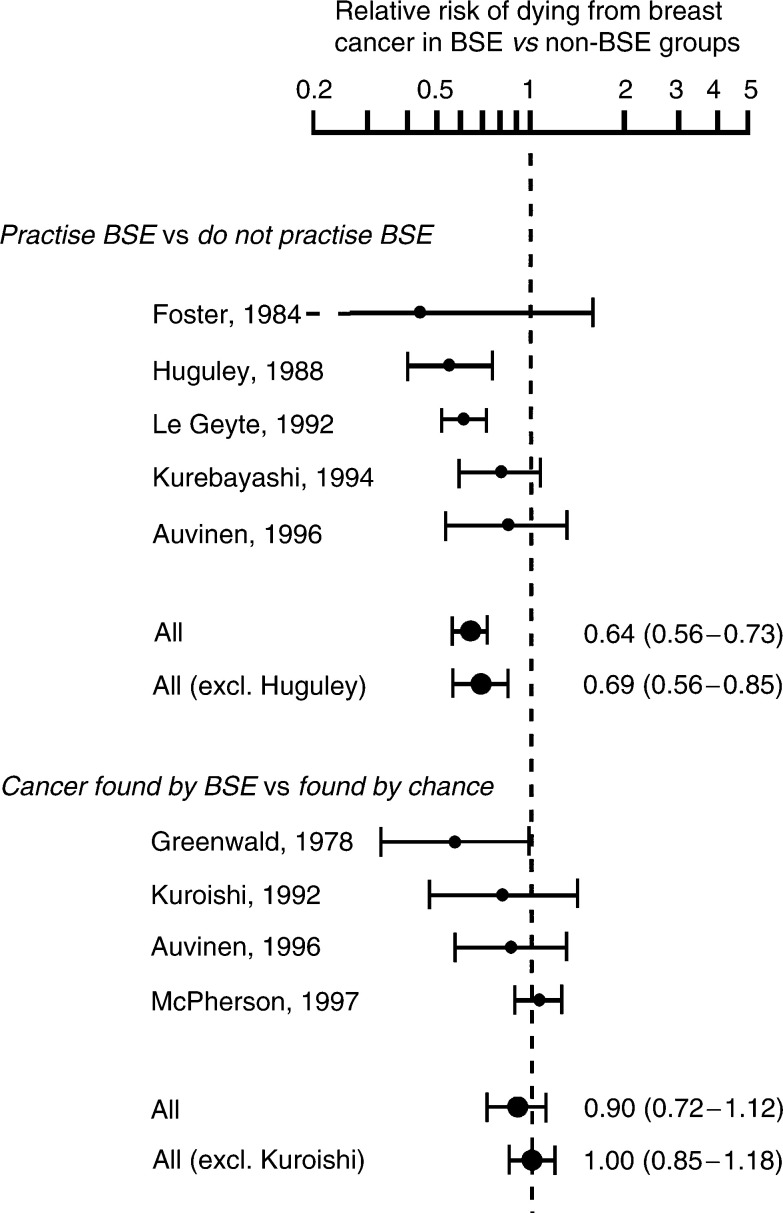
Figure 1 shows the individual relative risk of death from breast cancer and the pooled relative risks. Overall, there appears to be a statistically significant 36% reduction in the risk of death (relative risk 0.64, 95% CI 0.56–0.73, P<0.001) in those who practise BSE. There was no evidence of heterogeneity between the studies (P=0.41). If the study in which some women had mammo-graphy (Huguley et al, 1988) is excluded, the estimate is not substantially different (relative risk 0.69, 95% CI 0.56–0.85, P<0.001). In those women who reported that their cancer was detected during self-examination, there was no evidence of a reduction in the risk of death compared to those who found their cancer by chance (relative risk 0.90, 95% CI 0.72–1.12, P=0.34). Again there was no strong evidence of heterogeneity between the results (P=0.26). If the study (Kuroishi et al, 1992) in which some women had mass screening is excluded, the estimate is not much changed the pooled relative risk is 1.00 (95% CI 0.85–1.18, P=0.98).
Figure 1.
Observational studies of women with breast cancer, comparing the breast cancer death rates between the BSE and non-BSE groups. A test for heterogeneity between the studies yielded a P-value of 0.41 for those studies based on women who practise BSE and a P-value of 0.26 for those based on finding cancer by BSE.
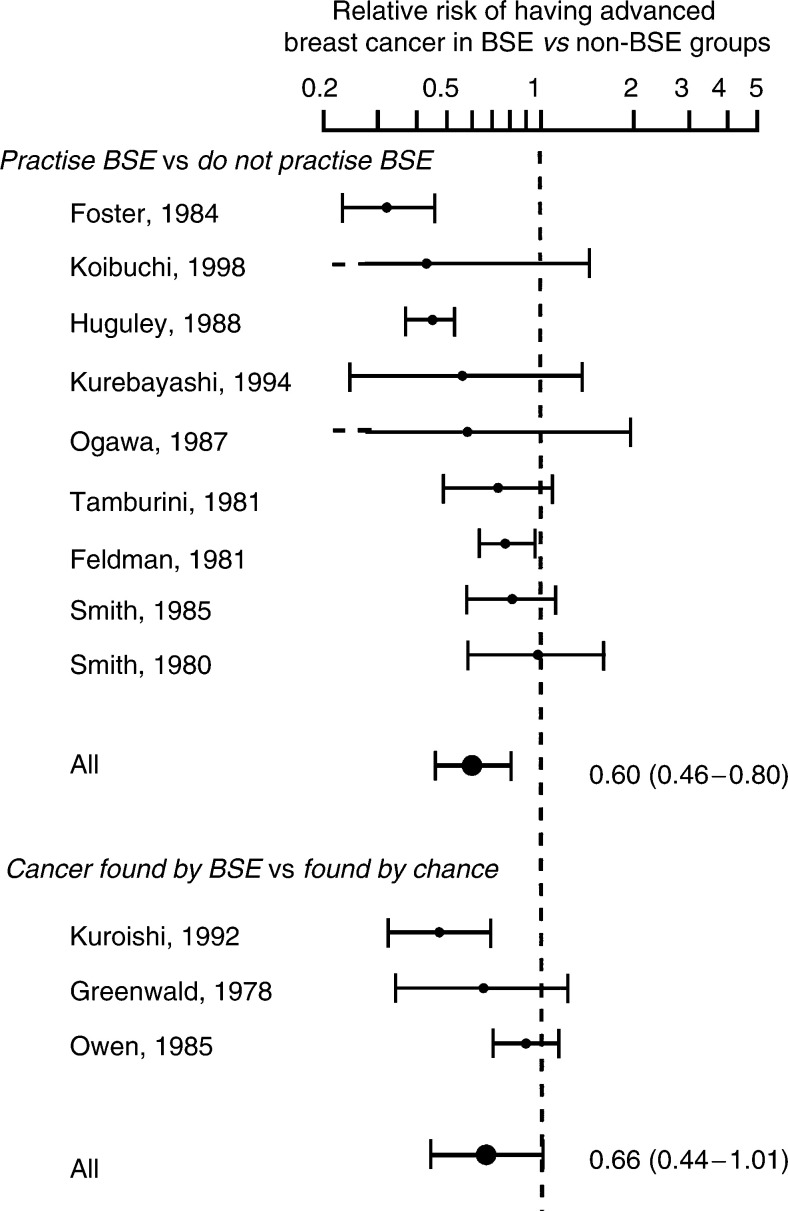
Figure 2 shows the relative risk of having advanced breast cancer in women who practise BSE compared to those who did not, among all women newly diagnosed with breast cancer. There is a 40% reduction in the risk (relative risk 0.60, 95% CI 0.46–0.80, P<0.001). Although there was evidence of hetero-geneity (P<0.001), all the studies reported a reduction in risk. In women who found their cancer during an examination, there was a 34% reduction in risk (relative risk 0.66, 95% CI 0.44–1.01, P=0.06).
Figure 2.
Observational studies of women with breast cancer, comparing the rates of advanced breast cancer between the BSE and non-BSE groups. A test for heterogeneity between the studies yielded a P-value of <0.001 for those studies based on women who practise BSE and a P-value of 0.051 for those based on finding cancer by BSE.
The results from the cohort and case–control studies of women with and without breast cancer according to BSE practice are shown in Table 2. The two cohort studies show inconsistent results; one indicates a statistically significant 29% reduction in the risk of death associated with BSE practice (relative risk 0.71, 95% CI 0.57–0.87) and the other shows no effect at all (relative risk 1.03, 95% CI 0.95–1.12). The pooled estimate is not statistically significant (relative risk 0.87, 95% CI 0.62–1.23, P=0.42). None of the case–control studies found statistically significant effects with only one suggesting a benefit (Harvey et al, 1997). The results for two of the case–control studies were not materially altered after adjustment for mammography use (Newcomb et al, 1991; Muscat and Huncharek, 1992).
Table 2. Observational studies of women with and without breast cancer; number of deaths and relative risk of dying from breast cancer in women who practise BSE compared to those who do not.
|
Women who practise BSE regularly |
Women who do not practise BSE |
|||||
|---|---|---|---|---|---|---|
| Study (first author), country | Age of women (years) | No. of breast cancer deathsa | No. of women without breast cancer | No. of breast cancer deathsa | No. of women without breast cancer | Relative risk or odds ratio of death (95% CI, if available) |
| Cohort studies | ||||||
| Gastrin, 1994, Finland | All (⩾20) | 95 | 28 780 | — | — | 0.71 (0.57–0.87) |
| 20–49 | 24 | — | — | — | 0.64 | |
| ⩾50 | 71 | — | — | — | 0.74 | |
| Holmberg, 1997, USA | All | 925 | 176 677 | 1375 | 271 179 | 1.03 (0.95–1.12) |
| ⩽39 | — | — | — | — | 0.95 | |
| 40–49 | — | — | — | — | 1.07 | |
| 50–59 | — | — | — | — | 1.03 | |
| ⩾60 | — | — | — | — | 1.02 | |
| Case–control studies | ||||||
| Muscat, 1992, USA | All (unspec.) | 251 | 430 | 184 | 457 | 1.45 (1.15–1.83) |
| Newcomb, 1991, USA | 20–80 | 168 | 344 | 41 | 89 | 1.06 (0.70–1.60) |
| Harvey, 1997, Canada | All (40+) | 97 | 1095 | 121 | 1091 | 0.79 (0.59–1.04) |
Or women with advanced breast cancer (Muscat, 1992; Newcomb, 1991). Dashes indicate that the data were not available from the published paper.
Table 3 provides the main results from the trials of teaching BSE and Figure 3 shows the relative risks and the pooled estimates for the main outcomes. The nonrandomised trial in the UK showed no effect overall (relative risk 0.99) even after 16 years of follow-up, although there was a difference between the two BSE centres; one showing a reduction in mortality (relative risk 0.79) and the other not (relative risk 1.09), which cannot readily be explained.
Table 3. Clinical trials of BSE; the number of biopsies, breast cancer cases and deaths and the relative risk of dying from breast cancer in women who practise BSE compared to those who do not.
|
BSE training |
No BSE training |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Study | Age of women (years) | Biopsies | Breast cancers | Deaths | Number randomised | Biopsies | Breast cancers | Deaths | Number randomised | Relative risk of death(95% CI) |
| Nonrandomised | ||||||||||
| UK Trial, 1999 | All (45–74) | — | — | 661 | 63 373b | — | — | 1312 | 127 123b | 0.99 (0.87–1.12)c |
| (after 16 years) | 45–49a | — | — | 236 | — | — | — | 511 | — | 0.94 (0.80–1.12) |
| 50–54 | — | — | 159 | — | — | — | 318 | — | 0.96 (0.78–1.18) | |
| 55–59 | — | — | 189 | — | — | — | 388 | — | 0.98 (0.81–1.19) | |
| 60–64 | — | — | 165 | — | — | — | 318 | — | 0.99 (0.81–1.22) | |
| Randomised | ||||||||||
| China | 30–69 | |||||||||
| (after 5 years) | 1788 | 331 | 25 | 133 375 | 945 | 322 | 25 | 133 665 | 1.00 (0.58–1.74) | |
| (after 10 years) | 3627 | 857 | 135 | 132 979 | 2398 | 890 | 131 | 133 085 | 1.03 (0.81–1.31) | |
| Russia | 40–64 | |||||||||
| (after 5 years) | 662 | 190 | — | 60 221 | 467 | 192 | — | 60 089 | — | |
| (after 9 years) | 1094 | 449 | 99 | 57 712 | 757 | 406 | 97 | 64 759 | 1.15 (0.87–1.52) | |
| (after 13 years) | 1138 | 493 | 157 | 57 712 | 797 | 446 | 164 | 64 759 | 1.07 (0.86–1.34) | |
Includes women from additional cohorts. Dashes indicate that the data were not available from the published paper. Publications: China (Thomas, 1997, 2002) and Russia (Semiglazov, 1992, 1996, 1999).
For the UK trial, this is the number of women invited to attend BSE training or the number that were not (i.e. in the comparison centres).
Age adjusted.
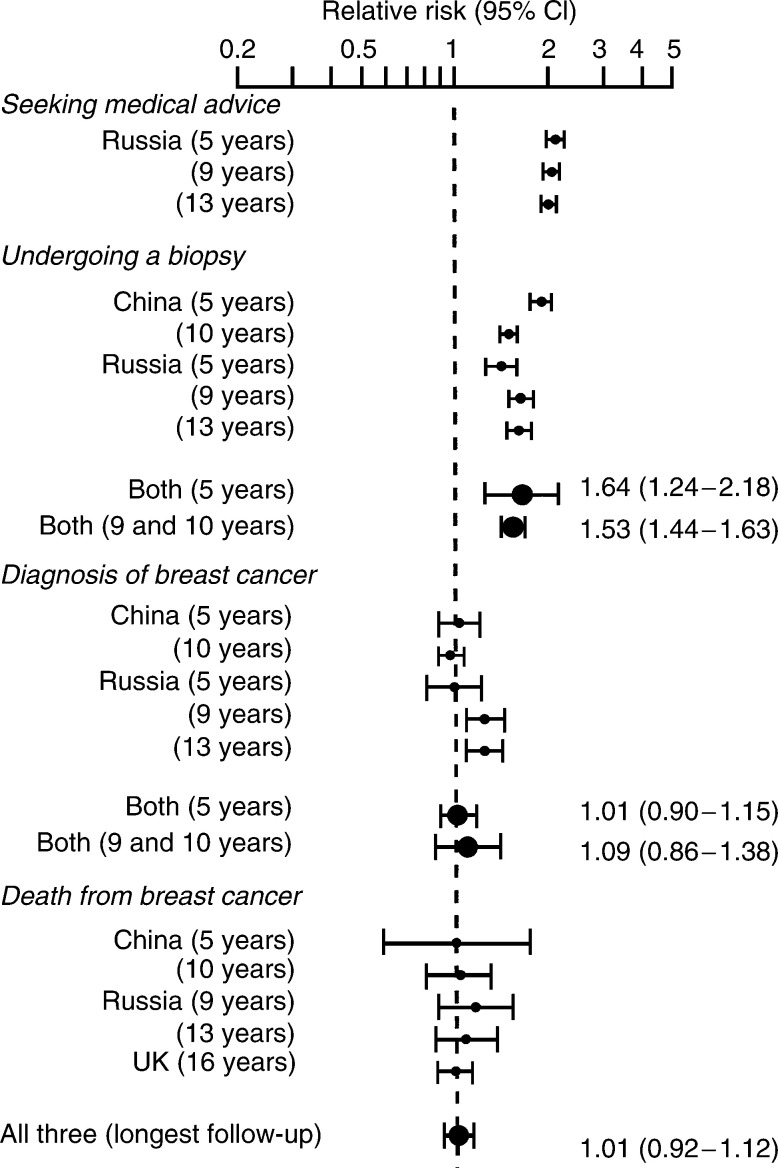
Figure 3.
Trials of BSE training. The rates for specified outcomes are compared between women invited for BSE training and those who were not. A test for heterogeneity between the trials yielded a P-value of 0.94 in relation to the results on mortality.
In the Russian trial, twice as many women in the BSE group sought medical advice compared to the non-BSE group (Figure 2), and this was consistent throughout the course of the trial (BSE vs non-BSE groups: 5.6 vs 2.8% at 5 years, 7.2 vs 3.5% at 9 years and 7.5 vs 3.8% at 13 years). After 10 years, the two trials (Russia and China) show that overall there were 53% more biopsies in women who were taught BSE compared to those who were not, this was highly statistically significant (relative risk of having a biopsy 1.53, 95% CI 1.44–1.63, P<0.001). The trials also suggest that at 5 years, women taught BSE were no more likely to be diagnosed with breast cancer than those not taught BSE (relative risk 1.01). After a longer follow-up (9–10 years), there is an indication from the Russian trial that more cancers were found in the BSE group (24% more women diagnosed with breast cancer), but this was not found in the trial from China.
The risk of dying from breast cancer was remarkably consistent between the three trials and over the different follow-up periods. There was no evidence of an advantage in the BSE group after any length of follow-up. The pooled relative risk was 1.01 with narrow 95% confidence limits (0.92–1.12, P=0.79); there was no evidence of heterogeneity (P=0.94). The results were not materially different if the nonrandomised trial from the UK was excluded, pooled relative risk 1.05 (95% CI 0.90–1.24, P=0.54).
There was little evidence that the effect of BSE varied between women in different age groups (Table 1 and Table 2).
DISCUSSION
Women who practise BSE
Only observational studies of women with breast cancer who were asked about their history of regular BSE practice consistently found a difference in breast cancer mortality associated with BSE. The studies are likely to be affected by several biases – publication bias, selection bias, recall bias, lead-time bias and length-biased sampling (there may be a larger proportion of slow-growing cancers diagnosed in women who practise BSE; slow-growing cancers tend to have better prognoses). Several studies have shown that various characteristics that are likely to be associated with dying from breast cancer were also associated with BSE practice, but analyses adjusting for the potential effect of such confounding on mortality were not reported. Women who practised BSE tended to be younger, premenopausal and of a higher socioeconomic status (Smith et al, 1980; Feldman et al, 1981; Tamburini et al, 1981; Huguley et al, 1988; Le Geyte et al, 1992; Auvinen et al, 1996). Much of the reduction in mortality observed in these studies might therefore be explained by a combination of these and other confounding factors as well as the aforementioned biases, rather than a real effect of BSE.
Women who found their cancer during an examination
No evidence of a reduction in mortality was found in women who reported that they found their cancer during self examination (Figure 1). There was an indication that there was an effect when advanced cancer was used as the outcome measure, but the overall result was not statistically significant (Figure 2).
Women who are taught BSE
The two randomised trials of mortality are unaffected by bias and both show no effect of BSE on breast cancer mortality, after 5 or 13 years. In the Russian trial, there was an increase in breast cancer diagnoses in women taught BSE after 9 and 13 years, but this was not reflected in a decrease in mortality at either time. Both trials also show that women in the BSE group are much more likely to be referred for a biopsy. At about 10 years, the overall malignant to benign biopsy ratio was 1 : 2.3 in the BSE group and 1 : 1.3 in the non-BSE group, indicating that in women who were taught BSE, there is one extra biopsy in women without cancer for every diagnosed case of breast cancer.
Despite the initial appeal of regular BSE, the evidence shows that it is likely to result in a considerable increase in women without breast cancer who have breast biopsy with its associated anxiety and counselling, but with no benefit. The two randomised and one nonrandomised trials were based on about 580 000 women and 2344 breast cancer deaths; the conclusions are therefore robust. Although the two randomised trials were based on BSE training, the negative results are also, to some extent, applicable to BSE practice since uptake was high and women reported practising BSE regularly (every 2 months in the Russian trial and every 4–5 months in the Chinese trial).
Breast self-examination as an alternative to mammography
The results presented here on BSE may have an impact on the current debate over the use of mammography screening. Despite clear evidence to the contrary (Wald et al, 1993; Nystrom et al, 1996; IARC 2002), it has been suggested recently that mammography screening is not effective in reducing mortality for breast cancer (Olsen and Gotzsche, 2000,2001). Breast self-examination may be considered to be an alternative. The conclusion that mammography was not worthwhile was based on only one out of the six existing randomised trials of breast-cancer mortality comparing mammography with no screening. The other five trials were rejected on the grounds of perceived differences between the screened and unscreened groups at baseline. When the one trial acceptable to the authors was combined with a trial that compared mammography with clinician examination, the reported relative risk was 1.04 (95% CI 0.84–1.27). As a result, there has been some confusion over whether mass mammography screening should continue. Several groups (Reply to Olsen and Gotzsche, 2000; Miettinen et al, 2002) have rejected the claim that mammography is not worthwhile with many valid criticisms of the reported analysis. Taking the evidence from all six trials, the relative risk is 0.76 (95% CI 0.67–0.87) in women aged ⩾50 years (Wald et al, 1993); a statistically significant 24% reduction in breast cancer deaths. Mammography screening is recommended to women over the age of 40 years in the US and 50–64 years in the UK. Without it, these women currently have no other means of reducing their chance of dying from breast cancer. Breast self-examination, perhaps the only other method that could be in widespread use, is unlikely to be a worthwhile alternative, even as a method of screening to be used in between mammographic examinations. The evidence presented here shows that it is ineffective in saving lives. Women should, of course, still be aware of changes in their breasts and seek advice if concerned, but being taught BSE and practising it regularly is no more effective at reducing breast cancer mortality than finding the tumour by chance.
References
- Auvinen A, Elovainio L, Hakama M (1996) Breast self-examination and survival from breast cancer; a prospective follow-up study. Breast Cancer Res Treat 38: 161–168 [DOI] [PubMed] [Google Scholar]
- Boyle P, Veronesi U, Tubiana M, Alexander FE, Calais da Silva F, Denis LJ, Freire JM, Hakama M, Hirsch A, Kroes R, La Vecchia C, Maisonneuve P, Martin-Moreno JM, Newton-Bishop J, Pindborg JJ, Saracci R, Scully C, Standaert B, Storm H, Blanco S, Malbois R, Bleehen N, Dictato M, Plesnicar S (1995) European School of Oncology Advisory Report to the European Commission for the “Europe against Cancer Programme”. Eur J Cancer 31A(9): 1395–1405 [DOI] [PubMed] [Google Scholar]
- DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Controlled Clin Trials 7: 177–188 [DOI] [PubMed] [Google Scholar]
- Feldman JG, Carter AC, Nicastri AD, Hosat ST (1981) Breast self-examination, relationship to stage of breast cancer at diagnosis. Cancer 47: 2740–2745 [DOI] [PubMed] [Google Scholar]
- Foster RS, Costanza MC (1984) Breast self-examination practises and breast cancer survival. Cancer 53: 999–1005 [DOI] [PubMed] [Google Scholar]
- Gastrin G, Miller AB, To T, Aronson KJ, Wall C, Hakama M, Louhivuori K, Pukkala E (1994) Incidence and mortality from breast cancer in the Mama Program for breast screening in Finland, 1973–1986. Cancer 73: 2168–2174 [DOI] [PubMed] [Google Scholar]
- Greenwald P, Nasca PC, Lawrence CE, Horton J, McGarrah RP, Gabrielle T, Carlton K (1978) Estimated effect of breast self-examination and routine physician examinations on breast cancer mortality. N Engl J Med 299: 271–273 [DOI] [PubMed] [Google Scholar]
- Hackshaw AK (1996) Screening for breast cancer in young women using breast self-examination. In Evidence-Guided Prescribing of the Pill, Hannaford PC, Webb AMC (eds). Royal College of General Practitioners. Parthenon Publishing Group, Lancs, UK [Google Scholar]
- Harvey BJ, Miller AB, Baines CJ, Corey PN (1997) Effect of breast self-examination techniques on the risk of death from breast cancer. Can Med Assoc J 157: 1205–1212 [PMC free article] [PubMed] [Google Scholar]
- Holmberg L, Ekbom A, Calle E, Mokdad A, Byers T (1997) Breast cancer mortality in relation to self-reported use of breast self-examination. A cohort study of 450,000 women. Breast Cancer Treat Res 43: 137–140 [DOI] [PubMed] [Google Scholar]
- Huguley CM, Brown RL, Greenberg RS, Clark WS (1988) Breast self-examination and survival from breast cancer. Cancer 62: 1389–1396 [DOI] [PubMed] [Google Scholar]
- International Agency for Research on Cancer (IARC) (2002) Efficacy of screening by self examination In Handbook of Cancer Prevention. Vol 7: Breast Cancer Screening, Vainio H, Bianchini F (eds). Lyon, France: IARC [Google Scholar]
- Koibuchi Y, Iino Y, Takei H, Maemura M, Horiguchi J, Yokoe T, Morishita Y (1998) The effect of mass screening by physical examination combined with regular breast self-examination on clinical stage and course of Japanese women with breast cancer. Oncol Rep 5: 151–155 [DOI] [PubMed] [Google Scholar]
- Kurebayashi J, Shimozuma K, Sonoo H (1994) The practise of breast self-examination results in the earlier detection and better clinical course of Japanese women with breast cancer. Jpn J Surg 24: 337–341 [DOI] [PubMed] [Google Scholar]
- Kuroishi T, Tominaga S, Ota J, Horino T, Taguchi T, Ishida T, Yokoe T, Masaru I, Ogita M, Itoh S, Abe R, Yoshida K, Morimoto T, Enomoto K, Tashiro H, Kashiki Y, Yamamoto S, Kido C, Honda K, Sasakawa M, Fukuda M, Watanabe H (1992) The effect of breast self-examination on early detection and survival. Jpn J Cancer Res 83: 344–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Geyte M, Mant D, Vessey MP, Jones L, Yudkin P (1992) Breast self-examination and survival from breast cancer. Br J Cancer 66: 917–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson CP, Swenson KK, Jolitz G, Murray CL (1997) Survival of women ages 40–49 with breast carcinoma according to method of detection. Cancer 79: 1923–1932 [DOI] [PubMed] [Google Scholar]
- Miettinen OS, Henschke C, Pasmantier MW, Smith JP, Libby DM, Yankelevitz DF (2002) Mammographic screening: no reliable supporting evidence? Lancet 359: 404–406 [DOI] [PubMed] [Google Scholar]
- Muscat JE, Huncharek MS (1992) Breast self-examination and extent of disease: a population-based study. Cancer Detect Prev 15: 155–159 [PubMed] [Google Scholar]
- Newcomb PA, Weiss NS, Storer BE, Scholes D, Young BE, Voigt LF (1991) Breast self-examination in relation to the occurrence of advanced breast cancer. J Natl Cancer Inst 83: 260–265 [DOI] [PubMed] [Google Scholar]
- Nystrom L, Larsson LG, Wall S (1996) An overview of the Swedish randomised mammography trials: total mortality pattern and the representivity of the study cohorts. J Med Screening 3: 85–87 [DOI] [PubMed] [Google Scholar]
- Ogawa H, Tominaga S, Yoshida M, Kubo K, Takeuchi S (1987) Breast self-examination practise and clinical stage of breast cancer. Jpn J Cancer Res 78: 447–452 [PubMed] [Google Scholar]
- Olsen O, Gotzsche PC (2000) Is screening for breast cancer with mammography justifiable? Lancet 355: 129–134 [DOI] [PubMed] [Google Scholar]
- Olsen O, Gotzsche PC (2001) Cochrane review on screening for breast cancer with mammography. Lancet 358: 1340–1342 [DOI] [PubMed] [Google Scholar]
- Owen WL, Hoge AF, Asal NR, Anderson PS, Owen AS, Cucchiara AJ (1985) Self-examination of the breast: use and effectiveness. Southern Med J 78: 1170–1173 [DOI] [PubMed] [Google Scholar]
- Reply to Olsen O, Gotzsche PC (various authors) (2000) Screening mammography re-evaluated. Lancet 355: 747–752 [DOI] [PubMed] [Google Scholar]
- Semiglazov VF, Moiseyenko VM, Bavli IL, Migmanova NS, Seleznyov NK, Popova RT, Ivanova OA, Orlov AA, Chagunava OA, Barash NJ, Matitzin AN, Dyatchenko OT, Kozhevnikov SY, Alexandrova GI, Sanchakova AV, Musayev BT (1992) The role of breast self-examination in early breast cancer detection (Results of the 5-years USSR/WHO randomised trial study in Leningrad). Eur J Epidemiol 8: 498–502 [DOI] [PubMed] [Google Scholar]
- Semiglazov VF, Moiseyenko VM, Manikhas AG, Protsenko SA, Kharikova RS, Popova RT, Migmanova NS, Orlov AA, Barash NI, Ivanova OA, Ivanov VG (1999) Interim results of a prospective randomised study of self-examination for early detection of breast cancer. Vopr Onkol 45: 265–271 [PubMed] [Google Scholar]
- Semiglazov VF, Moiseyenko VM, Protsenko SA, Bavli IL, Orlov AA, Ivanova OA, Barash NI, Chagunava OL, Golubeva OM, Migmanova NS, Seleznev IK, Popova RT, Diatchenko OT, Kozhevnikov SY, Aleksandrova GI, Sanchakova AV, Kharikova RS, Liubomirova NK, Ivanova GV, Azeev VF, Chuprakova IS (1996) Preliminary results of the Russia (St Petersburg)/WHO program for the evaluation of the effectiveness of breast self-examination. Vopr Onkol 42: 49–55 [PubMed] [Google Scholar]
- Shapiro S, Coleman EA, Broeders M, Codd M, de Konging H, Fracheboud J, Moss S, Paci E, Stachenko S, Ballard-Barbash R for the International Breast Cancer Screening Network (IBSN) and the European Network of Pilot Projects for Breast Cancer Screening (1998) Breast Cancer Screening Programmes in 22 countries: current policies administration and guidelines Int J Epidemiology 27: 735–742 [DOI] [PubMed] [Google Scholar]
- Smith EM, Burns TL (1985) The effects of breast self-examination in a population based cancer registry. Cancer 55: 432–437 [DOI] [PubMed] [Google Scholar]
- Smith EM, Francis AM, Polissar L (1980) The effect of breast self-exam practise and physician examinations on extent of disease at diagnosis. Prev Med 9: 409–417 [DOI] [PubMed] [Google Scholar]
- Tamburini M, Massara G, Bertario L, Re A, Di Pietro S (1981) Usefulness of breast self-examination for an early detection of breast cancer. Results of a study on 500 breast cancer patients and 652 controls. Tumori 67: 219–224 [DOI] [PubMed] [Google Scholar]
- Thomas DB, Gao DL, Ray RM, Wang WW, Allison CJ, Chen FL, Porter P, Hu YW, Zhao L, Pam D, Li W, Wu C, Coriaty Z, Evans I, Lin MG, Stalsberg H, Self SG (2002) Randomized trial of breast self-examination in Shanghai: final results. J Natl Cancer Inst 94: 1445–1457 [DOI] [PubMed] [Google Scholar]
- Thomas DB, Gao DL, Self SG, Allison CJ, Tao Y, Mahloch J, Ray R, Qin Q, Presley R, Porter P (1997) Randomised trial of breast self-examination in Shanghai: methodology and preliminary results. J Natl Cancer Inst 89: 355–365 [DOI] [PubMed] [Google Scholar]
- UK Trial of Early Detection of Breast Cancer Group (1999) 16-year mortality from breast cancer in the UK Trial of Early Detection of Breast Cancer. Lancet 353: 1909–1914 [PubMed] [Google Scholar]
- Wald NJ, Chamberlain J, Hackshaw AK on behalf of the Evaluation Committee Consensus statement (1993) Report of the European Society for Mastology Breast Cancer Screening Evaluation Committee. Breast 2: 209–216 [Google Scholar]