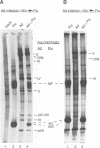
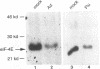
Abstract
Influenza virus infection of cells is accompanied by a striking shutoff of cellular protein synthesis, resulting in the exclusive translation of viral mRNAs. The mechanism for control of cellular protein synthesis by influenza virus is poorly understood, but several translation properties of influenza virus mRNAs which are potentially involved have been described. Influenza virus mRNAs possess the surprising ability to translate in the presence of inhibitory levels of inactive (phosphorylated) eukaryotic initiation factor 2 (eIF-2). In addition, influenza virus mRNAs were shown to be capable of translating in cells during the late phase of adenovirus infection but not in cells infected by poliovirus. Since both adenovirus and poliovirus facilitate virus-specific translation by impairing the activity of initiation factor eIF-4F (cap-binding protein complex) but through different mechanisms, we investigated the translation properties of influenza virus mRNAs in more detail. We show that influenza virus infection is associated with the significant dephosphorylation and inactivation of eIF-4E (cap-binding protein), a component of eIF-4F, and accordingly that influenza virus mRNAs possess a moderate ability to translate by using low levels of eIF-4F. We also confirm the ability of influenza virus mRNAs to translate in the presence of high levels of inactive (phosphorylated) eIF-2 but to a more limited extent than reported previously. We suggest a potential mechanism for the regulation of protein synthesis by influenza virus involving a decreased requirement for large pools of active eIF-4F and eIF-2.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abreu S. L., Lucas-Lenard J. Cellular protein synthesis shutoff by mengovirus: translation of nonviral and viral mRNA's in extracts from uninfected and infected Ehrlich ascites tumor cells. J Virol. 1976 Apr;18(1):182–194. doi: 10.1128/jvi.18.1.182-194.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso-Caplen F. V., Katze M. G., Krug R. M. Efficient transcription, not translation, is dependent on adenovirus tripartite leader sequences at late times of infection. J Virol. 1988 May;62(5):1606–1616. doi: 10.1128/jvi.62.5.1606-1616.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altmann M., Trachsel H. Altered mRNA cap recognition activity of initiation factor 4E in the yeast cell cycle division mutant cdc33. Nucleic Acids Res. 1989 Aug 11;17(15):5923–5931. doi: 10.1093/nar/17.15.5923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black T. L., Safer B., Hovanessian A., Katze M. G. The cellular 68,000-Mr protein kinase is highly autophosphorylated and activated yet significantly degraded during poliovirus infection: implications for translational regulation. J Virol. 1989 May;63(5):2244–2251. doi: 10.1128/jvi.63.5.2244-2251.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonneau A. M., Sonenberg N. Involvement of the 24-kDa cap-binding protein in regulation of protein synthesis in mitosis. J Biol Chem. 1987 Aug 15;262(23):11134–11139. [PubMed] [Google Scholar]
- Brendler T., Godefroy-Colburn T., Yu S., Thach R. E. The role of mRNA competition in regulating translation. III. Comparison of in vitro and in vivo results. J Biol Chem. 1981 Nov 25;256(22):11755–11761. [PubMed] [Google Scholar]
- Choi S. Y., Scherer B. J., Schnier J., Davies M. V., Kaufman R. J., Hershey J. W. Stimulation of protein synthesis in COS cells transfected with variants of the alpha-subunit of initiation factor eIF-2. J Biol Chem. 1992 Jan 5;267(1):286–293. [PubMed] [Google Scholar]
- Dever T. E., Feng L., Wek R. C., Cigan A. M., Donahue T. F., Hinnebusch A. G. Phosphorylation of initiation factor 2 alpha by protein kinase GCN2 mediates gene-specific translational control of GCN4 in yeast. Cell. 1992 Feb 7;68(3):585–596. doi: 10.1016/0092-8674(92)90193-g. [DOI] [PubMed] [Google Scholar]
- Dolph P. J., Huang J. T., Schneider R. J. Translation by the adenovirus tripartite leader: elements which determine independence from cap-binding protein complex. J Virol. 1990 Jun;64(6):2669–2677. doi: 10.1128/jvi.64.6.2669-2677.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolph P. J., Racaniello V., Villamarin A., Palladino F., Schneider R. J. The adenovirus tripartite leader may eliminate the requirement for cap-binding protein complex during translation initiation. J Virol. 1988 Jun;62(6):2059–2066. doi: 10.1128/jvi.62.6.2059-2066.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson R. W., Hagedorn C. H., Cohen S. Epidermal growth factor or okadaic acid stimulates phosphorylation of eukaryotic initiation factor 4F. J Biol Chem. 1991 Feb 15;266(5):3162–3166. [PubMed] [Google Scholar]
- Duncan R., Milburn S. C., Hershey J. W. Regulated phosphorylation and low abundance of HeLa cell initiation factor eIF-4F suggest a role in translational control. Heat shock effects on eIF-4F. J Biol Chem. 1987 Jan 5;262(1):380–388. [PubMed] [Google Scholar]
- Garfinkel M. S., Katze M. G. Translational control by influenza virus. Selective and cap-dependent translation of viral mRNAs in infected cells. J Biol Chem. 1992 May 5;267(13):9383–9390. [PubMed] [Google Scholar]
- Graham F. L., Smiley J., Russell W. C., Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977 Jul;36(1):59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- Hackett P. B., Egberts E., Traub P. Translation of ascites and mengovirus RNA in fractionated cell-free systems from uninfected and mengovirus-infected Ehrlich-ascites-tumor cells. Eur J Biochem. 1978 Feb;83(2):341–352. doi: 10.1111/j.1432-1033.1978.tb12100.x. [DOI] [PubMed] [Google Scholar]
- Herz C., Stavnezer E., Krug R., Gurney T., Jr Influenza virus, an RNA virus, synthesizes its messenger RNA in the nucleus of infected cells. Cell. 1981 Nov;26(3 Pt 1):391–400. doi: 10.1016/0092-8674(81)90208-7. [DOI] [PubMed] [Google Scholar]
- Hiremath L. S., Hiremath S. T., Rychlik W., Joshi S., Domier L. L., Rhoads R. E. In vitro synthesis, phosphorylation, and localization on 48 S initiation complexes of human protein synthesis initiation factor 4E. J Biol Chem. 1989 Jan 15;264(2):1132–1138. [PubMed] [Google Scholar]
- Huang J. T., Schneider R. J. Adenovirus inhibition of cellular protein synthesis involves inactivation of cap-binding protein. Cell. 1991 Apr 19;65(2):271–280. doi: 10.1016/0092-8674(91)90161-q. [DOI] [PubMed] [Google Scholar]
- Huang J. T., Schneider R. J. Adenovirus inhibition of cellular protein synthesis is prevented by the drug 2-aminopurine. Proc Natl Acad Sci U S A. 1990 Sep;87(18):7115–7119. doi: 10.1073/pnas.87.18.7115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson R. J. RNA translation. Picornaviruses break the rules. Nature. 1988 Jul 28;334(6180):292–293. doi: 10.1038/334292a0. [DOI] [PubMed] [Google Scholar]
- Jang S. K., Kräusslich H. G., Nicklin M. J., Duke G. M., Palmenberg A. C., Wimmer E. A segment of the 5' nontranslated region of encephalomyocarditis virus RNA directs internal entry of ribosomes during in vitro translation. J Virol. 1988 Aug;62(8):2636–2643. doi: 10.1128/jvi.62.8.2636-2643.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones N., Shenk T. Isolation of adenovirus type 5 host range deletion mutants defective for transformation of rat embryo cells. Cell. 1979 Jul;17(3):683–689. doi: 10.1016/0092-8674(79)90275-7. [DOI] [PubMed] [Google Scholar]
- Joshi-Barve S., De Benedetti A., Rhoads R. E. Preferential translation of heat shock mRNAs in HeLa cells deficient in protein synthesis initiation factors eIF-4E and eIF-4 gamma. J Biol Chem. 1992 Oct 15;267(29):21038–21043. [PubMed] [Google Scholar]
- Katze M. G., Chen Y. T., Krug R. M. Nuclear-cytoplasmic transport and VAI RNA-independent translation of influenza viral messenger RNAs in late adenovirus-infected cells. Cell. 1984 Jun;37(2):483–490. doi: 10.1016/0092-8674(84)90378-7. [DOI] [PubMed] [Google Scholar]
- Katze M. G., DeCorato D., Krug R. M. Cellular mRNA translation is blocked at both initiation and elongation after infection by influenza virus or adenovirus. J Virol. 1986 Dec;60(3):1027–1039. doi: 10.1128/jvi.60.3.1027-1039.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katze M. G., Detjen B. M., Safer B., Krug R. M. Translational control by influenza virus: suppression of the kinase that phosphorylates the alpha subunit of initiation factor eIF-2 and selective translation of influenza viral mRNAs. Mol Cell Biol. 1986 May;6(5):1741–1750. doi: 10.1128/mcb.6.5.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katze M. G., Krug R. M. Metabolism and expression of RNA polymerase II transcripts in influenza virus-infected cells. Mol Cell Biol. 1984 Oct;4(10):2198–2206. doi: 10.1128/mcb.4.10.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katze M. G., Krug R. M. Translational control in influenza virus-infected cells. Enzyme. 1990;44(1-4):265–277. doi: 10.1159/000468764. [DOI] [PubMed] [Google Scholar]
- Katze M. G., Tomita J., Black T., Krug R. M., Safer B., Hovanessian A. Influenza virus regulates protein synthesis during infection by repressing autophosphorylation and activity of the cellular 68,000-Mr protein kinase. J Virol. 1988 Oct;62(10):3710–3717. doi: 10.1128/jvi.62.10.3710-3717.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman R. J., Davies M. V., Pathak V. K., Hershey J. W. The phosphorylation state of eucaryotic initiation factor 2 alters translational efficiency of specific mRNAs. Mol Cell Biol. 1989 Mar;9(3):946–958. doi: 10.1128/mcb.9.3.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman R. J., Murtha P. Translational control mediated by eucaryotic initiation factor-2 is restricted to specific mRNAs in transfected cells. Mol Cell Biol. 1987 Apr;7(4):1568–1571. doi: 10.1128/mcb.7.4.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoller S., Kaempfer R. Isolation of a heme-controlled inhibitor of translation that blocks the interaction between messenger rna and eukaryotic initiation factor 2. Biochemistry. 1984 May 22;23(11):2462–2469. doi: 10.1021/bi00306a022. [DOI] [PubMed] [Google Scholar]
- Kozak M. Influence of mRNA secondary structure on binding and migration of 40S ribosomal subunits. Cell. 1980 Jan;19(1):79–90. doi: 10.1016/0092-8674(80)90390-6. [DOI] [PubMed] [Google Scholar]
- Kozak M. Leader length and secondary structure modulate mRNA function under conditions of stress. Mol Cell Biol. 1988 Jul;8(7):2737–2744. doi: 10.1128/mcb.8.7.2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krug R. M., Broni B. A., Bouloy M. Are the 5' ends of influenza viral mRNAs synthesized in vivo donated by host mRNAs? Cell. 1979 Oct;18(2):329–334. doi: 10.1016/0092-8674(79)90052-7. [DOI] [PubMed] [Google Scholar]
- Krug R. M., Broni B. A., LaFiandra A. J., Morgan M. A., Shatkin A. J. Priming and inhibitory activities of RNAs for the influenza viral transcriptase do not require base pairing with the virion template RNA. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5874–5878. doi: 10.1073/pnas.77.10.5874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamphear B. J., Panniers R. Cap binding protein complex that restores protein synthesis in heat-shocked Ehrlich cell lysates contains highly phosphorylated eIF-4E. J Biol Chem. 1990 Apr 5;265(10):5333–5336. [PubMed] [Google Scholar]
- Lamphear B. J., Panniers R. Heat shock impairs the interaction of cap-binding protein complex with 5' mRNA cap. J Biol Chem. 1991 Feb 15;266(5):2789–2794. [PubMed] [Google Scholar]
- Lawson T. G., Ray B. K., Dodds J. T., Grifo J. A., Abramson R. D., Merrick W. C., Betsch D. F., Weith H. L., Thach R. E. Influence of 5' proximal secondary structure on the translational efficiency of eukaryotic mRNAs and on their interaction with initiation factors. J Biol Chem. 1986 Oct 25;261(30):13979–13989. [PubMed] [Google Scholar]
- Lee T. G., Tomita J., Hovanessian A. G., Katze M. G. Purification and partial characterization of a cellular inhibitor of the interferon-induced protein kinase of Mr 68,000 from influenza virus-infected cells. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6208–6212. doi: 10.1073/pnas.87.16.6208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist S., Petersen R. Selective translation and degradation of heat-shock messenger RNAs in Drosophila. Enzyme. 1990;44(1-4):147–166. doi: 10.1159/000468754. [DOI] [PubMed] [Google Scholar]
- Macejak D. G., Sarnow P. Internal initiation of translation mediated by the 5' leader of a cellular mRNA. Nature. 1991 Sep 5;353(6339):90–94. doi: 10.1038/353090a0. [DOI] [PubMed] [Google Scholar]
- Marino M. W., Pfeffer L. M., Guidon P. T., Jr, Donner D. B. Tumor necrosis factor induces phosphorylation of a 28-kDa mRNA cap-binding protein in human cervical carcinoma cells. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8417–8421. doi: 10.1073/pnas.86.21.8417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroto F. G., Sierra J. M. Translational control in heat-shocked Drosophila embryos. Evidence for the inactivation of initiation factor(s) involved in the recognition of mRNA cap structure. J Biol Chem. 1988 Oct 25;263(30):15720–15725. [PubMed] [Google Scholar]
- Mathews M. B., Shenk T. Adenovirus virus-associated RNA and translation control. J Virol. 1991 Nov;65(11):5657–5662. doi: 10.1128/jvi.65.11.5657-5662.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley S. J., Traugh J. A. Differential stimulation of phosphorylation of initiation factors eIF-4F, eIF-4B, eIF-3, and ribosomal protein S6 by insulin and phorbol esters. J Biol Chem. 1990 Jun 25;265(18):10611–10616. [PubMed] [Google Scholar]
- Morley S. J., Traugh J. A. Phorbol esters stimulate phosphorylation of eukaryotic initiation factors 3, 4B, and 4F. J Biol Chem. 1989 Feb 15;264(5):2401–2404. [PubMed] [Google Scholar]
- Muñoz A., Alonso M. A., Carrasco L. Synthesis of heat-shock proteins in HeLa cells: inhibition by virus infection. Virology. 1984 Aug;137(1):150–159. doi: 10.1016/0042-6822(84)90018-7. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M., O'Farrell P. H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977 Dec;12(4):1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- O'Malley R. P., Duncan R. F., Hershey J. W., Mathews M. B. Modification of protein synthesis initiation factors and the shut-off of host protein synthesis in adenovirus-infected cells. Virology. 1989 Jan;168(1):112–118. doi: 10.1016/0042-6822(89)90409-1. [DOI] [PubMed] [Google Scholar]
- O'Neill R. E., Racaniello V. R. Inhibition of translation in cells infected with a poliovirus 2Apro mutant correlates with phosphorylation of the alpha subunit of eucaryotic initiation factor 2. J Virol. 1989 Dec;63(12):5069–5075. doi: 10.1128/jvi.63.12.5069-5075.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier J., Sonenberg N. Insertion mutagenesis to increase secondary structure within the 5' noncoding region of a eukaryotic mRNA reduces translational efficiency. Cell. 1985 Mar;40(3):515–526. doi: 10.1016/0092-8674(85)90200-4. [DOI] [PubMed] [Google Scholar]
- Ray B. K., Brendler T. G., Adya S., Daniels-McQueen S., Miller J. K., Hershey J. W., Grifo J. A., Merrick W. C., Thach R. E. Role of mRNA competition in regulating translation: further characterization of mRNA discriminatory initiation factors. Proc Natl Acad Sci U S A. 1983 Feb;80(3):663–667. doi: 10.1073/pnas.80.3.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray B. K., Lawson T. G., Kramer J. C., Cladaras M. H., Grifo J. A., Abramson R. D., Merrick W. C., Thach R. E. ATP-dependent unwinding of messenger RNA structure by eukaryotic initiation factors. J Biol Chem. 1985 Jun 25;260(12):7651–7658. [PubMed] [Google Scholar]
- Reichel P. A., Merrick W. C., Siekierka J., Mathews M. B. Regulation of a protein synthesis initiation factor by adenovirus virus-associated RNA. Nature. 1985 Jan 17;313(5999):196–200. doi: 10.1038/313196a0. [DOI] [PubMed] [Google Scholar]
- Rhoads R. E. Cap recognition and the entry of mRNA into the protein synthesis initiation cycle. Trends Biochem Sci. 1988 Feb;13(2):52–56. doi: 10.1016/0968-0004(88)90028-x. [DOI] [PubMed] [Google Scholar]
- Rychlik W., Gardner P. R., Vanaman T. C., Rhoads R. E. Structural analysis of the messenger RNA cap-binding protein. Presence of phosphate, sulfhydryl, and disulfide groups. J Biol Chem. 1986 Jan 5;261(1):71–75. [PubMed] [Google Scholar]
- Sarnow P. Translation of glucose-regulated protein 78/immunoglobulin heavy-chain binding protein mRNA is increased in poliovirus-infected cells at a time when cap-dependent translation of cellular mRNAs is inhibited. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5795–5799. doi: 10.1073/pnas.86.15.5795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider R. J., Safer B., Munemitsu S. M., Samuel C. E., Shenk T. Adenovirus VAI RNA prevents phosphorylation of the eukaryotic initiation factor 2 alpha subunit subsequent to infection. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4321–4325. doi: 10.1073/pnas.82.13.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider R. J., Shenk T. Impact of virus infection on host cell protein synthesis. Annu Rev Biochem. 1987;56:317–332. doi: 10.1146/annurev.bi.56.070187.001533. [DOI] [PubMed] [Google Scholar]
- Schneider R. J., Weinberger C., Shenk T. Adenovirus VAI RNA facilitates the initiation of translation in virus-infected cells. Cell. 1984 May;37(1):291–298. doi: 10.1016/0092-8674(84)90325-8. [DOI] [PubMed] [Google Scholar]
- Siekierka J., Mariano T. M., Reichel P. A., Mathews M. B. Translational control by adenovirus: lack of virus-associated RNAI during adenovirus infection results in phosphorylation of initiation factor eIF-2 and inhibition of protein synthesis. Proc Natl Acad Sci U S A. 1985 Apr;82(7):1959–1963. doi: 10.1073/pnas.82.7.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skehel J. J., Hay A. J. Nucleotide sequences at the 5' termini of influenza virus RNAs and their transcripts. Nucleic Acids Res. 1978 Apr;5(4):1207–1219. doi: 10.1093/nar/5.4.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonenberg N. Regulation of translation by poliovirus. Adv Virus Res. 1987;33:175–204. doi: 10.1016/s0065-3527(08)60318-8. [DOI] [PubMed] [Google Scholar]
- Thach R. E. Cap recap: the involvement of eIF-4F in regulating gene expression. Cell. 1992 Jan 24;68(2):177–180. doi: 10.1016/0092-8674(92)90461-k. [DOI] [PubMed] [Google Scholar]
- Thimmappaya B., Weinberger C., Schneider R. J., Shenk T. Adenovirus VAI RNA is required for efficient translation of viral mRNAs at late times after infection. Cell. 1982 Dec;31(3 Pt 2):543–551. doi: 10.1016/0092-8674(82)90310-5. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka K., Nagata K., Ishihama A. Temporal control for translation of influenza virus mRNAs. Arch Virol. 1991;120(1-2):33–42. doi: 10.1007/BF01310947. [DOI] [PubMed] [Google Scholar]
- Zapata J. M., Maroto F. G., Sierra J. M. Inactivation of mRNA cap-binding protein complex in Drosophila melanogaster embryos under heat shock. J Biol Chem. 1991 Aug 25;266(24):16007–16014. [PubMed] [Google Scholar]
- Zhang Y., Dolph P. J., Schneider R. J. Secondary structure analysis of adenovirus tripartite leader. J Biol Chem. 1989 Jun 25;264(18):10679–10684. [PubMed] [Google Scholar]