Abstract
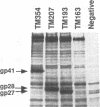
We have investigated how truncation of the cytoplasmic domain of the transmembrane (TM) glycoprotein of simian immunodeficiency virus (SIV) modulates the host range of this virus. Termination codons were introduced into the env gene of SIVmac239 which resulted in the truncation of the transmembrane protein from a wild-type 354 amino acids (TM354) to 207 (TM207) and 193 (TM193) amino acids. Expression of the wild-type and mutant env genes from a simian virus 40-based vector resulted in normal biosynthesis and processing of the glycoproteins to gp130 and gp41 or the truncated TM proteins (gp28 and gp27). When expressed on the surface of COS-1 cells, all three glycoproteins mediated fusion of both CEMX174 and HUT78 cells. Virions containing the wild-type and mutant glycoproteins were capable of efficient replication in macaque peripheral blood lymphocytes and CEMX174 cells; in contrast, only virions that contained TM207 were capable of rapid infection of HUT78 cells. Both truncated glycoproteins were capable of efficiently mediating infection of both CEMX174 and HUT78 cells by an env-deficient human immunodeficiency virus. The wild-type SIV glycoprotein, however, was unable to mediate human immunodeficiency virus infection of HUT78 cells when assayed with this system. An analysis of the protein composition of SIV released from infected CEMX174 cells showed that the mutant virions contained significantly higher levels of glycoprotein compared with the wild type. These results demonstrate that truncation of the SIV cytoplasmic domain removes a block at the level of glycoprotein-mediated virus entry into HUT78 cells and points to a role for glycoprotein density in determining virus tropism.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albert J., Bredberg U., Chiodi F., Böttiger B., Fenyö E. M., Norrby E., Biberfeld G. A new human retrovirus isolate of West African origin (SBL-6669) and its relationship to HTLV-IV, LAV-II, and HTLV-IIIB. AIDS Res Hum Retroviruses. 1987 Spring;3(1):3–10. doi: 10.1089/aid.1987.3.3. [DOI] [PubMed] [Google Scholar]
- Benveniste R. E., Arthur L. O., Tsai C. C., Sowder R., Copeland T. D., Henderson L. E., Oroszlan S. Isolation of a lentivirus from a macaque with lymphoma: comparison with HTLV-III/LAV and other lentiviruses. J Virol. 1986 Nov;60(2):483–490. doi: 10.1128/jvi.60.2.483-490.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti L., Emerman M., Tiollais P., Sonigo P. The cytoplasmic domain of simian immunodeficiency virus transmembrane protein modulates infectivity. J Virol. 1989 Oct;63(10):4395–4403. doi: 10.1128/jvi.63.10.4395-4403.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti L., Guyader M., Alizon M., Daniel M. D., Desrosiers R. C., Tiollais P., Sonigo P. Sequence of simian immunodeficiency virus from macaque and its relationship to other human and simian retroviruses. Nature. 1987 Aug 6;328(6130):543–547. doi: 10.1038/328543a0. [DOI] [PubMed] [Google Scholar]
- Daniel M. D., Letvin N. L., King N. W., Kannagi M., Sehgal P. K., Hunt R. D., Kanki P. J., Essex M., Desrosiers R. C. Isolation of T-cell tropic HTLV-III-like retrovirus from macaques. Science. 1985 Jun 7;228(4704):1201–1204. doi: 10.1126/science.3159089. [DOI] [PubMed] [Google Scholar]
- Daniel M. D., Li Y., Naidu Y. M., Durda P. J., Schmidt D. K., Troup C. D., Silva D. P., MacKey J. J., Kestler H. W., 3rd, Sehgal P. K. Simian immunodeficiency virus from African green monkeys. J Virol. 1988 Nov;62(11):4123–4128. doi: 10.1128/jvi.62.11.4123-4128.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubay J. W., Roberts S. J., Brody B., Hunter E. Mutations in the leucine zipper of the human immunodeficiency virus type 1 transmembrane glycoprotein affect fusion and infectivity. J Virol. 1992 Aug;66(8):4748–4756. doi: 10.1128/jvi.66.8.4748-4756.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubay J. W., Roberts S. J., Hahn B. H., Hunter E. Truncation of the human immunodeficiency virus type 1 transmembrane glycoprotein cytoplasmic domain blocks virus infectivity. J Virol. 1992 Nov;66(11):6616–6625. doi: 10.1128/jvi.66.11.6616-6625.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans L. A., Moreau J., Odehouri K., Legg H., Barboza A., Cheng-Mayer C., Levy J. A. Characterization of a noncytopathic HIV-2 strain with unusual effects on CD4 expression. Science. 1988 Jun 10;240(4858):1522–1525. doi: 10.1126/science.2836951. [DOI] [PubMed] [Google Scholar]
- Franchini G., Gurgo C., Guo H. G., Gallo R. C., Collalti E., Fargnoli K. A., Hall L. F., Wong-Staal F., Reitz M. S., Jr Sequence of simian immunodeficiency virus and its relationship to the human immunodeficiency viruses. Nature. 1987 Aug 6;328(6130):539–543. doi: 10.1038/328539a0. [DOI] [PubMed] [Google Scholar]
- Fukasawa M., Miura T., Hasegawa A., Morikawa S., Tsujimoto H., Miki K., Kitamura T., Hayami M. Sequence of simian immunodeficiency virus from African green monkey, a new member of the HIV/SIV group. Nature. 1988 Jun 2;333(6172):457–461. doi: 10.1038/333457a0. [DOI] [PubMed] [Google Scholar]
- Fultz P. N., McClure H. M., Anderson D. C., Swenson R. B., Anand R., Srinivasan A. Isolation of a T-lymphotropic retrovirus from naturally infected sooty mangabey monkeys (Cercocebus atys). Proc Natl Acad Sci U S A. 1986 Jul;83(14):5286–5290. doi: 10.1073/pnas.83.14.5286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyader M., Emerman M., Sonigo P., Clavel F., Montagnier L., Alizon M. Genome organization and transactivation of the human immunodeficiency virus type 2. Nature. 1987 Apr 16;326(6114):662–669. doi: 10.1038/326662a0. [DOI] [PubMed] [Google Scholar]
- Helseth E., Kowalski M., Gabuzda D., Olshevsky U., Haseltine W., Sodroski J. Rapid complementation assays measuring replicative potential of human immunodeficiency virus type 1 envelope glycoprotein mutants. J Virol. 1990 May;64(5):2416–2420. doi: 10.1128/jvi.64.5.2416-2420.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch V. M., Edmondson P., Murphey-Corb M., Arbeille B., Johnson P. R., Mullins J. I. SIV adaptation to human cells. Nature. 1989 Oct 19;341(6243):573–574. doi: 10.1038/341573a0. [DOI] [PubMed] [Google Scholar]
- Hirsch V., Riedel N., Mullins J. I. The genome organization of STLV-3 is similar to that of the AIDS virus except for a truncated transmembrane protein. Cell. 1987 May 8;49(3):307–319. doi: 10.1016/0092-8674(87)90283-2. [DOI] [PubMed] [Google Scholar]
- Kannagi M., Yetz J. M., Letvin N. L. In vitro growth characteristics of simian T-lymphotropic virus type III. Proc Natl Acad Sci U S A. 1985 Oct;82(20):7053–7057. doi: 10.1073/pnas.82.20.7053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama T., Wooley D. P., Naidu Y. M., Kestler H. W., 3rd, Daniel M. D., Li Y., Desrosiers R. C. Significance of premature stop codons in env of simian immunodeficiency virus. J Virol. 1989 Nov;63(11):4709–4714. doi: 10.1128/jvi.63.11.4709-4714.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong L. I., Lee S. W., Kappes J. C., Parkin J. S., Decker D., Hoxie J. A., Hahn B. H., Shaw G. M. West African HIV-2-related human retrovirus with attenuated cytopathicity. Science. 1988 Jun 10;240(4858):1525–1529. doi: 10.1126/science.3375832. [DOI] [PubMed] [Google Scholar]
- Kornfeld H., Riedel N., Viglianti G. A., Hirsch V., Mullins J. I. Cloning of HTLV-4 and its relation to simian and human immunodeficiency viruses. Nature. 1987 Apr 9;326(6113):610–613. doi: 10.1038/326610a0. [DOI] [PubMed] [Google Scholar]
- Letvin N. L., Daniel M. D., Sehgal P. K., Desrosiers R. C., Hunt R. D., Waldron L. M., MacKey J. J., Schmidt D. K., Chalifoux L. V., King N. W. Induction of AIDS-like disease in macaque monkeys with T-cell tropic retrovirus STLV-III. Science. 1985 Oct 4;230(4721):71–73. doi: 10.1126/science.2412295. [DOI] [PubMed] [Google Scholar]
- Lowenstine L. J., Pedersen N. C., Higgins J., Pallis K. C., Uyeda A., Marx P., Lerche N. W., Munn R. J., Gardner M. B. Seroepidemiologic survey of captive Old-World primates for antibodies to human and simian retroviruses, and isolation of a lentivirus from sooty mangabeys (Cercocebus atys). Int J Cancer. 1986 Oct 15;38(4):563–574. doi: 10.1002/ijc.2910380417. [DOI] [PubMed] [Google Scholar]
- Mulligan M. J., Yamshchikov G. V., Ritter G. D., Jr, Gao F., Jin M. J., Nail C. D., Spies C. P., Hahn B. H., Compans R. W. Cytoplasmic domain truncation enhances fusion activity by the exterior glycoprotein complex of human immunodeficiency virus type 2 in selected cell types. J Virol. 1992 Jun;66(6):3971–3975. doi: 10.1128/jvi.66.6.3971-3975.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphey-Corb M., Martin L. N., Rangan S. R., Baskin G. B., Gormus B. J., Wolf R. H., Andes W. A., West M., Montelaro R. C. Isolation of an HTLV-III-related retrovirus from macaques with simian AIDS and its possible origin in asymptomatic mangabeys. Nature. 1986 May 22;321(6068):435–437. doi: 10.1038/321435a0. [DOI] [PubMed] [Google Scholar]
- Naidu Y. M., Kestler H. W., 3rd, Li Y., Butler C. V., Silva D. P., Schmidt D. K., Troup C. D., Sehgal P. K., Sonigo P., Daniel M. D. Characterization of infectious molecular clones of simian immunodeficiency virus (SIVmac) and human immunodeficiency virus type 2: persistent infection of rhesus monkeys with molecularly cloned SIVmac. J Virol. 1988 Dec;62(12):4691–4696. doi: 10.1128/jvi.62.12.4691-4696.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta Y., Masuda T., Tsujimoto H., Ishikawa K., Kodama T., Morikawa S., Nakai M., Honjo S., Hayami M. Isolation of simian immunodeficiency virus from African green monkeys and seroepidemiologic survey of the virus in various non-human primates. Int J Cancer. 1988 Jan 15;41(1):115–122. doi: 10.1002/ijc.2910410121. [DOI] [PubMed] [Google Scholar]
- Shimizu H., Morikawa S., Yamaguchi K., Tsuchie H., Hachimori K., Ushijima H., Kitamura T. Shorter size of transmembrane glycoprotein of an HIV-1 isolate. AIDS. 1990 Jun;4(6):575–576. doi: 10.1097/00002030-199006000-00013. [DOI] [PubMed] [Google Scholar]
- Simon M. A., Chalifoux L. V., Ringler D. J. Pathologic features of SIV-induced disease and the association of macrophage infection with disease evolution. AIDS Res Hum Retroviruses. 1992 Mar;8(3):327–337. doi: 10.1089/aid.1992.8.327. [DOI] [PubMed] [Google Scholar]
- Sodroski J. G., Rosen C. A., Haseltine W. A. Trans-acting transcriptional activation of the long terminal repeat of human T lymphotropic viruses in infected cells. Science. 1984 Jul 27;225(4660):381–385. doi: 10.1126/science.6330891. [DOI] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Tsujimoto H., Cooper R. W., Kodama T., Fukasawa M., Miura T., Ohta Y., Ishikawa K., Nakai M., Frost E., Roelants G. E. Isolation and characterization of simian immunodeficiency virus from mandrills in Africa and its relationship to other human and simian immunodeficiency viruses. J Virol. 1988 Nov;62(11):4044–4050. doi: 10.1128/jvi.62.11.4044-4050.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]