Abstract
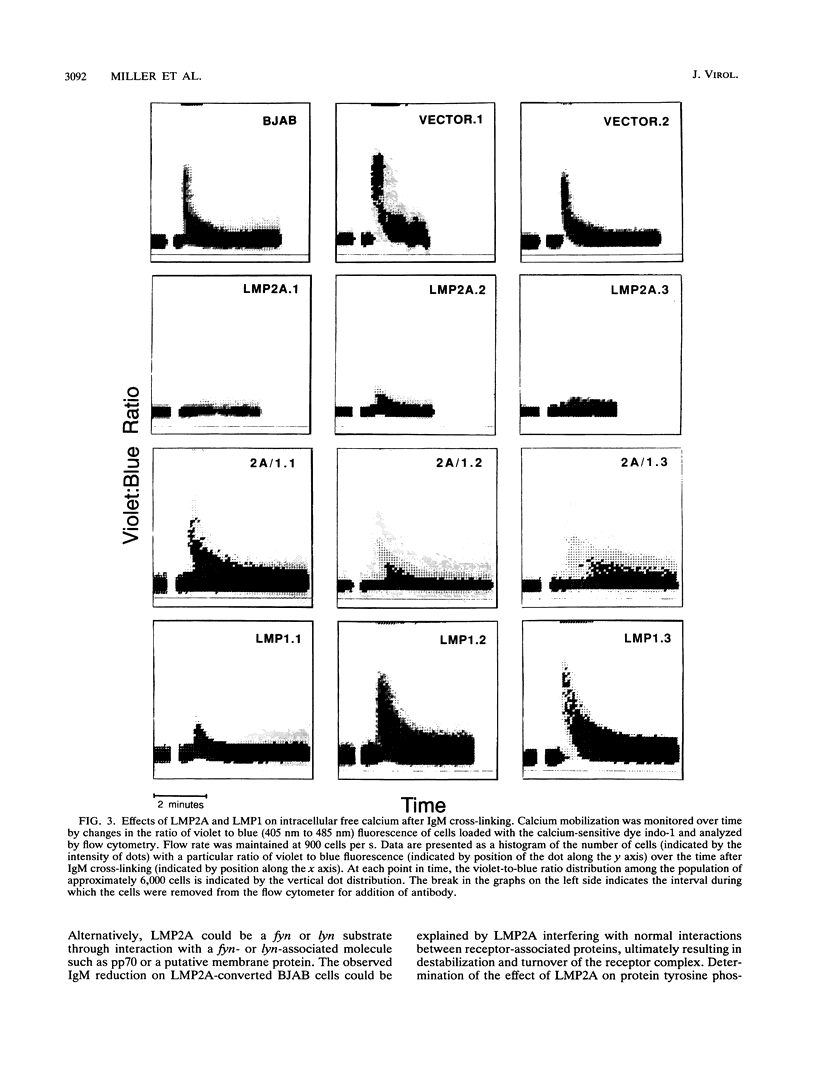
LMP2A is expressed in latent Epstein-Barr virus (EBV) infection and interacts with LMP1 and members of the src tyrosine kinase family in the plasma membrane. Since tyrosine kinase mediate receptor-induced changes in intracellular free calcium, the effect of LMP2A on receptor-mediated intracellular calcium mobilization was evaluated by stably expressing LMP2A in an EBV-negative Burkitt tumor cell line (BJAB) or in LMP1-converted BJAB cells. LMP2A significantly blocked calcium mobilization following class II, CD19, or immunoglobulin M cross-linking. LMP2A effects were partially reversed in LMP1-converted cell lines. These results are compatible with LMP2A acting in latent B-lymphocyte infection to downmodulate LMP1 effects on cell growth or to inhibit induction of lytic EBV infection in specific human tissues following receptor ligation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baichwal V. R., Sugden B. Transformation of Balb 3T3 cells by the BNLF-1 gene of Epstein-Barr virus. Oncogene. 1988 May;2(5):461–467. [PubMed] [Google Scholar]
- Brooks L., Yao Q. Y., Rickinson A. B., Young L. S. Epstein-Barr virus latent gene transcription in nasopharyngeal carcinoma cells: coexpression of EBNA1, LMP1, and LMP2 transcripts. J Virol. 1992 May;66(5):2689–2697. doi: 10.1128/jvi.66.5.2689-2697.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunswick M., Samelson L. E., Mond J. J. Surface immunoglobulin crosslinking activates a tyrosine kinase pathway in B cells that is independent of protein kinase C. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1311–1314. doi: 10.1073/pnas.88.4.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhardt A. L., Bolen J. B., Kieff E., Longnecker R. An Epstein-Barr virus transformation-associated membrane protein interacts with src family tyrosine kinases. J Virol. 1992 Aug;66(8):5161–5167. doi: 10.1128/jvi.66.8.5161-5167.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhardt A. L., Brunswick M., Bolen J. B., Mond J. J. Anti-immunoglobulin stimulation of B lymphocytes activates src-related protein-tyrosine kinases. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7410–7414. doi: 10.1073/pnas.88.16.7410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busson P., McCoy R., Sadler R., Gilligan K., Tursz T., Raab-Traub N. Consistent transcription of the Epstein-Barr virus LMP2 gene in nasopharyngeal carcinoma. J Virol. 1992 May;66(5):3257–3262. doi: 10.1128/jvi.66.5.3257-3262.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambier J. C., Campbell K. S. Membrane immunoglobulin and its accomplices: new lessons from an old receptor. FASEB J. 1992 Oct;6(13):3207–3217. doi: 10.1096/fasebj.6.13.1397843. [DOI] [PubMed] [Google Scholar]
- Cambier J. C., Justement L. B., Newell M. K., Chen Z. Z., Harris L. K., Sandoval V. M., Klemsz M. J., Ransom J. T. Transmembrane signals and intracellular "second messengers" in the regulation of quiescent B-lymphocyte activation. Immunol Rev. 1987 Feb;95:37–57. doi: 10.1111/j.1600-065x.1987.tb00499.x. [DOI] [PubMed] [Google Scholar]
- Campbell K. S., Cambier J. C. B lymphocyte antigen receptors (mIg) are non-covalently associated with a disulfide linked, inducibly phosphorylated glycoprotein complex. EMBO J. 1990 Feb;9(2):441–448. doi: 10.1002/j.1460-2075.1990.tb08129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell M. A., Sefton B. M. Protein tyrosine phosphorylation is induced in murine B lymphocytes in response to stimulation with anti-immunoglobulin. EMBO J. 1990 Jul;9(7):2125–2131. doi: 10.1002/j.1460-2075.1990.tb07381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter R. H., Park D. J., Rhee S. G., Fearon D. T. Tyrosine phosphorylation of phospholipase C induced by membrane immunoglobulin in B lymphocytes. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2745–2749. doi: 10.1073/pnas.88.7.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang H. C., Moingeon P., Lopez P., Krasnow H., Stebbins C., Reinherz E. L. Dissection of the human CD2 intracellular domain. Identification of a segment required for signal transduction and interleukin 2 production. J Exp Med. 1989 Jun 1;169(6):2073–2083. doi: 10.1084/jem.169.6.2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. Z., Stall A. M., Herzenberg L. A., Herzenberg L. A. Differences in glycoprotein complexes associated with IgM and IgD on normal murine B cells potentially enable transduction of different signals. EMBO J. 1990 Jul;9(7):2117–2124. doi: 10.1002/j.1460-2075.1990.tb07380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z. Z., Coggeshall K. M., Cambier J. C. Translocation of protein kinase C during membrane immunoglobulin-mediated transmembrane signaling in B lymphocytes. J Immunol. 1986 Mar 15;136(6):2300–2304. [PubMed] [Google Scholar]
- Coggeshall K. M., McHugh J. C., Altman A. Predominant expression and activation-induced tyrosine phosphorylation of phospholipase C-gamma 2 in B lymphocytes. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5660–5664. doi: 10.1073/pnas.89.12.5660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daibata M., Humphreys R. E., Takada K., Sairenji T. Activation of latent EBV via anti-IgG-triggered, second messenger pathways in the Burkitt's lymphoma cell line Akata. J Immunol. 1990 Jun 15;144(12):4788–4793. [PubMed] [Google Scholar]
- DeFranco A. L., Gold M. R., Jakway J. P. B-lymphocyte signal transduction in response to anti-immunoglobulin and bacterial lipopolysaccharide. Immunol Rev. 1987 Feb;95:161–176. doi: 10.1111/j.1600-065x.1987.tb00504.x. [DOI] [PubMed] [Google Scholar]
- Delsol G., Brousset P., Chittal S., Rigal-Huguet F. Correlation of the expression of Epstein-Barr virus latent membrane protein and in situ hybridization with biotinylated BamHI-W probes in Hodgkin's disease. Am J Pathol. 1992 Feb;140(2):247–253. [PMC free article] [PubMed] [Google Scholar]
- Gold M. R., Law D. A., DeFranco A. L. Stimulation of protein tyrosine phosphorylation by the B-lymphocyte antigen receptor. Nature. 1990 Jun 28;345(6278):810–813. doi: 10.1038/345810a0. [DOI] [PubMed] [Google Scholar]
- Harabuchi Y., Yamanaka N., Kataura A., Imai S., Kinoshita T., Mizuno F., Osato T. Epstein-Barr virus in nasal T-cell lymphomas in patients with lethal midline granuloma. Lancet. 1990 Jan 20;335(8682):128–130. doi: 10.1016/0140-6736(90)90002-m. [DOI] [PubMed] [Google Scholar]
- Henderson S., Rowe M., Gregory C., Croom-Carter D., Wang F., Longnecker R., Kieff E., Rickinson A. Induction of bcl-2 expression by Epstein-Barr virus latent membrane protein 1 protects infected B cells from programmed cell death. Cell. 1991 Jun 28;65(7):1107–1115. doi: 10.1016/0092-8674(91)90007-l. [DOI] [PubMed] [Google Scholar]
- Heston L., Rabson M., Brown N., Miller G. New Epstein-Barr virus variants from cellular subclones of P3J-HR-1 Burkitt lymphoma. Nature. 1982 Jan 14;295(5845):160–163. doi: 10.1038/295160a0. [DOI] [PubMed] [Google Scholar]
- Hombach J., Leclercq L., Radbruch A., Rajewsky K., Reth M. A novel 34-kd protein co-isolated with the IgM molecule in surface IgM-expressing cells. EMBO J. 1988 Nov;7(11):3451–3456. doi: 10.1002/j.1460-2075.1988.tb03219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hombach J., Tsubata T., Leclercq L., Stappert H., Reth M. Molecular components of the B-cell antigen receptor complex of the IgM class. Nature. 1990 Feb 22;343(6260):760–762. doi: 10.1038/343760a0. [DOI] [PubMed] [Google Scholar]
- Hutchcroft J. E., Harrison M. L., Geahlen R. L. B lymphocyte activation is accompanied by phosphorylation of a 72-kDa protein-tyrosine kinase. J Biol Chem. 1991 Aug 15;266(23):14846–14849. [PubMed] [Google Scholar]
- Lane P. J., McConnell F. M., Schieven G. L., Clark E. A., Ledbetter J. A. The role of class II molecules in human B cell activation. Association with phosphatidyl inositol turnover, protein tyrosine phosphorylation, and proliferation. J Immunol. 1990 May 15;144(10):3684–3692. [PubMed] [Google Scholar]
- Laux G., Economou A., Farrell P. J. The terminal protein gene 2 of Epstein-Barr virus is transcribed from a bidirectional latent promoter region. J Gen Virol. 1989 Nov;70(Pt 11):3079–3084. doi: 10.1099/0022-1317-70-11-3079. [DOI] [PubMed] [Google Scholar]
- Ledbetter J. A., Rabinovitch P. S., June C. H., Song C. W., Clark E. A., Uckun F. M. Antigen-independent regulation of cytoplasmic calcium in B cells with a 12-kDa B-cell growth factor and anti-CD19. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1897–1901. doi: 10.1073/pnas.85.6.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebowitz D., Kieff E. Epstein-Barr virus latent membrane protein: induction of B-cell activation antigens and membrane patch formation does not require vimentin. J Virol. 1989 Sep;63(9):4051–4054. doi: 10.1128/jvi.63.9.4051-4054.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longnecker R., Druker B., Roberts T. M., Kieff E. An Epstein-Barr virus protein associated with cell growth transformation interacts with a tyrosine kinase. J Virol. 1991 Jul;65(7):3681–3692. doi: 10.1128/jvi.65.7.3681-3692.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longnecker R., Kieff E. A second Epstein-Barr virus membrane protein (LMP2) is expressed in latent infection and colocalizes with LMP1. J Virol. 1990 May;64(5):2319–2326. doi: 10.1128/jvi.64.5.2319-2326.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longnecker R., Miller C. L., Miao X. Q., Marchini A., Kieff E. The only domain which distinguishes Epstein-Barr virus latent membrane protein 2A (LMP2A) from LMP2B is dispensable for lymphocyte infection and growth transformation in vitro; LMP2A is therefore nonessential. J Virol. 1992 Nov;66(11):6461–6469. doi: 10.1128/jvi.66.11.6461-6469.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longnecker R., Miller C. L., Miao X. Q., Tomkinson B., Kieff E. The last seven transmembrane and carboxy-terminal cytoplasmic domains of Epstein-Barr virus latent membrane protein 2 (LMP2) are dispensable for lymphocyte infection and growth transformation in vitro. J Virol. 1993 Apr;67(4):2006–2013. doi: 10.1128/jvi.67.4.2006-2013.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menezes J., Leibold W., Klein G., Clements G. Establishment and characterization of an Epstein-Barr virus (EBC)-negative lymphoblastoid B cell line (BJA-B) from an exceptional, EBV-genome-negative African Burkitt's lymphoma. Biomedicine. 1975 Jul;22(4):276–284. [PubMed] [Google Scholar]
- Murray R. J., Kurilla M. G., Brooks J. M., Thomas W. A., Rowe M., Kieff E., Rickinson A. B. Identification of target antigens for the human cytotoxic T cell response to Epstein-Barr virus (EBV): implications for the immune control of EBV-positive malignancies. J Exp Med. 1992 Jul 1;176(1):157–168. doi: 10.1084/jem.176.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nel A. E., Wooten M. W., Landreth G. E., Goldschmidt-Clermont P. J., Stevenson H. C., Miller P. J., Galbraith R. M. Translocation of phospholipid/Ca2+-dependent protein kinase in B-lymphocytes activated by phorbol ester or cross-linking of membrane immunoglobulin. Biochem J. 1986 Jan 1;233(1):145–149. doi: 10.1042/bj2330145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkhouse R. M. Three B-cell surface molecules associating with membrane immunoglobulin. Immunology. 1990 Feb;69(2):298–302. [PMC free article] [PubMed] [Google Scholar]
- Partain K., Jensen K., Aldo-Benson M. Inositol phospholipid and intracellular calcium metabolism in B lymphocytes stimulated with antigen. Biochem Biophys Res Commun. 1986 Nov 14;140(3):1079–1085. doi: 10.1016/0006-291x(86)90745-x. [DOI] [PubMed] [Google Scholar]
- Pezzutto A., Dörken B., Moldenhauer G., Clark E. A. Amplification of human B cell activation by a monoclonal antibody to the B cell-specific antigen CD22, Bp 130/140. J Immunol. 1987 Jan 1;138(1):98–103. [PubMed] [Google Scholar]
- Qu L., Rowe D. T. Epstein-Barr virus latent gene expression in uncultured peripheral blood lymphocytes. J Virol. 1992 Jun;66(6):3715–3724. doi: 10.1128/jvi.66.6.3715-3724.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovitch P. S., June C. H., Grossmann A., Ledbetter J. A. Heterogeneity among T cells in intracellular free calcium responses after mitogen stimulation with PHA or anti-CD3. Simultaneous use of indo-1 and immunofluorescence with flow cytometry. J Immunol. 1986 Aug 1;137(3):952–961. [PubMed] [Google Scholar]
- Sample J., Liebowitz D., Kieff E. Two related Epstein-Barr virus membrane proteins are encoded by separate genes. J Virol. 1989 Feb;63(2):933–937. doi: 10.1128/jvi.63.2.933-937.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada K. Cross-linking of cell surface immunoglobulins induces Epstein-Barr virus in Burkitt lymphoma lines. Int J Cancer. 1984 Jan 15;33(1):27–32. doi: 10.1002/ijc.2910330106. [DOI] [PubMed] [Google Scholar]
- Wang D., Liebowitz D., Kieff E. An EBV membrane protein expressed in immortalized lymphocytes transforms established rodent cells. Cell. 1985 Dec;43(3 Pt 2):831–840. doi: 10.1016/0092-8674(85)90256-9. [DOI] [PubMed] [Google Scholar]
- Wang D., Liebowitz D., Kieff E. The truncated form of the Epstein-Barr virus latent-infection membrane protein expressed in virus replication does not transform rodent fibroblasts. J Virol. 1988 Jul;62(7):2337–2346. doi: 10.1128/jvi.62.7.2337-2346.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Liebowitz D., Wang F., Gregory C., Rickinson A., Larson R., Springer T., Kieff E. Epstein-Barr virus latent infection membrane protein alters the human B-lymphocyte phenotype: deletion of the amino terminus abolishes activity. J Virol. 1988 Nov;62(11):4173–4184. doi: 10.1128/jvi.62.11.4173-4184.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F., Gregory C., Sample C., Rowe M., Liebowitz D., Murray R., Rickinson A., Kieff E. Epstein-Barr virus latent membrane protein (LMP1) and nuclear proteins 2 and 3C are effectors of phenotypic changes in B lymphocytes: EBNA-2 and LMP1 cooperatively induce CD23. J Virol. 1990 May;64(5):2309–2318. doi: 10.1128/jvi.64.5.2309-2318.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanashi Y., Fukui Y., Wongsasant B., Kinoshita Y., Ichimori Y., Toyoshima K., Yamamoto T. Activation of Src-like protein-tyrosine kinase Lyn and its association with phosphatidylinositol 3-kinase upon B-cell antigen receptor-mediated signaling. Proc Natl Acad Sci U S A. 1992 Feb 1;89(3):1118–1122. doi: 10.1073/pnas.89.3.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanashi Y., Kakiuchi T., Mizuguchi J., Yamamoto T., Toyoshima K. Association of B cell antigen receptor with protein tyrosine kinase Lyn. Science. 1991 Jan 11;251(4990):192–194. doi: 10.1126/science.1702903. [DOI] [PubMed] [Google Scholar]
- Yang Z., Korman A. J., Cooper J., Pious D., Accolla R. S., Mulligan R. C., Strominger J. L. Expression of HLA-DR antigen in human class II mutant B-cell lines by double infection with retrovirus vectors. Mol Cell Biol. 1987 Nov;7(11):3923–3928. doi: 10.1128/mcb.7.11.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]