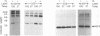Abstract
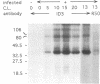
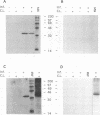
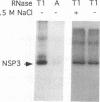
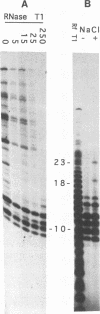
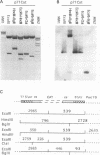
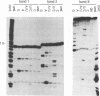
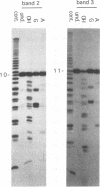
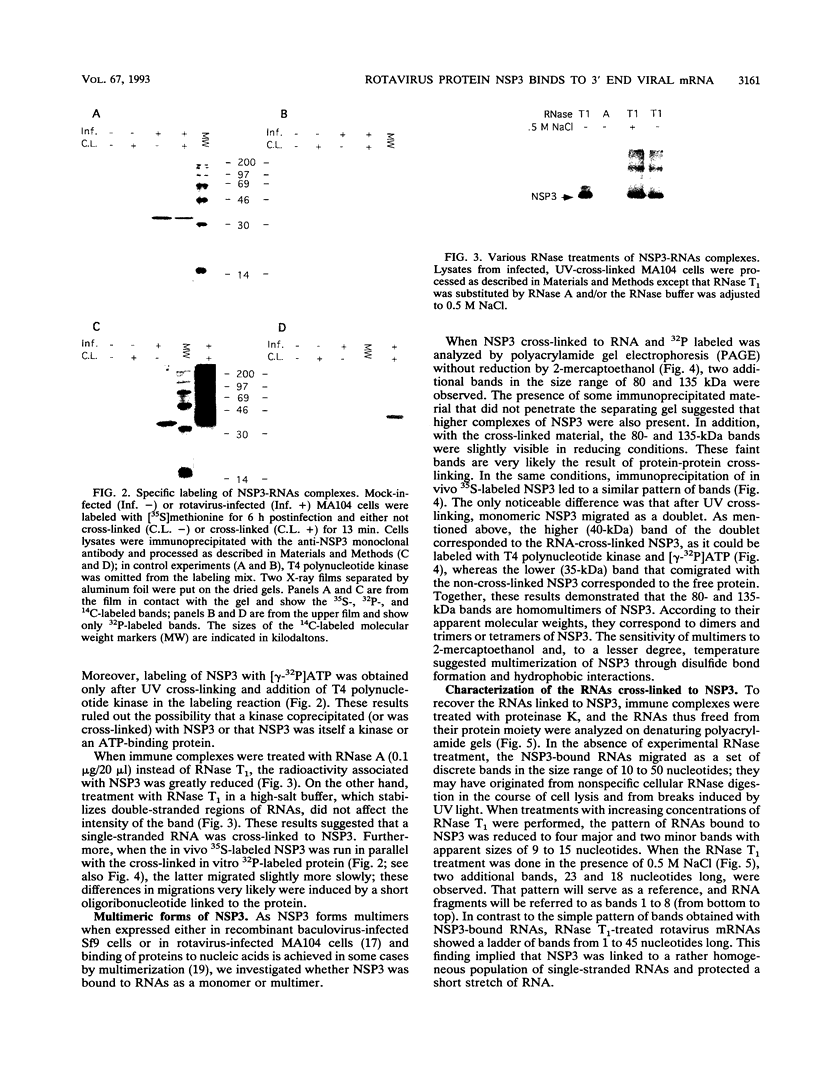
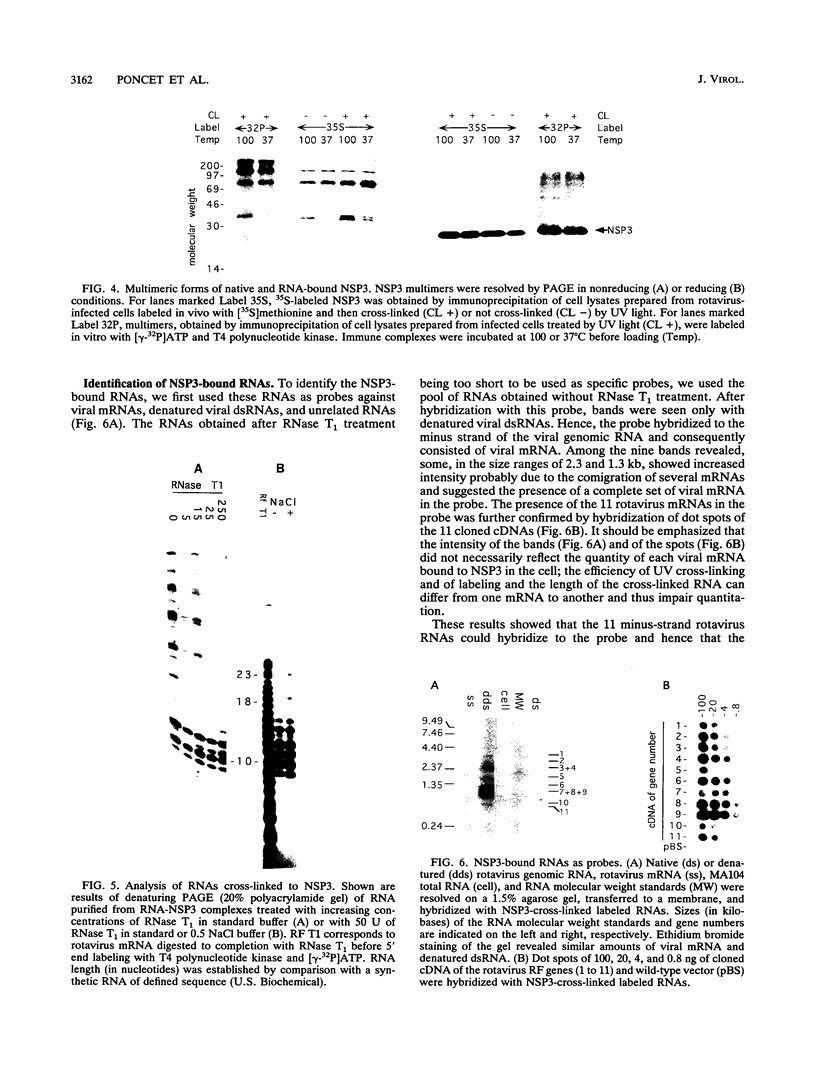
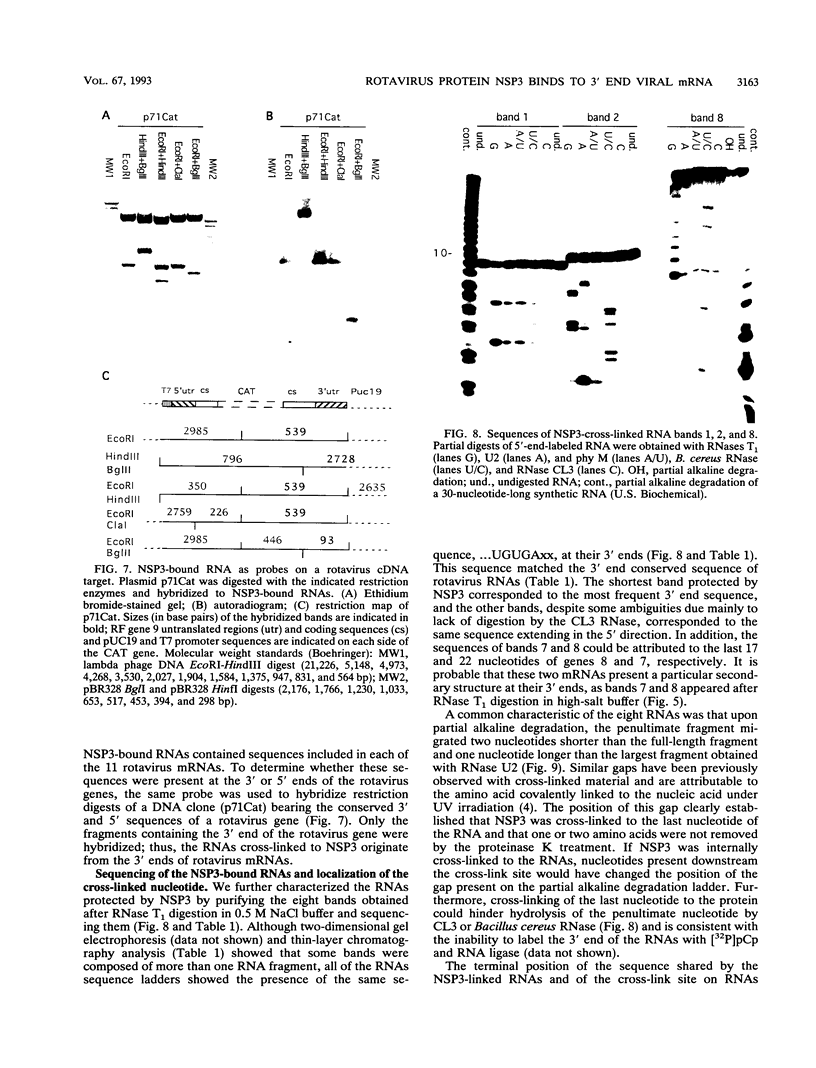
Interaction between viral proteins and RNAs has been studied in rotavirus-infected cells. The use of UV cross-linking followed by immunoprecipitation and labeling with T4 polynucleotide kinase allowed us to detect interactions between RNA and nonstructural viral proteins. The RNAs linked to the nonstructural protein NSP3 have been identified as rotavirus mRNAs, and the sequences of the RNase T1-protected fragments have been established. These sequences correspond to the 3' end sequence common to all rotavirus group A genes. We also show that the last 3' nucleotide is cross-linked to the protein and that monomeric and multimeric forms of NSP3 are bound to rotavirus mRNA. The role of NSP3 in rotavirus replication is discussed in the light of our results and by comparison with other RNA-binding proteins of members of the Reoviridae family.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antczak J. B., Joklik W. K. Reovirus genome segment assortment into progeny genomes studied by the use of monoclonal antibodies directed against reovirus proteins. Virology. 1992 Apr;187(2):760–776. doi: 10.1016/0042-6822(92)90478-8. [DOI] [PubMed] [Google Scholar]
- Boyle J. F., Holmes K. V. RNA-binding proteins of bovine rotavirus. J Virol. 1986 May;58(2):561–568. doi: 10.1128/jvi.58.2.561-568.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brottier P., Nandi P., Bremont M., Cohen J. Bovine rotavirus segment 5 protein expressed in the baculovirus system interacts with zinc and RNA. J Gen Virol. 1992 Aug;73(Pt 8):1931–1938. doi: 10.1099/0022-1317-73-8-1931. [DOI] [PubMed] [Google Scholar]
- Budowsky E. I., Abdurashidova G. G. Polynucleotide-protein cross-links induced by ultraviolet light and their use for structural investigation of nucleoproteins. Prog Nucleic Acid Res Mol Biol. 1989;37:1–65. doi: 10.1016/s0079-6603(08)60694-7. [DOI] [PubMed] [Google Scholar]
- Cohen J., Lefevre F., Estes M. K., Bremont M. Cloning of bovine rotavirus (RF strain): nucleotide sequence of the gene coding for the major capsid protein. Virology. 1984 Oct 15;138(1):178–182. doi: 10.1016/0042-6822(84)90159-4. [DOI] [PubMed] [Google Scholar]
- Cohen J. Ribonucleic acid polymerase activity associated with purified calf rotavirus. J Gen Virol. 1977 Sep;36(3):395–402. doi: 10.1099/0022-1317-36-3-395. [DOI] [PubMed] [Google Scholar]
- Dall D. J., Anzola J. V., Xu Z. K., Nuss D. L. Structure-specific binding of wound tumor virus transcripts by a host factor: involvement of both terminal nucleotide domains. Virology. 1990 Dec;179(2):599–608. doi: 10.1016/0042-6822(90)90127-d. [DOI] [PubMed] [Google Scholar]
- Donis-Keller H. Site specific enzymatic cleavage of RNA. Nucleic Acids Res. 1979 Sep 11;7(1):179–192. doi: 10.1093/nar/7.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes M. K., Cohen J. Rotavirus gene structure and function. Microbiol Rev. 1989 Dec;53(4):410–449. doi: 10.1128/mr.53.4.410-449.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallegos C. O., Patton J. T. Characterization of rotavirus replication intermediates: a model for the assembly of single-shelled particles. Virology. 1989 Oct;172(2):616–627. doi: 10.1016/0042-6822(89)90204-3. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorziglia M. I., Collins P. L. Intracellular amplification and expression of a synthetic analog of rotavirus genomic RNA bearing a foreign marker gene: mapping cis-acting nucleotides in the 3'-noncoding region. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):5784–5788. doi: 10.1073/pnas.89.13.5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins C. F. Stability and degradation of mRNA. Curr Opin Cell Biol. 1991 Dec;3(6):1013–1018. doi: 10.1016/0955-0674(91)90122-f. [DOI] [PubMed] [Google Scholar]
- Kattoura M. D., Clapp L. L., Patton J. T. The rotavirus nonstructural protein, NS35, possesses RNA-binding activity in vitro and in vivo. Virology. 1992 Dec;191(2):698–708. doi: 10.1016/0042-6822(92)90245-k. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Mattion N. M., Cohen J., Aponte C., Estes M. K. Characterization of an oligomerization domain and RNA-binding properties on rotavirus nonstructural protein NS34. Virology. 1992 Sep;190(1):68–83. doi: 10.1016/0042-6822(92)91193-x. [DOI] [PubMed] [Google Scholar]
- Mitchell P. J., Tjian R. Transcriptional regulation in mammalian cells by sequence-specific DNA binding proteins. Science. 1989 Jul 28;245(4916):371–378. doi: 10.1126/science.2667136. [DOI] [PubMed] [Google Scholar]
- Patton J. T. Synthesis of simian rotavirus SA11 double-stranded RNA in a cell-free system. Virus Res. 1986 Dec;6(3):217–233. doi: 10.1016/0168-1702(86)90071-7. [DOI] [PubMed] [Google Scholar]
- Singer R. H. The cytoskeleton and mRNA localization. Curr Opin Cell Biol. 1992 Feb;4(1):15–19. doi: 10.1016/0955-0674(92)90053-f. [DOI] [PubMed] [Google Scholar]
- Stacy-Phipps S., Patton J. T. Synthesis of plus- and minus-strand RNA in rotavirus-infected cells. J Virol. 1987 Nov;61(11):3479–3484. doi: 10.1128/jvi.61.11.3479-3484.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatos N. M., Gomatos P. J. Binding to selected regions of reovirus mRNAs by a nonstructural reovirus protein. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3457–3461. doi: 10.1073/pnas.79.11.3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart L., Schatz G., Vogt V. M. Properties of avian retrovirus particles defective in viral protease. J Virol. 1990 Oct;64(10):5076–5092. doi: 10.1128/jvi.64.10.5076-5092.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Staden V., Theron J., Greyling B. J., Huismans H., Nel L. H. A comparison of the nucleotide sequences of cognate NS2 genes of three different orbiviruses. Virology. 1991 Nov;185(1):500–504. doi: 10.1016/0042-6822(91)90808-o. [DOI] [PubMed] [Google Scholar]