Abstract
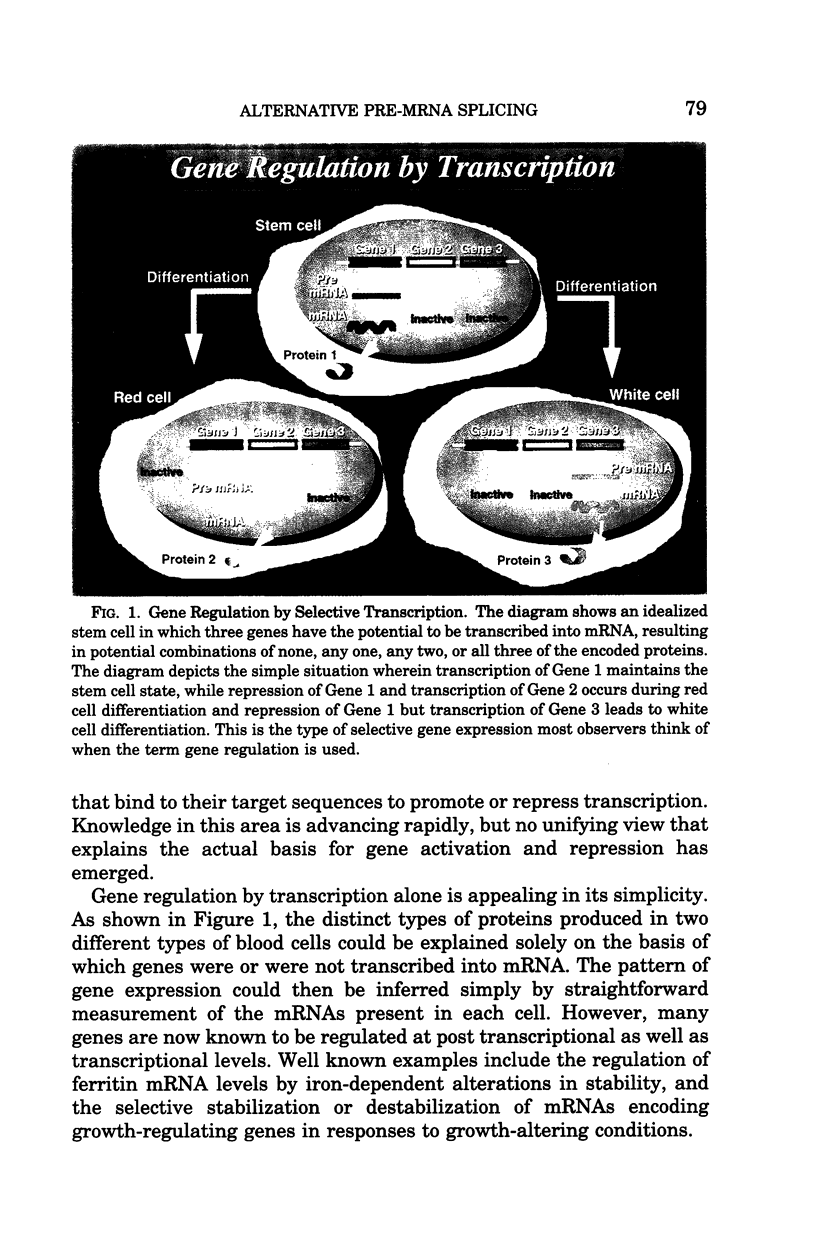
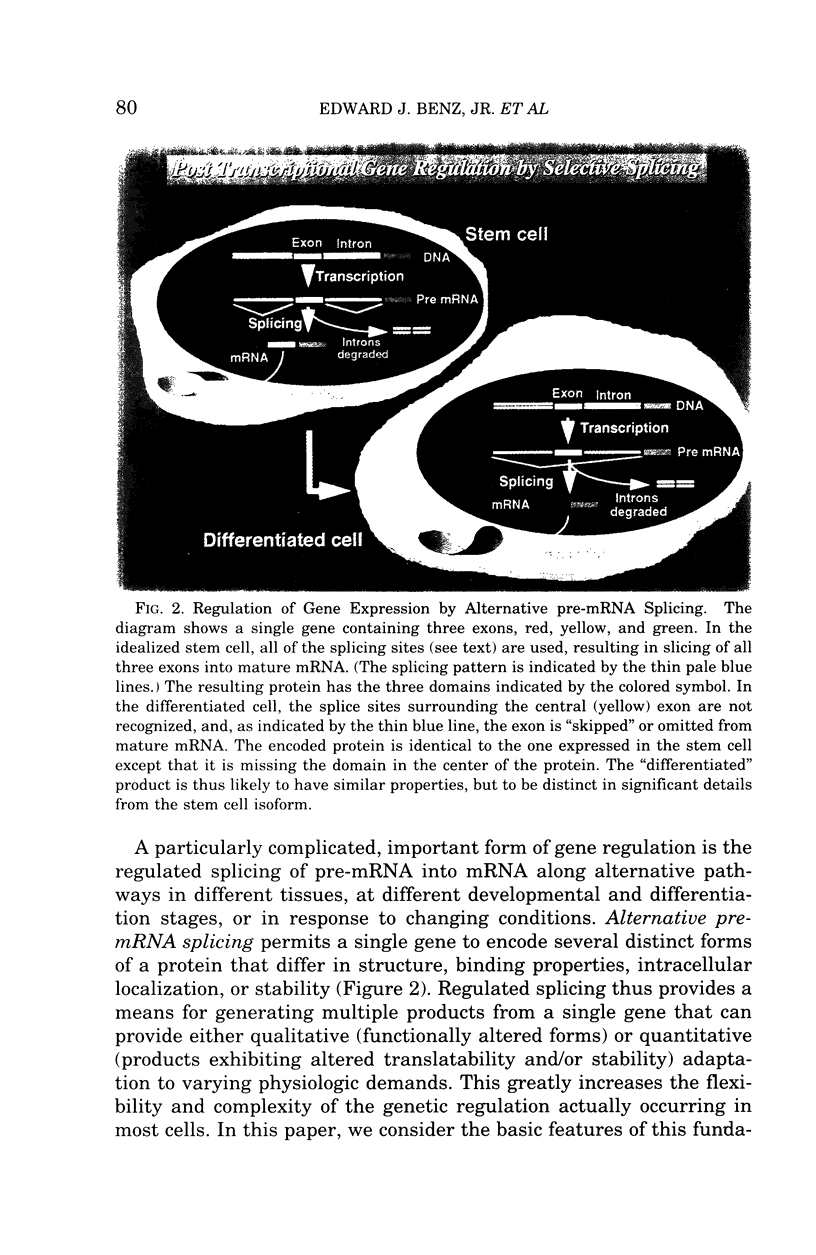
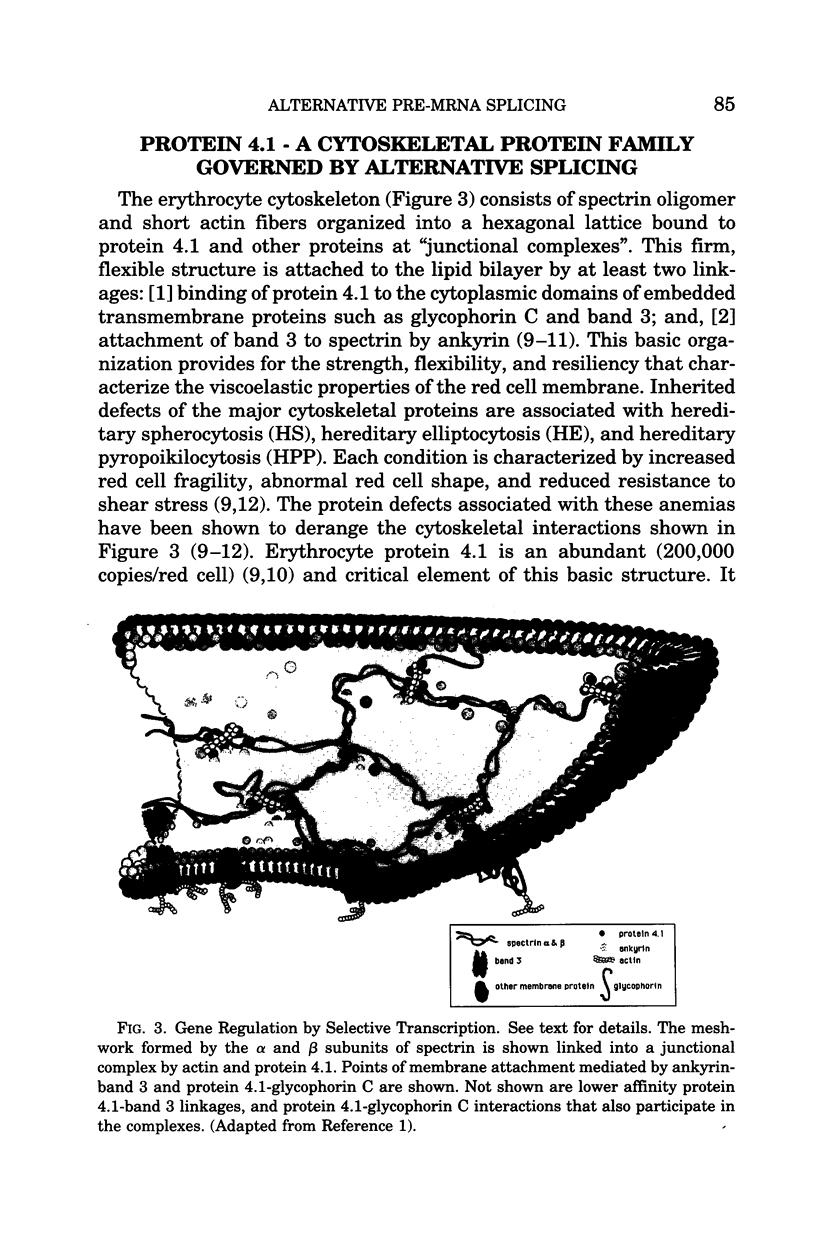
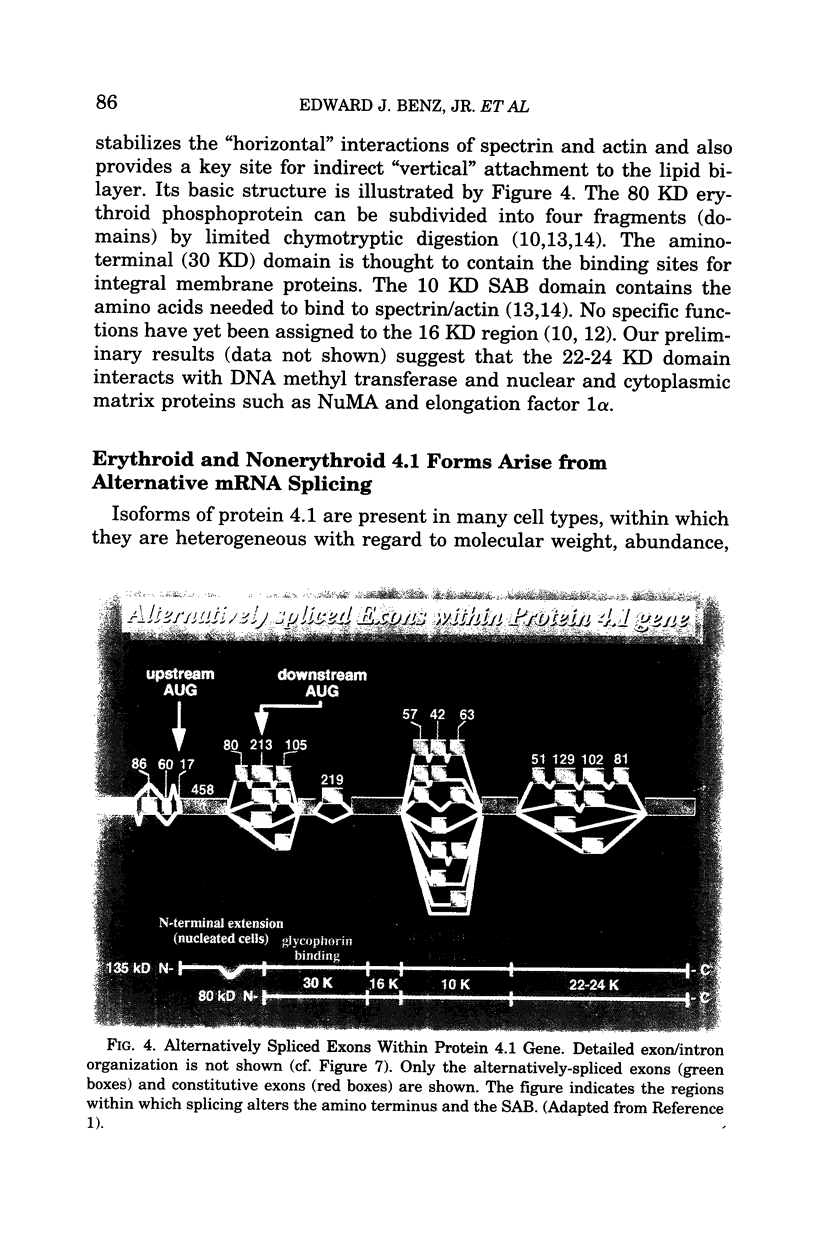
Regulated alternative pre-mRNA splicing is neither as widely appreciated as a fundamental aspect of controlled gene expression nor as thoroughly studied as transcriptional regulation. However, as exemplified by the phenomena cited in this review, alternative splicing is a fundamentally important mechanism used in the eukaryotic world to enhance the range, versatility and plasticity of the structural information contained within a gene, and to create additional strategies by which the net quantitative output of a given gene product can be controlled. Regulation of RNA splicing gives genes a modularity that adds flexibility, and, therefore, selective advantage, to eukaryotes. It is likely, though unproven, that this opportunity for refined regulation and diversification provides at least one basis for the existence of the tandem exon-intron-exon structure found in the vast majority of eukaryotic genes and many viral genes. Many examples of alternative splicing are known, but, for the majority, no obvious biological impact of the alternatively spliced proteins on known cellular functions can be appreciated. Examples by which selectively regulated splicing pathways alter both the physiology and pathology of a major cellular event, such as differentiation and mechanical function of the red cell membrane, are thus relatively rare. The protein 4.1 gene and mRNA products thus provide an instructive and unusual system in which to explore the broader issue of the role of these regulatory mechanisms in the overall scheme of gene regulation and adaptation. The fact that hereditary hemolytic anemias result from mutations that directly or indirectly disrupt the splicing system emphasized the relevance of these mechanisms to molecular medicine. The features of splicing that we have reviewed in this paper, and the specific impact that regulated splicing exerts on differentiating red cells have, we hope, convinced the reader that RNA splicing is an important, fascinating, and potentially fruitful area for future study of human disease processes.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R. A., Correas I., Mazzucco C., Castle J. D., Marchesi V. T. Tissue-specific analogues of erythrocyte protein 4.1 retain functional domains. J Cell Biochem. 1988 Jul;37(3):269–284. doi: 10.1002/jcb.240370303. [DOI] [PubMed] [Google Scholar]
- Baker B. S. Sex in flies: the splice of life. Nature. 1989 Aug 17;340(6234):521–524. doi: 10.1038/340521a0. [DOI] [PubMed] [Google Scholar]
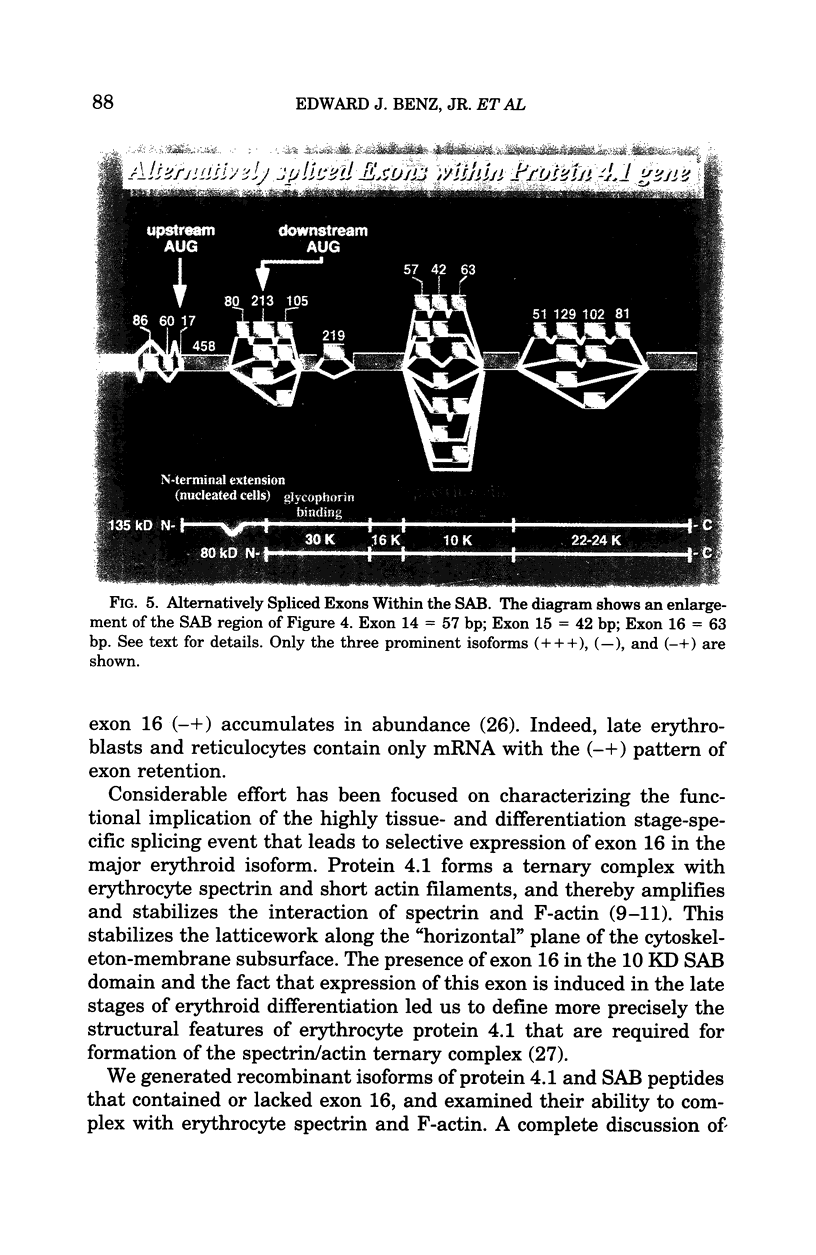
- Baklouti F., Huang S. C., Tang T. K., Delaunay J., Marchesi V. T., Benz E. J., Jr Asynchronous regulation of splicing events within protein 4.1 pre-mRNA during erythroid differentiation. Blood. 1996 May 1;87(9):3934–3941. [PubMed] [Google Scholar]
- Chasis J. A., Coulombel L., Conboy J., McGee S., Andrews K., Kan Y. W., Mohandas N. Differentiation-associated switches in protein 4.1 expression. Synthesis of multiple structural isoforms during normal human erythropoiesis. J Clin Invest. 1993 Jan;91(1):329–338. doi: 10.1172/JCI116189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen C. M., Foley S. F., Korsgren C. A protein immunologically related to erythrocyte band 4.1 is found on stress fibres on non-erythroid cells. Nature. 1982 Oct 14;299(5884):648–650. doi: 10.1038/299648a0. [DOI] [PubMed] [Google Scholar]
- Compton D. A., Cleveland D. W. NuMA is required for the proper completion of mitosis. J Cell Biol. 1993 Feb;120(4):947–957. doi: 10.1083/jcb.120.4.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conboy J. G., Chan J., Mohandas N., Kan Y. W. Multiple protein 4.1 isoforms produced by alternative splicing in human erythroid cells. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9062–9065. doi: 10.1073/pnas.85.23.9062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conboy J. G., Shitamoto R., Parra M., Winardi R., Kabra A., Smith J., Mohandas N. Hereditary elliptocytosis due to both qualitative and quantitative defects in membrane skeletal protein 4.1. Blood. 1991 Nov 1;78(9):2438–2443. [PubMed] [Google Scholar]
- Conboy J. G. Structure, function, and molecular genetics of erythroid membrane skeletal protein 4.1 in normal and abnormal red blood cells. Semin Hematol. 1993 Jan;30(1):58–73. [PubMed] [Google Scholar]
- Correas I. Characterization of isoforms of protein 4.1 present in the nucleus. Biochem J. 1991 Oct 15;279(Pt 2):581–585. doi: 10.1042/bj2790581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correas I., Leto T. L., Speicher D. W., Marchesi V. T. Identification of the functional site of erythrocyte protein 4.1 involved in spectrin-actin associations. J Biol Chem. 1986 Mar 5;261(7):3310–3315. [PubMed] [Google Scholar]
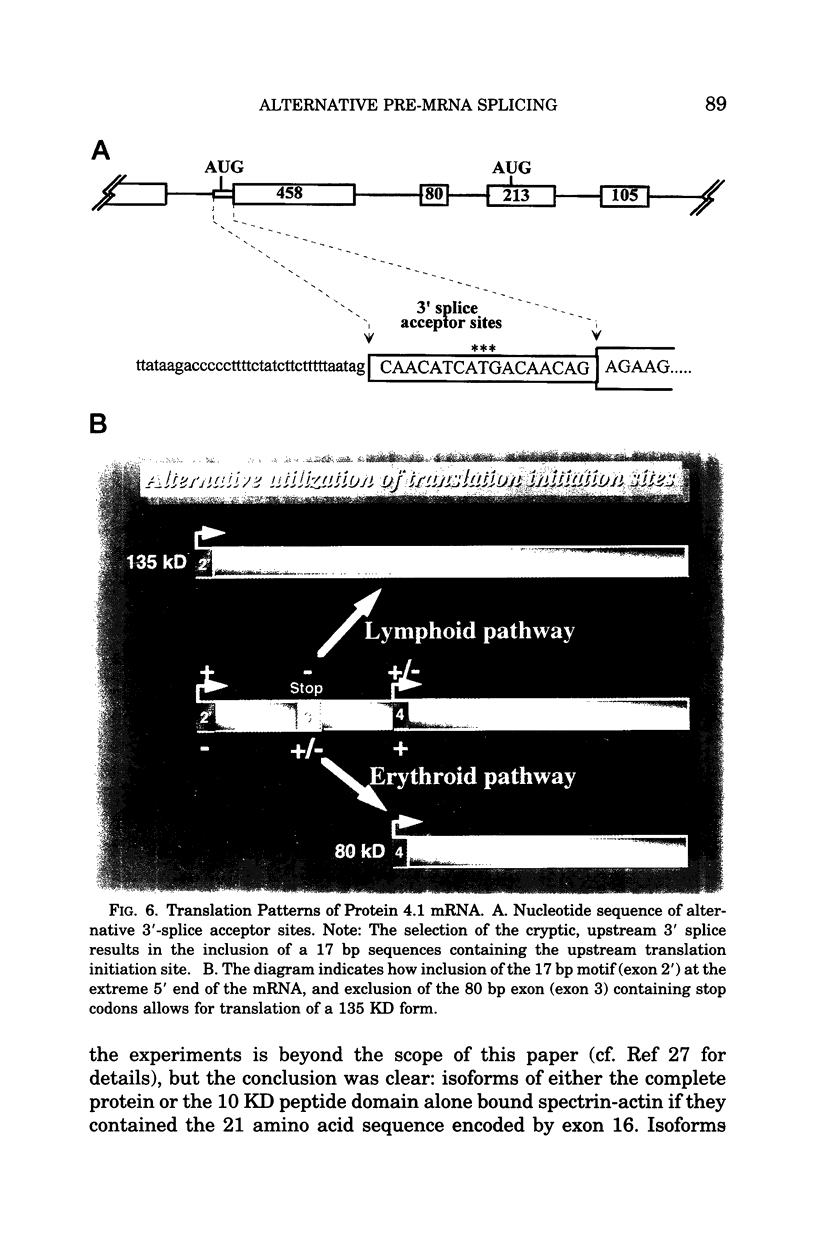
- Dalla Venezia N., Gilsanz F., Alloisio N., Ducluzeau M. T., Benz E. J., Jr, Delaunay J. Homozygous 4.1(-) hereditary elliptocytosis associated with a point mutation in the downstream initiation codon of protein 4.1 gene. J Clin Invest. 1992 Nov;90(5):1713–1717. doi: 10.1172/JCI116044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Cesaris P., Filippini A., Stefanini M., Ziparo E. Spectrin, fodrin and protein 4.1-like proteins in differentiating rat germ cells. Differentiation. 1989 Sep;41(3):216–222. doi: 10.1111/j.1432-0436.1989.tb00750.x. [DOI] [PubMed] [Google Scholar]
- Discher D., Parra M., Conboy J. G., Mohandas N. Mechanochemistry of the alternatively spliced spectrin-actin binding domain in membrane skeletal protein 4.1. J Biol Chem. 1993 Apr 5;268(10):7186–7195. [PubMed] [Google Scholar]
- Giebelhaus D. H., Eib D. W., Moon R. T. Antisense RNA inhibits expression of membrane skeleton protein 4.1 during embryonic development of Xenopus. Cell. 1988 May 20;53(4):601–615. doi: 10.1016/0092-8674(88)90576-4. [DOI] [PubMed] [Google Scholar]
- Green M. R. Biochemical mechanisms of constitutive and regulated pre-mRNA splicing. Annu Rev Cell Biol. 1991;7:559–599. doi: 10.1146/annurev.cb.07.110191.003015. [DOI] [PubMed] [Google Scholar]
- Horne W. C., Huang S. C., Becker P. S., Tang T. K., Benz E. J., Jr Tissue-specific alternative splicing of protein 4.1 inserts an exon necessary for formation of the ternary complex with erythrocyte spectrin and F-actin. Blood. 1993 Oct 15;82(8):2558–2563. [PubMed] [Google Scholar]
- Huang J. P., Tang C. J., Kou G. H., Marchesi V. T., Benz E. J., Jr, Tang T. K. Genomic structure of the locus encoding protein 4.1. Structural basis for complex combinational patterns of tissue-specific alternative RNA splicing. J Biol Chem. 1993 Feb 15;268(5):3758–3766. [PubMed] [Google Scholar]
- Kuo H. C., Nasim F. H., Grabowski P. J. Control of alternative splicing by the differential binding of U1 small nuclear ribonucleoprotein particle. Science. 1991 Mar 1;251(4997):1045–1050. doi: 10.1126/science.1825520. [DOI] [PubMed] [Google Scholar]
- Leto T. L., Marchesi V. T. A structural model of human erythrocyte protein 4.1. J Biol Chem. 1984 Apr 10;259(7):4603–4608. [PubMed] [Google Scholar]
- Leto T. L., Pratt B. M., Madri J. A. Mechanisms of cytoskeletal regulation: modulation of aortic endothelial cell protein band 4.1 by the extracellular matrix. J Cell Physiol. 1986 Jun;127(3):423–431. doi: 10.1002/jcp.1041270311. [DOI] [PubMed] [Google Scholar]
- Malek S. N., Katumuluwa A. I., Pasternack G. R. Identification and preliminary characterization of two related proliferation-associated nuclear phosphoproteins. J Biol Chem. 1990 Aug 5;265(22):13400–13409. [PubMed] [Google Scholar]
- Marchesi V. T., Ngo N. In vitro assembly of multiprotein complexes containing alpha, beta, and gamma tubulin, heat shock protein HSP70, and elongation factor 1 alpha. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):3028–3032. doi: 10.1073/pnas.90.7.3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohandas N., Chasis J. A. Red blood cell deformability, membrane material properties and shape: regulation by transmembrane, skeletal and cytosolic proteins and lipids. Semin Hematol. 1993 Jul;30(3):171–192. [PubMed] [Google Scholar]
- Ngai J., Stack J. H., Moon R. T., Lazarides E. Regulated expression of multiple chicken erythroid membrane skeletal protein 4.1 variants is governed by differential RNA processing and translational control. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4432–4436. doi: 10.1073/pnas.84.13.4432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternack G. R., Racusen R. H. Erythrocyte protein 4.1 binds and regulates myosin. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9712–9716. doi: 10.1073/pnas.86.24.9712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racusen R. H., Pasternack G. R. Microscale, filtration-type binding assay for studying myosin-erythrocyte protein 4.1 interactions. Anal Biochem. 1990 Aug 1;188(2):344–348. doi: 10.1016/0003-2697(90)90618-j. [DOI] [PubMed] [Google Scholar]
- Rio D. C. Splicing of pre-mRNA: mechanism, regulation and role in development. Curr Opin Genet Dev. 1993 Aug;3(4):574–584. doi: 10.1016/0959-437x(93)90093-5. [DOI] [PubMed] [Google Scholar]
- Rousseaux-Prévost R., Lesur P., Collier F., Rigot J. M., Dalla Venezia N., Pol P. S., Delaunay J., Gauthier A., Rousseaux J. Abnormal expression of protein 4.1 in spermatozoa of infertile men with teratospermia. Lancet. 1994 Mar 26;343(8900):764–765. doi: 10.1016/s0140-6736(94)91840-6. [DOI] [PubMed] [Google Scholar]
- Sharp P. A. Split genes and RNA splicing. Cell. 1994 Jun 17;77(6):805–815. doi: 10.1016/0092-8674(94)90130-9. [DOI] [PubMed] [Google Scholar]
- Tang C. J., Tang T. K. Rapid localization of membrane skeletal protein 4.1 (EL1) to human chromosome 1p33----p34.2 by nonradioactive in situ hybridization. Cytogenet Cell Genet. 1991;57(2-3):119–119. doi: 10.1159/000133128. [DOI] [PubMed] [Google Scholar]
- Tang T. K., Leto T. L., Correas I., Alonso M. A., Marchesi V. T., Benz E. J., Jr Selective expression of an erythroid-specific isoform of protein 4.1. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3713–3717. doi: 10.1073/pnas.85.11.3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang T. K., Qin Z., Leto T., Marchesi V. T., Benz E. J., Jr Heterogeneity of mRNA and protein products arising from the protein 4.1 gene in erythroid and nonerythroid tissues. J Cell Biol. 1990 Mar;110(3):617–624. doi: 10.1083/jcb.110.3.617. [DOI] [PMC free article] [PubMed] [Google Scholar]









