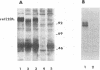
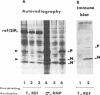
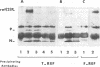
Abstract
The ref(2)P gene is one of the Drosophila melanogaster genes involved in the inhibition of sigma rhabdovirus multiplication. The partial restriction of viral replication varies according to the ref(2)P alleles and virus strains and involves intracellular interactions between parasite and host products. We identified the protein encoded by the ref(2)P gene and produced polyclonal antibodies directed against the whole ref(2)P protein obtained from a recombinant baculovirus and against a part of the protein expressed as a fusion protein. These antibodies were used to study the interactions with sigma virus proteins by different immunoprecipitation techniques. We showed that the native ref(2)P protein shared conformation-dependent common epitopes with the viral structural genome-associated N protein. Furthermore, the cellular protein was found to be associated in complexes with the viral P protein required for RNA polymerase activity. The significance of these observations in the control of sigma virus multiplication by its host is discussed.
Full text
PDF


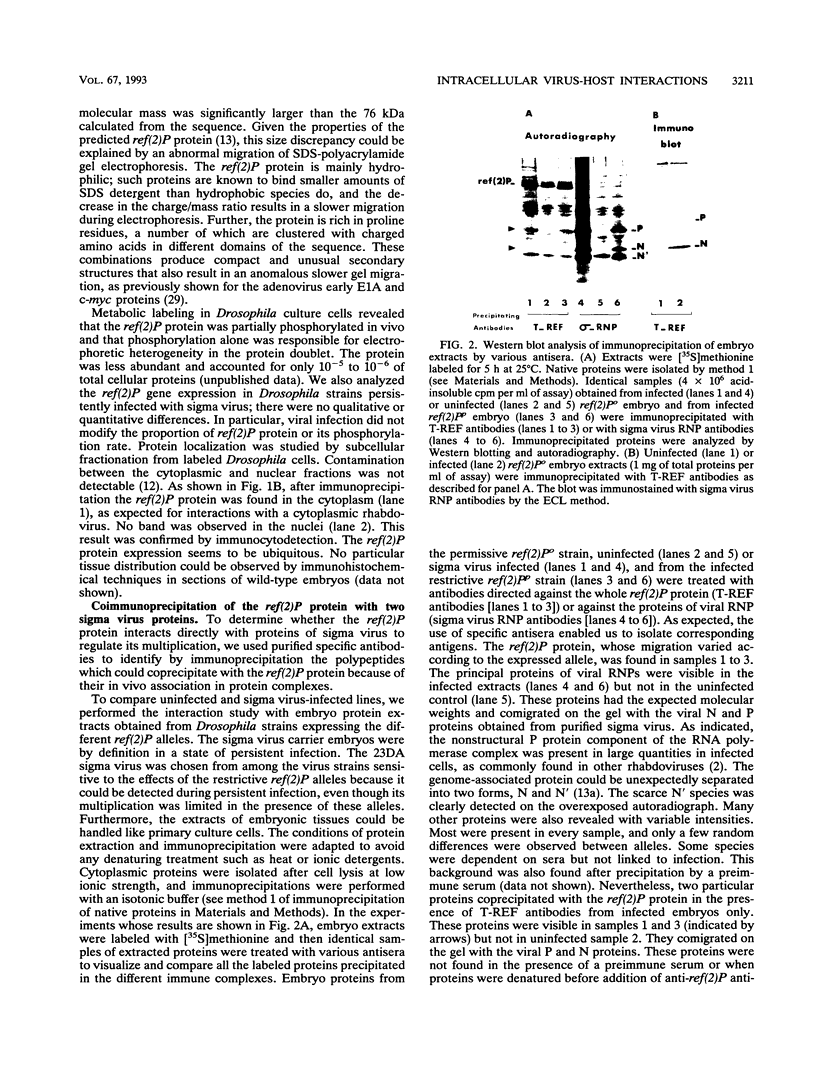





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRUN G., SIGOT A. Etude de la sensibilité héréditaire au gaz carbonique chez la drosophile. II. Installation du virus sigma dans la lignée germinale á la suite d'une inoculation. Ann Inst Pasteur (Paris) 1955 Apr;88(4):488–512. [PubMed] [Google Scholar]
- Baltimore D. Gene therapy. Intracellular immunization. Nature. 1988 Sep 29;335(6189):395–396. doi: 10.1038/335395a0. [DOI] [PubMed] [Google Scholar]
- Banerjee A. K., Chattopadhyay D. Structure and function of the RNA polymerase of vesicular stomatitis virus. Adv Virus Res. 1990;38:99–124. doi: 10.1016/s0065-3527(08)60860-x. [DOI] [PubMed] [Google Scholar]
- Blumenthal T., Hill D. Roles of the host polypeptides in Q beta RNA replication. Host factor and ribosomal protein S1 allow initiation at reduced GTP concentration. J Biol Chem. 1980 Dec 25;255(24):11713–11716. [PubMed] [Google Scholar]
- Contamine D., Petitjean A. M., Ashburner M. Genetic resistance to viral infection: the molecular cloning of a Drosophila gene that restricts infection by the rhabdovirus sigma. Genetics. 1989 Nov;123(3):525–533. doi: 10.1093/genetics/123.3.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damian R. T. Molecular mimicry: parasite evasion and host defense. Curr Top Microbiol Immunol. 1989;145:101–115. doi: 10.1007/978-3-642-74594-2_9. [DOI] [PubMed] [Google Scholar]
- Dezelee S., Bras F., Contamine D., Lopez-Ferber M., Segretain D., Teninges D. Molecular analysis of ref(2)P, a Drosophila gene implicated in sigma rhabdovirus multiplication and necessary for male fertility. EMBO J. 1989 Nov;8(11):3437–3446. doi: 10.1002/j.1460-2075.1989.tb08508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dezélée S., Blondel D., Wyers F., Petitjean A. M. Vesicular stomatitis virus in Drosophila melanogaster cells: lack of leader RNA transport into the nuclei and frequent abortion of the replication step. J Virol. 1987 May;61(5):1391–1397. doi: 10.1128/jvi.61.5.1391-1397.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman A. D., Triezenberg S. J., McKnight S. L. Expression of a truncated viral trans-activator selectively impedes lytic infection by its cognate virus. Nature. 1988 Sep 29;335(6189):452–454. doi: 10.1038/335452a0. [DOI] [PubMed] [Google Scholar]
- Gay P. Les gènes de la Drosophile qui interviennent dans la multiplication du virus sigma. Mol Gen Genet. 1978 Feb 27;159(3):269–283. doi: 10.1007/BF00268263. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lafay F., Benejean J. Temperature sensitive mutants of vesicular stomatitis virus: tryptic peptide maps of the proteins modified in complementation groups II and IV. Virology. 1981 May;111(1):93–102. doi: 10.1016/0042-6822(81)90656-5. [DOI] [PubMed] [Google Scholar]
- Masters P. S., Banerjee A. K. Resolution of multiple complexes of phosphoprotein NS with nucleocapsid protein N of vesicular stomatitis virus. J Virol. 1988 Aug;62(8):2651–2657. doi: 10.1128/jvi.62.8.2651-2657.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuura Y., Possee R. D., Bishop D. H. Expression of the S-coded genes of lymphocytic choriomeningitis arenavirus using a baculovirus vector. J Gen Virol. 1986 Aug;67(Pt 8):1515–1529. doi: 10.1099/0022-1317-67-8-1515. [DOI] [PubMed] [Google Scholar]
- Nakamura N. Influence du dosage de l'allèle non permissif du gène ref(2)P de la Drosophile sur les souches sensibles du virus sigma. Mol Gen Genet. 1978 Feb 27;159(3):285–292. doi: 10.1007/BF00268264. [DOI] [PubMed] [Google Scholar]
- Oldstone M. B. Molecular mimicry and autoimmune disease. Cell. 1987 Sep 11;50(6):819–820. doi: 10.1016/0092-8674(87)90507-1. [DOI] [PubMed] [Google Scholar]
- Pavlovic J., Staeheli P. The antiviral potentials of Mx proteins. J Interferon Res. 1991 Aug;11(4):215–219. doi: 10.1089/jir.1991.11.215. [DOI] [PubMed] [Google Scholar]
- Persson H., Hennighausen L., Taub R., DeGrado W., Leder P. Antibodies to human c-myc oncogene product: evidence of an evolutionarily conserved protein induced during cell proliferation. Science. 1984 Aug 17;225(4663):687–693. doi: 10.1126/science.6431612. [DOI] [PubMed] [Google Scholar]
- Srinivasappa J., Saegusa J., Prabhakar B. S., Gentry M. K., Buchmeier M. J., Wiktor T. J., Koprowski H., Oldstone M. B., Notkins A. L. Molecular mimicry: frequency of reactivity of monoclonal antiviral antibodies with normal tissues. J Virol. 1986 Jan;57(1):397–401. doi: 10.1128/jvi.57.1.397-401.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley K. K., Luzio J. P. Construction of a new family of high efficiency bacterial expression vectors: identification of cDNA clones coding for human liver proteins. EMBO J. 1984 Jun;3(6):1429–1434. doi: 10.1002/j.1460-2075.1984.tb01988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teninges D., Bras-Herreng F. Rhabdovirus sigma, the hereditary CO2 sensitivity agent of Drosophila: nucleotide sequence of a cDNA clone encoding the glycoprotein. J Gen Virol. 1987 Oct;68(Pt 10):2625–2638. doi: 10.1099/0022-1317-68-10-2625. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyers F., Richard-Molard C., Blondel D., Dezelee S. Vesicular stomatitis virus growth in Drosophila melanogaster cells: G protein deficiency. J Virol. 1980 Jan;33(1):411–422. doi: 10.1128/jvi.33.1.411-422.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- di Mauro E., Synder L., Marino P., Lamberti A., Coppo A., Tocchini-Valentini G. P. Rifampicin sensitivity of the components of DNA-dependent RNA polymerase. Nature. 1969 May 10;222(5193):533–537. doi: 10.1038/222533a0. [DOI] [PubMed] [Google Scholar]