Abstract
The most abundant polyadenylated viral transcripts in the Epstein-Barr virus (EBV)-associated tumor nasopharyngeal carcinoma are a family (apparent sizes, 4.8, 5.2, 6.2, and 7.0 kb) of highly spliced cytoplasmic RNAs expressed from the BamHI-I and -A regions of the viral genome in an antisense direction with respect to several viral lytic functions encoded within the same region and concerned with the lytic cycle of the virus. We have called these complementary-strand transcripts. They are also expressed in B cells, including Burkitt's lymphoma and EBV-immortalized marmoset cell lines, and tumors generated in cottontop tamarins in response to EBV infection, but at a lower level. The complete structure of the major 4.8-kb RNAs (seven or eight exons) was determined in this study; the larger, but related, transcripts appear to be produced by differential splicing. The transcriptional promoter for the major complementary-strand transcripts, located in BamHI-I, contains several well-characterized transcriptional control elements (E2A, SP1, and AP1) and is functionally active in both B lymphocytes and epithelial cells. It appears to be a bifunctional viral promoter, as it also contains the initiation codon for a gene (BILF2) that encodes a glycoprotein that is expressed off the other strand. Splicing events create a number of small AUG-initiated open reading frames, one of which has homology to functionally significant regions of the EBV-encoded nuclear antigen 2 and to E2 (in papillomavirus). The complex nature of these transcripts and their potential role in the virus association with malignancy are considered.
Full text
PDF

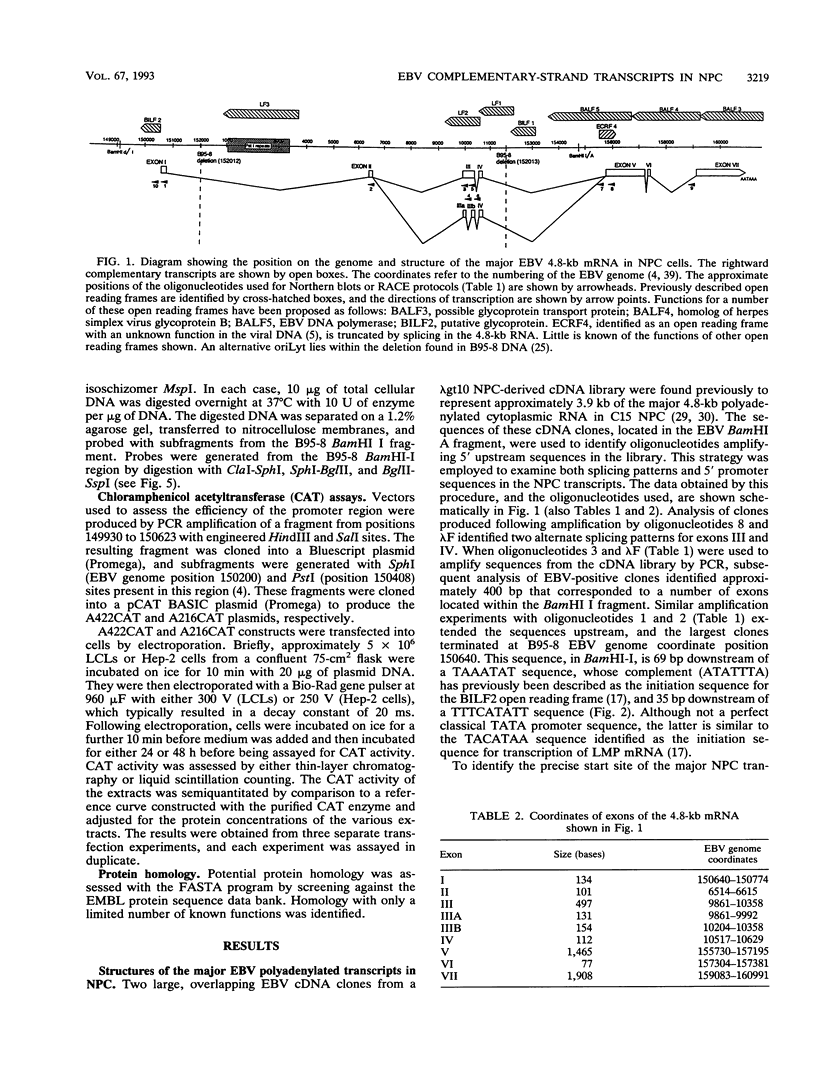
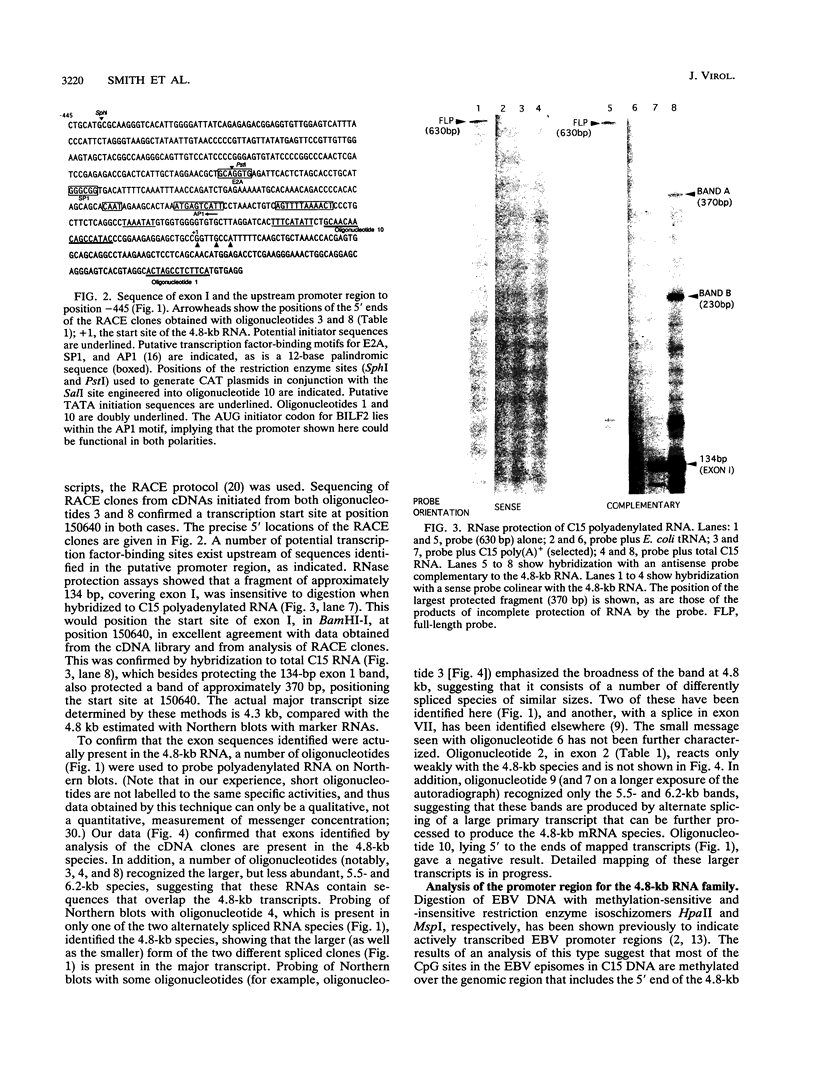
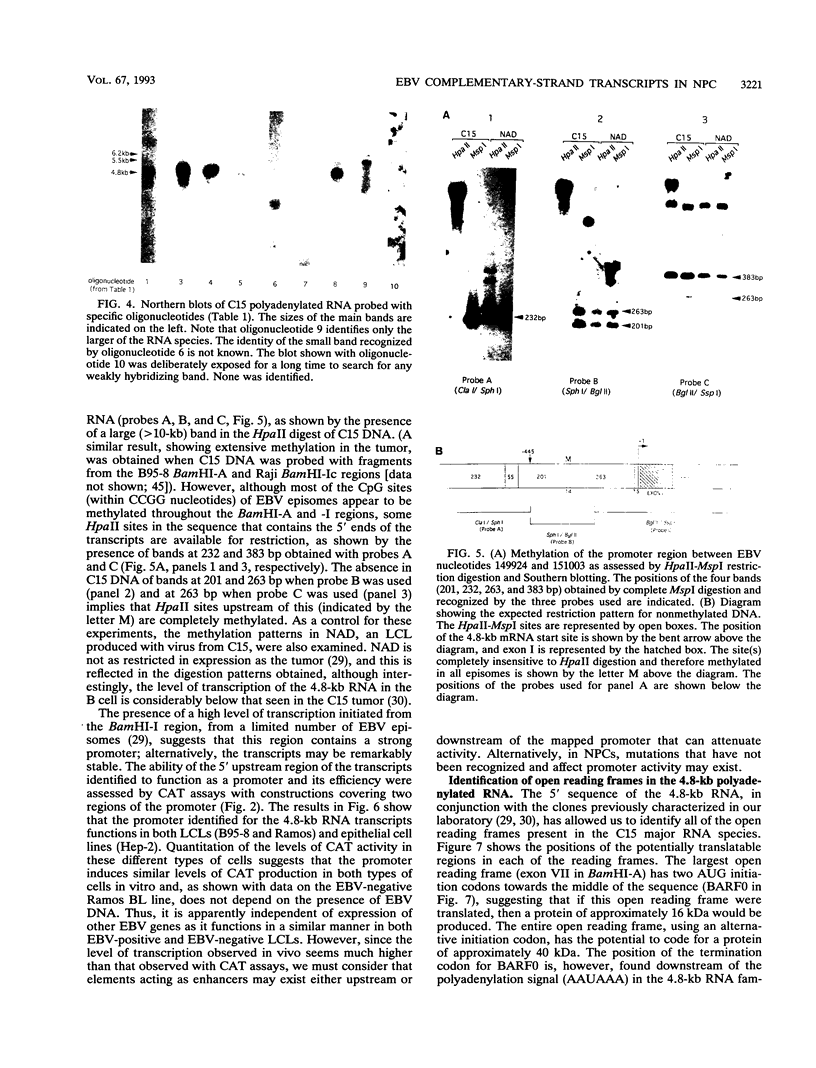



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbot S. D., Rowe M., Cadwallader K., Ricksten A., Gordon J., Wang F., Rymo L., Rickinson A. B. Epstein-Barr virus nuclear antigen 2 induces expression of the virus-encoded latent membrane protein. J Virol. 1990 May;64(5):2126–2134. doi: 10.1128/jvi.64.5.2126-2134.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allday M. J., Kundu D., Finerty S., Griffin B. E. CpG methylation of viral DNA in EBV-associated tumours. Int J Cancer. 1990 Jun 15;45(6):1125–1130. doi: 10.1002/ijc.2910450623. [DOI] [PubMed] [Google Scholar]
- Arrand J. R., Walsh-Arrand J. E., Rymo L. Cytoplasmic RNA from normal and malignant human cells shows homology to the DNAs of Epstein-Barr virus and human adenoviruses. EMBO J. 1983;2(10):1673–1683. doi: 10.1002/j.1460-2075.1983.tb01642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer R., Bankier A. T., Biggin M. D., Deininger P. L., Farrell P. J., Gibson T. J., Hatfull G., Hudson G. S., Satchwell S. C., Séguin C. DNA sequence and expression of the B95-8 Epstein-Barr virus genome. Nature. 1984 Jul 19;310(5974):207–211. doi: 10.1038/310207a0. [DOI] [PubMed] [Google Scholar]
- Bankier A. T., Deininger P. L., Satchwell S. C., Baer R., Farrell P. J., Barrell B. G. DNA sequence analysis of the EcoRI Dhet fragment of B95-8 Epstein-Barr virus containing the terminal repeat sequences. Mol Biol Med. 1983 Nov;1(4):425–445. [PubMed] [Google Scholar]
- Burkitt's lymphoma: a human cancer model. Proceedings of a symposium. Lyon, 6-9 December 1983. IARC Sci Publ. 1985;(60):1–484. [PubMed] [Google Scholar]
- Busson P., Ganem G., Flores P., Mugneret F., Clausse B., Caillou B., Braham K., Wakasugi H., Lipinski M., Tursz T. Establishment and characterization of three transplantable EBV-containing nasopharyngeal carcinomas. Int J Cancer. 1988 Oct 15;42(4):599–606. doi: 10.1002/ijc.2910420422. [DOI] [PubMed] [Google Scholar]
- Cai W., Schaffer P. A. Herpes simplex virus type 1 ICP0 regulates expression of immediate-early, early, and late genes in productively infected cells. J Virol. 1992 May;66(5):2904–2915. doi: 10.1128/jvi.66.5.2904-2915.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlin C. R., Tollefson A. E., Brady H. A., Hoffman B. L., Wold W. S. Epidermal growth factor receptor is down-regulated by a 10,400 MW protein encoded by the E3 region of adenovirus. Cell. 1989 Apr 7;57(1):135–144. doi: 10.1016/0092-8674(89)90179-7. [DOI] [PubMed] [Google Scholar]
- Chen H. L., Lung M. M., Sham J. S., Choy D. T., Griffin B. E., Ng M. H. Transcription of BamHI-A region of the EBV genome in NPC tissues and B cells. Virology. 1992 Nov;191(1):193–201. doi: 10.1016/0042-6822(92)90181-n. [DOI] [PubMed] [Google Scholar]
- Cohen J. I., Wang F., Kieff E. Epstein-Barr virus nuclear protein 2 mutations define essential domains for transformation and transactivation. J Virol. 1991 May;65(5):2545–2554. doi: 10.1128/jvi.65.5.2545-2554.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. I., Wang F., Mannick J., Kieff E. Epstein-Barr virus nuclear protein 2 is a key determinant of lymphocyte transformation. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9558–9562. doi: 10.1073/pnas.86.23.9558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernberg I., Falk K., Minarovits J., Busson P., Tursz T., Masucci M. G., Klein G. The role of methylation in the phenotype-dependent modulation of Epstein-Barr nuclear antigen 2 and latent membrane protein genes in cells latently infected with Epstein-Barr virus. J Gen Virol. 1989 Nov;70(Pt 11):2989–3002. doi: 10.1099/0022-1317-70-11-2989. [DOI] [PubMed] [Google Scholar]
- Faisst S., Meyer S. Compilation of vertebrate-encoded transcription factors. Nucleic Acids Res. 1992 Jan 11;20(1):3–26. doi: 10.1093/nar/20.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feramisco J. R., Glass D. B., Krebs E. G. Optimal spatial requirements for the location of basic residues in peptide substrates for the cyclic AMP-dependent protein kinase. J Biol Chem. 1980 May 10;255(9):4240–4245. [PubMed] [Google Scholar]
- Frohman M. A., Dush M. K., Martin G. R. Rapid production of full-length cDNAs from rare transcripts: amplification using a single gene-specific oligonucleotide primer. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8998–9002. doi: 10.1073/pnas.85.23.8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fåhraeus R., Fu H. L., Ernberg I., Finke J., Rowe M., Klein G., Falk K., Nilsson E., Yadav M., Busson P. Expression of Epstein-Barr virus-encoded proteins in nasopharyngeal carcinoma. Int J Cancer. 1988 Sep 15;42(3):329–338. doi: 10.1002/ijc.2910420305. [DOI] [PubMed] [Google Scholar]
- Fåhraeus R., Rymo L., Rhim J. S., Klein G. Morphological transformation of human keratinocytes expressing the LMP gene of Epstein-Barr virus. Nature. 1990 May 31;345(6274):447–449. doi: 10.1038/345447a0. [DOI] [PubMed] [Google Scholar]
- Gilligan K. J., Rajadurai P., Lin J. C., Busson P., Abdel-Hamid M., Prasad U., Tursz T., Raab-Traub N. Expression of the Epstein-Barr virus BamHI A fragment in nasopharyngeal carcinoma: evidence for a viral protein expressed in vivo. J Virol. 1991 Nov;65(11):6252–6259. doi: 10.1128/jvi.65.11.6252-6259.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilligan K., Sato H., Rajadurai P., Busson P., Young L., Rickinson A., Tursz T., Raab-Traub N. Novel transcription from the Epstein-Barr virus terminal EcoRI fragment, DIJhet, in a nasopharyngeal carcinoma. J Virol. 1990 Oct;64(10):4948–4956. doi: 10.1128/jvi.64.10.4948-4956.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green P. J., Pines O., Inouye M. The role of antisense RNA in gene regulation. Annu Rev Biochem. 1986;55:569–597. doi: 10.1146/annurev.bi.55.070186.003033. [DOI] [PubMed] [Google Scholar]
- Griffin B. E., Karran L. Immortalization of monkey epithelial cells by specific fragments of Epstein-Barr virus DNA. Nature. 1984 May 3;309(5963):78–82. doi: 10.1038/309078a0. [DOI] [PubMed] [Google Scholar]
- Hammerschmidt W., Sugden B. Genetic analysis of immortalizing functions of Epstein-Barr virus in human B lymphocytes. Nature. 1989 Aug 3;340(6232):393–397. doi: 10.1038/340393a0. [DOI] [PubMed] [Google Scholar]
- Hammerschmidt W., Sugden B. Identification and characterization of oriLyt, a lytic origin of DNA replication of Epstein-Barr virus. Cell. 1988 Nov 4;55(3):427–433. doi: 10.1016/0092-8674(88)90028-1. [DOI] [PubMed] [Google Scholar]
- Herbst H., Dallenbach F., Hummel M., Niedobitek G., Pileri S., Müller-Lantzsch N., Stein H. Epstein-Barr virus latent membrane protein expression in Hodgkin and Reed-Sternberg cells. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4766–4770. doi: 10.1073/pnas.88.11.4766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitt M. M., Allday M. J., Hara T., Karran L., Jones M. D., Busson P., Tursz T., Ernberg I., Griffin B. E. EBV gene expression in an NPC-related tumour. EMBO J. 1989 Sep;8(9):2639–2651. doi: 10.1002/j.1460-2075.1989.tb08404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karran L., Gao Y., Smith P. R., Griffin B. E. Expression of a family of complementary-strand transcripts in Epstein-Barr virus-infected cells. Proc Natl Acad Sci U S A. 1992 Sep 1;89(17):8058–8062. doi: 10.1073/pnas.89.17.8058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karran L., Teo C. G., King D., Hitt M. M., Gao Y. N., Wedderburn N., Griffin B. E. Establishment of immortalized primate epithelial cells with sub-genomic EBV DNA. Int J Cancer. 1990 Apr 15;45(4):763–772. doi: 10.1002/ijc.2910450432. [DOI] [PubMed] [Google Scholar]
- Khochbin S., Lawrence J. J. An antisense RNA involved in p53 mRNA maturation in murine erythroleukemia cells induced to differentiate. EMBO J. 1989 Dec 20;8(13):4107–4114. doi: 10.1002/j.1460-2075.1989.tb08595.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimelman D., Kirschner M. W. An antisense mRNA directs the covalent modification of the transcript encoding fibroblast growth factor in Xenopus oocytes. Cell. 1989 Nov 17;59(4):687–696. doi: 10.1016/0092-8674(89)90015-9. [DOI] [PubMed] [Google Scholar]
- MacMahon E. M., Glass J. D., Hayward S. D., Mann R. B., Becker P. S., Charache P., McArthur J. C., Ambinder R. F. Epstein-Barr virus in AIDS-related primary central nervous system lymphoma. Lancet. 1991 Oct 19;338(8773):969–973. doi: 10.1016/0140-6736(91)91837-k. [DOI] [PubMed] [Google Scholar]
- Mackett M., Conway M. J., Arrand J. R., Haddad R. S., Hutt-Fletcher L. M. Characterization and expression of a glycoprotein encoded by the Epstein-Barr virus BamHI I fragment. J Virol. 1990 Jun;64(6):2545–2552. doi: 10.1128/jvi.64.6.2545-2552.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Old L. J., Boyse E. A., Oettgen H. F., Harven E. D., Geering G., Williamson B., Clifford P. Precipitating antibody in human serum to an antigen present in cultured burkitt's lymphoma cells. Proc Natl Acad Sci U S A. 1966 Dec;56(6):1699–1704. doi: 10.1073/pnas.56.6.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker B. D., Bankier A., Satchwell S., Barrell B., Farrell P. J. Sequence and transcription of Raji Epstein-Barr virus DNA spanning the B95-8 deletion region. Virology. 1990 Nov;179(1):339–346. doi: 10.1016/0042-6822(90)90302-8. [DOI] [PubMed] [Google Scholar]
- Pellett P. E., Jenkins F. J., Ackermann M., Sarmiento M., Roizman B. Transcription initiation sites and nucleotide sequence of a herpes simplex virus 1 gene conserved in the Epstein-Barr virus genome and reported to affect the transport of viral glycoproteins. J Virol. 1986 Dec;60(3):1134–1140. doi: 10.1128/jvi.60.3.1134-1140.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raab-Traub N., Hood R., Yang C. S., Henry B., 2nd, Pagano J. S. Epstein-Barr virus transcription in nasopharyngeal carcinoma. J Virol. 1983 Dec;48(3):580–590. doi: 10.1128/jvi.48.3.580-590.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisman D., Sugden B. trans activation of an Epstein-Barr viral transcriptional enhancer by the Epstein-Barr viral nuclear antigen 1. Mol Cell Biol. 1986 Nov;6(11):3838–3846. doi: 10.1128/mcb.6.11.3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisman D., Yates J., Sugden B. A putative origin of replication of plasmids derived from Epstein-Barr virus is composed of two cis-acting components. Mol Cell Biol. 1985 Aug;5(8):1822–1832. doi: 10.1128/mcb.5.8.1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. R., Griffin B. E. Differential expression of Epstein Barr viral transcripts for two proteins (TP1 and LMP) in lymphocyte and epithelial cells. Nucleic Acids Res. 1991 May 11;19(9):2435–2440. doi: 10.1093/nar/19.9.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. R., Griffin B. E. Transcription of the Epstein-Barr virus gene EBNA-1 from different promoters in nasopharyngeal carcinoma and B-lymphoblastoid cells. J Virol. 1992 Feb;66(2):706–714. doi: 10.1128/jvi.66.2.706-714.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens J. G., Wagner E. K., Devi-Rao G. B., Cook M. L., Feldman L. T. RNA complementary to a herpesvirus alpha gene mRNA is prominent in latently infected neurons. Science. 1987 Feb 27;235(4792):1056–1059. doi: 10.1126/science.2434993. [DOI] [PubMed] [Google Scholar]
- Sugden B., Warren N. A promoter of Epstein-Barr virus that can function during latent infection can be transactivated by EBNA-1, a viral protein required for viral DNA replication during latent infection. J Virol. 1989 Jun;63(6):2644–2649. doi: 10.1128/jvi.63.6.2644-2649.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vormwald-Dogan V., Fischer B., Bludau H., Freese U. K., Gissmann L., Glitz D., Schwartz E., Dürst M. Sense and antisense transcripts of human papillomavirus type 16 in cervical cancers. J Gen Virol. 1992 Jul;73(Pt 7):1833–1838. doi: 10.1099/0022-1317-73-7-1833. [DOI] [PubMed] [Google Scholar]
- Wang D., Liebowitz D., Kieff E. An EBV membrane protein expressed in immortalized lymphocytes transforms established rodent cells. Cell. 1985 Dec;43(3 Pt 2):831–840. doi: 10.1016/0092-8674(85)90256-9. [DOI] [PubMed] [Google Scholar]
- Wang F., Tsang S. F., Kurilla M. G., Cohen J. I., Kieff E. Epstein-Barr virus nuclear antigen 2 transactivates latent membrane protein LMP1. J Virol. 1990 Jul;64(7):3407–3416. doi: 10.1128/jvi.64.7.3407-3416.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei M. X., Ooka T. A transforming function of the BARF1 gene encoded by Epstein-Barr virus. EMBO J. 1989 Oct;8(10):2897–2903. doi: 10.1002/j.1460-2075.1989.tb08438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates J., Warren N., Reisman D., Sugden B. A cis-acting element from the Epstein-Barr viral genome that permits stable replication of recombinant plasmids in latently infected cells. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3806–3810. doi: 10.1073/pnas.81.12.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C. X., Lowrey P., Finerty S., Morgan A. J. Analysis of Epstein-Barr virus gene transcription in lymphoma induced by the virus in the cottontop tamarin by construction of a cDNA library with RNA extracted from a tumour biopsy. J Gen Virol. 1993 Mar;74(Pt 3):509–514. doi: 10.1099/0022-1317-74-3-509. [DOI] [PubMed] [Google Scholar]