Abstract
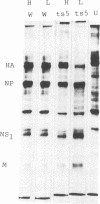
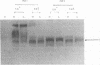
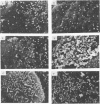
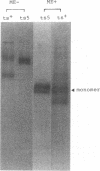
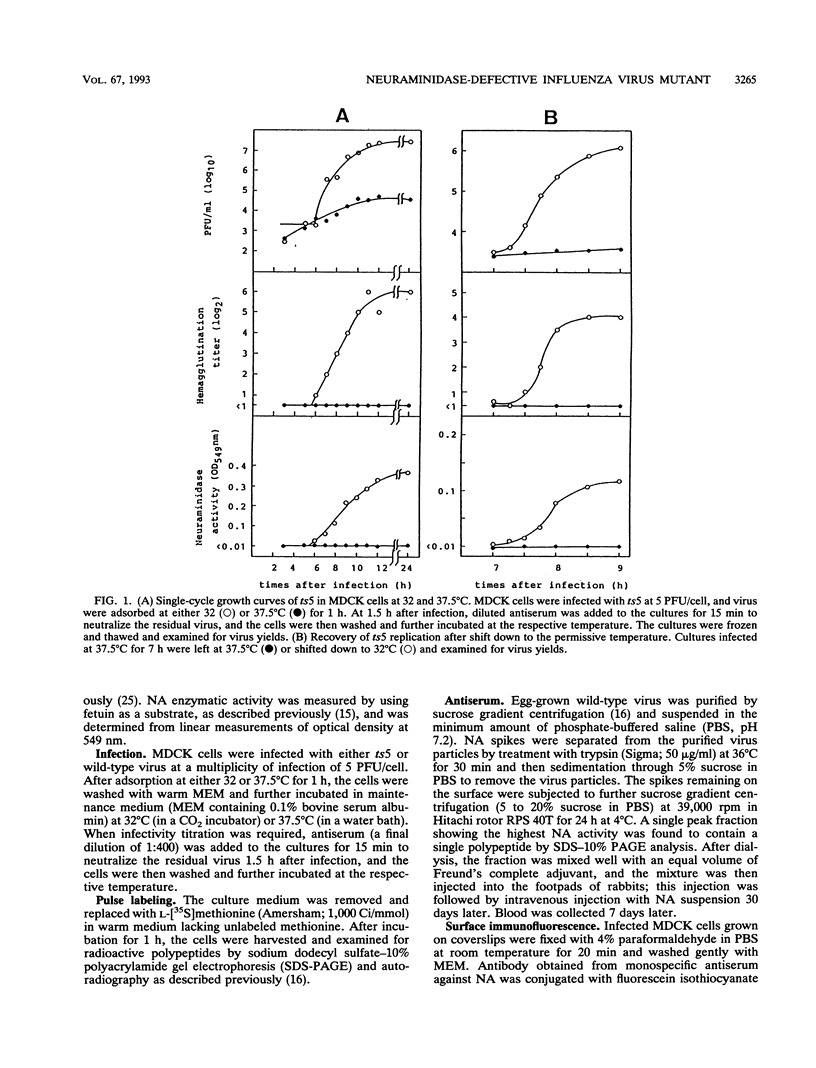
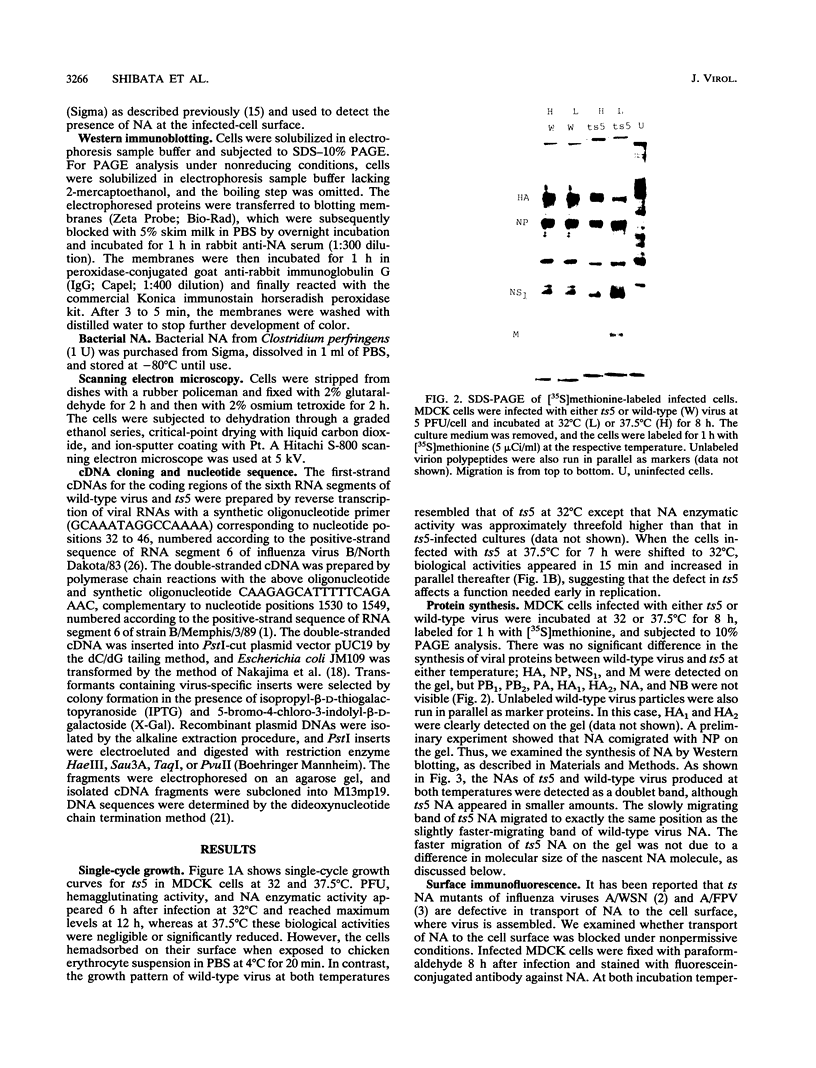
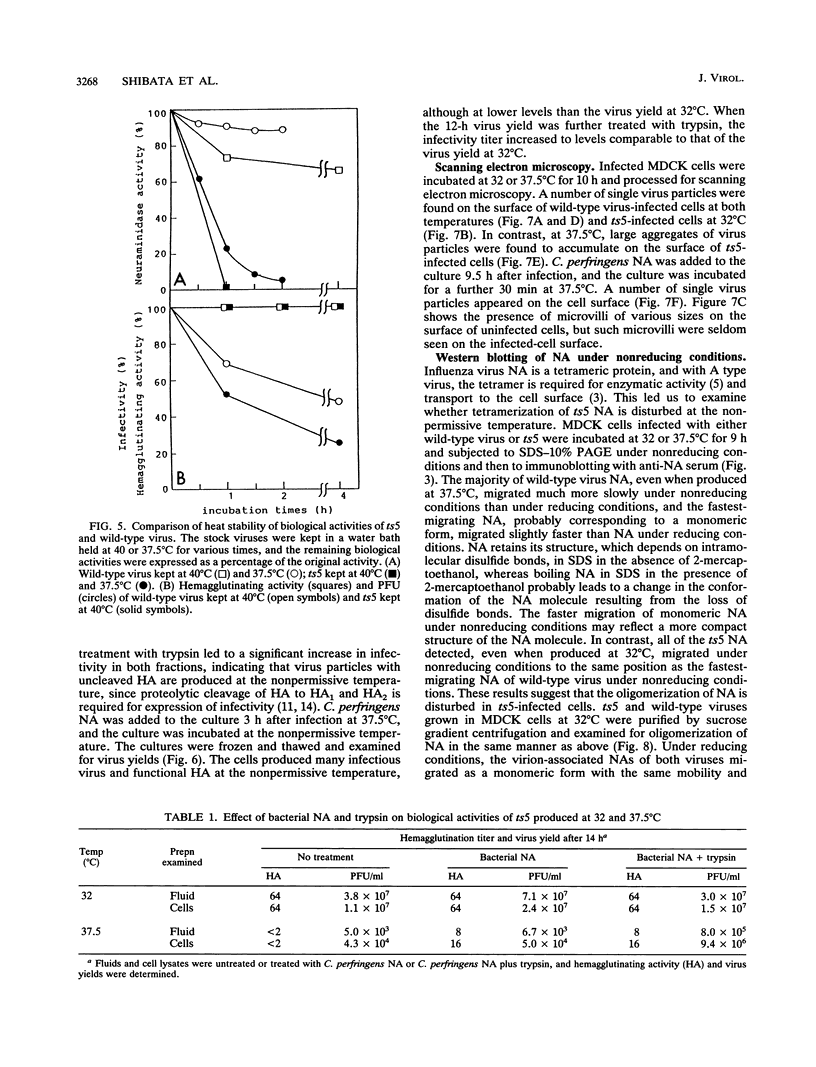
ts5, a temperature-sensitive mutant of influenza B virus, belongs to one of seven recombination groups. When the mutant infected MDCK cells at the nonpermissive temperature (37.5 degrees C), infectious virus was produced at very low levels compared with the yield at the permissive temperature (32 degrees C) and hemagglutinating and enzymatic activities were undetectable. However, viral protein synthesis and transport of hemagglutinin (HA) and neuraminidase (NA) to the cell surface were not affected. The NA was found as a monomer within cells even at 32 degrees C, in contrast to wild-type virus NA, existing mostly as an oligomer, but the mutant had oligomeric NA, like the wild-type virus. Its enzymatic activity was more thermolabile than that of wild-type virus. Despite the low yield, large aggregates of progeny virus particles were found to accumulate on the cell surface at the nonpermissive temperature, and these aggregates were broken by treatment with bacterial neuraminidase, with the concomitant appearance of hemagglutinating activity, suggesting that NA prevents the aggregation of progeny virus by removal of neuraminic acid from HA and cell receptor, allowing its release from the cells. Further treatment with trypsin resulted in the recovery of infectivity. When bacterial NA was added to the culture early in infection, many hemagglutinable infectious virus was produced. We also suggest that the removal of neuraminic acid from HA by NA is essential for the subsequent cleavage of HA by cellular protease. Nucleotide sequence analysis of RNA segment 6 revealed that ts5 encoded five amino acid changes in the NA molecule but not in NB.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Air G. M., Laver W. G., Luo M., Stray S. J., Legrone G., Webster R. G. Antigenic, sequence, and crystal variation in influenza B neuraminidase. Virology. 1990 Aug;177(2):578–587. doi: 10.1016/0042-6822(90)90523-t. [DOI] [PubMed] [Google Scholar]
- Bos T. J., Nayak D. P. Identification of defects in the neuraminidase gene of four temperature-sensitive mutants of A/WSN/33 influenza virus. Virology. 1986 Oct 15;154(1):85–96. doi: 10.1016/0042-6822(86)90432-0. [DOI] [PubMed] [Google Scholar]
- Breuning A., Müller K., Scholtissek C. Mutants of an influenza A reassortant which are cold-sensitive (cs) as well as temperature-sensitive (ts): on the role of the neuraminidase activity for influenza virus infection. Virology. 1987 Jan;156(1):101–106. doi: 10.1016/0042-6822(87)90440-5. [DOI] [PubMed] [Google Scholar]
- Breuning A., Scholtissek C. A reassortant between influenza A viruses (H7N2) synthesizing an enzymatically inactive neuraminidase at 40 degrees which is not incorporated into infectious particles. Virology. 1986 Apr 15;150(1):65–74. doi: 10.1016/0042-6822(86)90266-7. [DOI] [PubMed] [Google Scholar]
- Bucher D. J., Kilbourne E. D. A 2 (N2) neuraminidase of the X-7 influenza virus recombinant: determination of molecular size and subunit composition of the active unit. J Virol. 1972 Jul;10(1):60–66. doi: 10.1128/jvi.10.1.60-66.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burmeister W. P., Ruigrok R. W., Cusack S. The 2.2 A resolution crystal structure of influenza B neuraminidase and its complex with sialic acid. EMBO J. 1992 Jan;11(1):49–56. doi: 10.1002/j.1460-2075.1992.tb05026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOTTSCHALK A. Neuraminidase: the specific enzyme of influenza virus and Vibrio cholerae. Biochim Biophys Acta. 1957 Mar;23(3):645–646. doi: 10.1016/0006-3002(57)90389-x. [DOI] [PubMed] [Google Scholar]
- Ghendon Y., Markushin S., Ginzburg V., Hay A. Functional defects of fowl plague virus temperature-sensitive mutant having mutation in the neuraminidase. Arch Virol. 1983;75(1-2):55–70. doi: 10.1007/BF01314127. [DOI] [PubMed] [Google Scholar]
- Kawaoka Y., Naeve C. W., Webster R. G. Is virulence of H5N2 influenza viruses in chickens associated with loss of carbohydrate from the hemagglutinin? Virology. 1984 Dec;139(2):303–316. doi: 10.1016/0042-6822(84)90376-3. [DOI] [PubMed] [Google Scholar]
- Klenk H. D., Rott R., Orlich M., Blödorn J. Activation of influenza A viruses by trypsin treatment. Virology. 1975 Dec;68(2):426–439. doi: 10.1016/0042-6822(75)90284-6. [DOI] [PubMed] [Google Scholar]
- Krystal M., Elliott R. M., Benz E. W., Jr, Young J. F., Palese P. Evolution of influenza A and B viruses: conservation of structural features in the hemagglutinin genes. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4800–4804. doi: 10.1073/pnas.79.15.4800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal M., Young J. F., Palese P., Wilson I. A., Skehel J. J., Wiley D. C. Sequential mutations in hemagglutinins of influenza B virus isolates: definition of antigenic domains. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4527–4531. doi: 10.1073/pnas.80.14.4527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarowitz S. G., Choppin P. W. Enhancement of the infectivity of influenza A and B viruses by proteolytic cleavage of the hemagglutinin polypeptide. Virology. 1975 Dec;68(2):440–454. doi: 10.1016/0042-6822(75)90285-8. [DOI] [PubMed] [Google Scholar]
- Maeno K., Kilbourne E. D. Developmental sequence and intracellular sites of synthesis of three structural protein antigens of influenza A2 virus. J Virol. 1970 Feb;5(2):153–164. doi: 10.1128/jvi.5.2.153-164.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeno K., Yoshii S., Mita K., Hamaguchi M., Yoshida T., Iinuma M., Nagai Y., Matsumoto T. Analysis of the inhibitory effect of canavanine on the replication of influenza RI/5+ virus. I. Inhibition of assembly of RNP. Virology. 1979 Apr 15;94(1):128–137. doi: 10.1016/0042-6822(79)90443-4. [DOI] [PubMed] [Google Scholar]
- Nakajima K., Nobusawa E., Nakajima S. Genetic relatedness between A/Swine/Iowa/15/30(H1N1) and human influenza viruses. Virology. 1984 Nov;139(1):194–198. doi: 10.1016/0042-6822(84)90341-6. [DOI] [PubMed] [Google Scholar]
- Ohuchi M., Orlich M., Ohuchi R., Simpson B. E., Garten W., Klenk H. D., Rott R. Mutations at the cleavage site of the hemagglutinin after the pathogenicity of influenza virus A/chick/Penn/83 (H5N2). Virology. 1989 Feb;168(2):274–280. doi: 10.1016/0042-6822(89)90267-5. [DOI] [PubMed] [Google Scholar]
- Palese P., Tobita K., Ueda M., Compans R. W. Characterization of temperature sensitive influenza virus mutants defective in neuraminidase. Virology. 1974 Oct;61(2):397–410. doi: 10.1016/0042-6822(74)90276-1. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulman J. L., Palese P. Virulence factors of influenza A viruses: WSN virus neuraminidase required for plaque production in MDBK cells. J Virol. 1977 Oct;24(1):170–176. doi: 10.1128/jvi.24.1.170-176.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw M. W., Choppin P. W., Lamb R. A. A previously unrecognized influenza B virus glycoprotein from a bicistronic mRNA that also encodes the viral neuraminidase. Proc Natl Acad Sci U S A. 1983 Aug;80(16):4879–4883. doi: 10.1073/pnas.80.16.4879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw M. W., Lamb R. A., Erickson B. W., Briedis D. J., Choppin P. W. Complete nucleotide sequence of the neuraminidase gene of influenza B virus. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6817–6821. doi: 10.1073/pnas.79.22.6817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata M., Maeno K., Isomura S., Tsurumi T., Aoki H., Suzuki S. Plaque formation by influenza B virus in a porcine kidney cell line. Microbiol Immunol. 1982;26(5):441–444. doi: 10.1111/j.1348-0421.1982.tb00195.x. [DOI] [PubMed] [Google Scholar]
- Stoeckle M. Y., Shaw M. W., Choppin P. W. Segment-specific and common nucleotide sequences in the noncoding regions of influenza B virus genome RNAs. Proc Natl Acad Sci U S A. 1987 May;84(9):2703–2707. doi: 10.1073/pnas.84.9.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura A., Ueda M. Neurovirulence of influenza virus in mice. I. Neurovirulence of recombinants between virulent and avirulent virus strains. Virology. 1980 Mar;101(2):440–449. doi: 10.1016/0042-6822(80)90457-2. [DOI] [PubMed] [Google Scholar]
- Verhoeyen M., Van Rompuy L., Jou W. M., Huylebroeck D., Fiers W. Complete nucleotide sequence of the influenza B/Singapore/222/79 virus hemagglutinin gene and comparison with the B/Lee/40 hemagglutinin. Nucleic Acids Res. 1983 Jul 25;11(14):4703–4712. doi: 10.1093/nar/11.14.4703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams M. A., Lamb R. A. Determination of the orientation of an integral membrane protein and sites of glycosylation by oligonucleotide-directed mutagenesis: influenza B virus NB glycoprotein lacks a cleavable signal sequence and has an extracellular NH2-terminal region. Mol Cell Biol. 1986 Dec;6(12):4317–4328. doi: 10.1128/mcb.6.12.4317. [DOI] [PMC free article] [PubMed] [Google Scholar]