Abstract
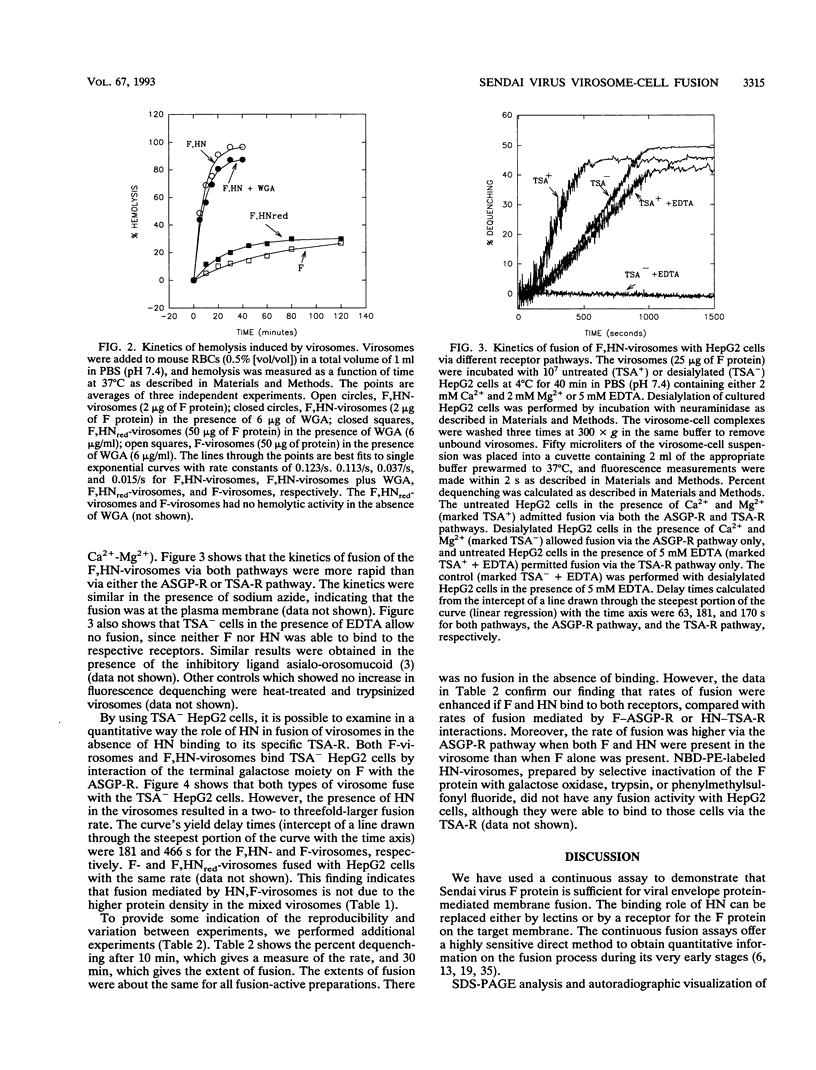
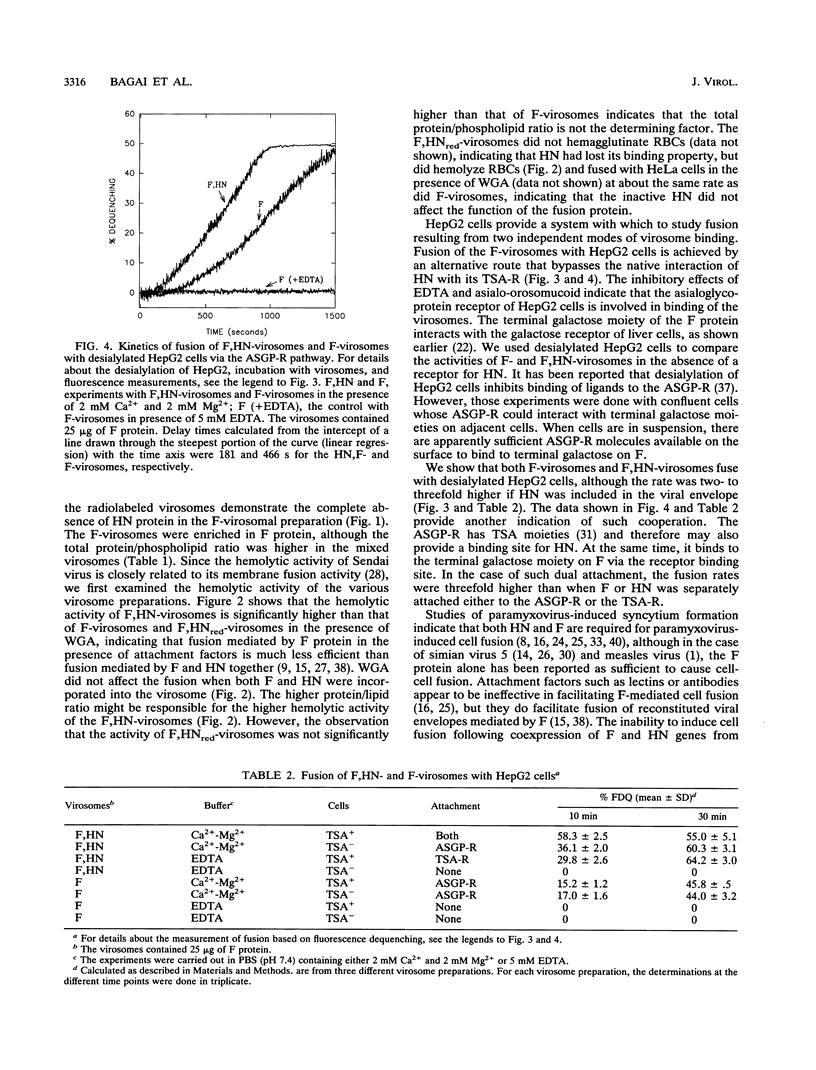
Reconstituted Sendai virus envelopes containing both the fusion (F) protein and the hemagglutinin-neuraminidase (HN) (F,HN-virosomes) or only the F protein (F-virosomes) were prepared by solubilization of the intact virus with Triton X-100 followed by its removal by using SM2 Bio-Beads. Viral envelopes containing HN whose disulfide bonds were irreversibly reduced (HNred) were also prepared by treating the envelopes with dithiothreitol followed by dialysis (F,HNred-virosomes). Both F-virosomes and F,HNred-virosomes induced hemolysis of erythrocytes in the presence of wheat germ agglutinin, but the rates and extents were markedly lower than those for hemolysis induced by F,HN-virosomes. Using an assay based on the relief of self-quenching of a lipid probe incorporated in the Sendai virus envelopes, we demonstrate the fusion of both F,HN-virosomes and F-virosomes with cultured HepG2 cells containing the asialoglycoprotein receptor, which binds to a terminal galactose moiety of F. By desialylating the HepG2 cells, the entry mediated by HN-terminal sialic acid receptor interactions was bypassed. We show that both F-virosomes and F,HN-virosomes fuse with desialylated HepG2 cells, although the rate was two- to threefold higher if HN was included in the viral envelope. We also observed enhancement of fusion rates when both F and HN envelope proteins were attached to their specific receptors.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alkhatib G., Richardson C., Shen S. H. Intracellular processing, glycosylation, and cell-surface expression of the measles virus fusion protein (F) encoded by a recombinant adenovirus. Virology. 1990 Mar;175(1):262–270. doi: 10.1016/0042-6822(90)90207-8. [DOI] [PubMed] [Google Scholar]
- Aroeti B., Henis Y. I. Accumulation of Sendai virus glycoproteins in cell-cell contact regions and its role in cell fusion. J Biol Chem. 1991 Aug 25;266(24):15845–15849. [PubMed] [Google Scholar]
- Ashwell G., Harford J. Carbohydrate-specific receptors of the liver. Annu Rev Biochem. 1982;51:531–554. doi: 10.1146/annurev.bi.51.070182.002531. [DOI] [PubMed] [Google Scholar]
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Blumenthal R., Loyter A. Reconstituted viral envelopes--'Trojan horses' for drug delivery and gene therapy? Trends Biotechnol. 1991 Feb;9(2):41–45. doi: 10.1016/0167-7799(91)90184-J. [DOI] [PubMed] [Google Scholar]
- Blumenthal R., Schoch C., Puri A., Clague M. J. A dissection of steps leading to viral envelope protein-mediated membrane fusion. Ann N Y Acad Sci. 1991;635:285–296. doi: 10.1111/j.1749-6632.1991.tb36499.x. [DOI] [PubMed] [Google Scholar]
- Citovsky V., Loyter A. Fusion of Sendai virions or reconstituted Sendai virus envelopes with liposomes or erythrocyte membranes lacking virus receptors. J Biol Chem. 1985 Oct 5;260(22):12072–12077. [PubMed] [Google Scholar]
- Ebata S. N., Côté M. J., Kang C. Y., Dimock K. The fusion and hemagglutinin-neuraminidase glycoproteins of human parainfluenza virus 3 are both required for fusion. Virology. 1991 Jul;183(1):437–441. doi: 10.1016/0042-6822(91)90162-5. [DOI] [PubMed] [Google Scholar]
- Gitman A. G., Graessmann A., Loyter A. Targeting of loaded Sendai virus envelopes by covalently attached insulin molecules to virus receptor-depleted cells: fusion-mediated microinjection of ricin A and simian virus 40 DNA. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7309–7313. doi: 10.1073/pnas.82.21.7309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitman A. G., Kahane I., Loyter A. Use of virus-attached antibodies or insulin molecules to mediate fusion between Sendai virus envelopes and neuraminidase-treated cells. Biochemistry. 1985 May 21;24(11):2762–2768. doi: 10.1021/bi00332a025. [DOI] [PubMed] [Google Scholar]
- Gitman A. G., Loyter A. Construction of fusogenic vesicles bearing specific antibodies. Targeting of reconstituted Sendai virus envelopes towards neuraminidase-treated human erythrocytes. J Biol Chem. 1984 Aug 10;259(15):9813–9820. [PubMed] [Google Scholar]
- Henis Y. I., Herman-Barhom Y., Aroeti B., Gutman O. Lateral mobility of both envelope proteins (F and HN) of Sendai virus in the cell membrane is essential for cell-cell fusion. J Biol Chem. 1989 Oct 15;264(29):17119–17125. [PubMed] [Google Scholar]
- Hoekstra D., de Boer T., Klappe K., Wilschut J. Fluorescence method for measuring the kinetics of fusion between biological membranes. Biochemistry. 1984 Nov 20;23(24):5675–5681. doi: 10.1021/bi00319a002. [DOI] [PubMed] [Google Scholar]
- Horvath C. M., Paterson R. G., Shaughnessy M. A., Wood R., Lamb R. A. Biological activity of paramyxovirus fusion proteins: factors influencing formation of syncytia. J Virol. 1992 Jul;66(7):4564–4569. doi: 10.1128/jvi.66.7.4564-4569.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu M. C., Scheid A., Choppin P. W. Reconstitution of membranes with individual paramyxovirus glycoproteins and phospholipid in cholate solution. Virology. 1979 Jun;95(2):476–491. doi: 10.1016/0042-6822(79)90502-6. [DOI] [PubMed] [Google Scholar]
- Hu X. L., Ray R., Compans R. W. Functional interactions between the fusion protein and hemagglutinin-neuraminidase of human parainfluenza viruses. J Virol. 1992 Mar;66(3):1528–1534. doi: 10.1128/jvi.66.3.1528-1534.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Loyter A., Citovsky V., Blumenthal R. The use of fluorescence dequenching measurements to follow viral membrane fusion events. Methods Biochem Anal. 1988;33:129–164. doi: 10.1002/9780470110546.ch4. [DOI] [PubMed] [Google Scholar]
- Loyter A., Tomasi M., Gitman A. G., Etinger L., Nussbaum O. The use of specific antibodies to mediate fusion between Sendai virus envelopes and living cells. Ciba Found Symp. 1984;103:163–180. doi: 10.1002/9780470720844.ch11. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Tolbert N. E., Bieber L. L. Protein determination in membrane and lipoprotein samples: manual and automated procedures. Methods Enzymol. 1981;72:296–303. doi: 10.1016/s0076-6879(81)72018-4. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Portner A., Schwartz A. L. An alternative route of infection for viruses: entry by means of the asialoglycoprotein receptor of a Sendai virus mutant lacking its attachment protein. Proc Natl Acad Sci U S A. 1985 Feb;82(4):978–982. doi: 10.1073/pnas.82.4.978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura N., Uchida T., Okada Y. HVJ (Sendai virus)-induced envelope fusion and cell fusion are blocked by monoclonal anti-HN protein antibody that does not inhibit hemagglutination activity of HVJ. Exp Cell Res. 1982 Oct;141(2):409–420. doi: 10.1016/0014-4827(82)90229-4. [DOI] [PubMed] [Google Scholar]
- Morrison T., McQuain C., McGinnes L. Complementation between avirulent Newcastle disease virus and a fusion protein gene expressed from a retrovirus vector: requirements for membrane fusion. J Virol. 1991 Feb;65(2):813–822. doi: 10.1128/jvi.65.2.813-822.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscona A., Peluso R. W. Fusion properties of cells infected with human parainfluenza virus type 3: receptor requirements for viral spread and virus-mediated membrane fusion. J Virol. 1992 Nov;66(11):6280–6287. doi: 10.1128/jvi.66.11.6280-6287.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscona A., Peluso R. W. Fusion properties of cells persistently infected with human parainfluenza virus type 3: participation of hemagglutinin-neuraminidase in membrane fusion. J Virol. 1991 Jun;65(6):2773–2777. doi: 10.1128/jvi.65.6.2773-2777.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi M., Uchida T., Kim J., Okada Y. Glycoproteins of Sendai virus (HVJ) have a critical ratio for fusion between virus envelopes and cell membranes. Exp Cell Res. 1982 Nov;142(1):95–101. doi: 10.1016/0014-4827(82)90413-x. [DOI] [PubMed] [Google Scholar]
- Paternostre M. T., Lowy R. J., Blumenthal R. pH-dependent fusion of reconstituted vesicular stomatitis virus envelopes with Vero cells. Measurement by dequenching of fluorescence. FEBS Lett. 1989 Jan 30;243(2):251–258. doi: 10.1016/0014-5793(89)80139-5. [DOI] [PubMed] [Google Scholar]
- Paterson R. G., Hiebert S. W., Lamb R. A. Expression at the cell surface of biologically active fusion and hemagglutinin/neuraminidase proteins of the paramyxovirus simian virus 5 from cloned cDNA. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7520–7524. doi: 10.1073/pnas.82.22.7520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulson J. C., Hill R. L., Tanabe T., Ashwell G. Reactivation of asialo-rabbit liver binding protein by resialylation with beta-D-galactoside alpha2 leads to 6 sialyltransferase. J Biol Chem. 1977 Dec 10;252(23):8624–8628. [PubMed] [Google Scholar]
- Peretz H., Toister Z., Laster Y., Loyter A. Fusion of intact human erythrocytes and erythrocyte ghosts. J Cell Biol. 1974 Oct;63(1):1–11. doi: 10.1083/jcb.63.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai Y., Shibuta H. Syncytium formation by recombinant vaccinia viruses carrying bovine parainfluenza 3 virus envelope protein genes. J Virol. 1989 Sep;63(9):3661–3668. doi: 10.1128/jvi.63.9.3661-3668.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar D. P., Blumenthal R. The role of the target membrane structure in fusion with Sendai virus. Membr Biochem. 1987;7(4):231–247. doi: 10.3109/09687688709029434. [DOI] [PubMed] [Google Scholar]
- Sarkar D. P., Morris S. J., Eidelman O., Zimmerberg J., Blumenthal R. Initial stages of influenza hemagglutinin-induced cell fusion monitored simultaneously by two fluorescent events: cytoplasmic continuity and lipid mixing. J Cell Biol. 1989 Jul;109(1):113–122. doi: 10.1083/jcb.109.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheid A., Choppin P. W. Two disulfide-linked polypeptide chains constitute the active F protein of paramyxoviruses. Virology. 1977 Jul 1;80(1):54–66. doi: 10.1016/0042-6822(77)90380-4. [DOI] [PubMed] [Google Scholar]
- Schwartz A. L., Fridovich S. E., Knowles B. B., Lodish H. F. Characterization of the asialoglycoprotein receptor in a continuous hepatoma line. J Biol Chem. 1981 Sep 10;256(17):8878–8881. [PubMed] [Google Scholar]
- Sechoy O., Vidal M., Philippot J. R., Bienvenue A. Interactions of human lymphoblasts with targeted vesicles containing Sendai virus envelope proteins. Exp Cell Res. 1989 Nov;185(1):122–131. doi: 10.1016/0014-4827(89)90042-6. [DOI] [PubMed] [Google Scholar]
- Tomasi M., Loyter A. Selective extraction of biologically active F-glycoprotein from dithiothreitol reduced Sendai virus particles. FEBS Lett. 1981 Aug 31;131(2):381–385. doi: 10.1016/0014-5793(81)80409-7. [DOI] [PubMed] [Google Scholar]
- Wild T. F., Malvoisin E., Buckland R. Measles virus: both the haemagglutinin and fusion glycoproteins are required for fusion. J Gen Virol. 1991 Feb;72(Pt 2):439–442. doi: 10.1099/0022-1317-72-2-439. [DOI] [PubMed] [Google Scholar]
- Wolf D., Kahana I., Nir S., Loyter A. The interaction between Sendai virus and cell membranes. A quantitative analysis of 125I-sendai virus particles' association with human red blood cells. Exp Cell Res. 1980 Dec;130(2):361–369. doi: 10.1016/0014-4827(80)90013-0. [DOI] [PubMed] [Google Scholar]
- Yoshima H., Nakanishi M., Okada Y., Kobata A. Carbohydrate structures of HVJ (Sendai virus) glycoproteins. J Biol Chem. 1981 Jun 10;256(11):5355–5361. [PubMed] [Google Scholar]




