Abstract
One of the most pervasive weight loss rules is that a cumulative energy deficit of 3500 kcal is required per pound of body weight loss, or equivalently 32.2 MJ per kg. Under what conditions is it appropriate to use this rule of thumb and what are the factors that determine the cumulative energy deficit required per unit weight loss? Here, I examine this question using a modification of the classic Forbes equation that predicts the composition of weight loss as a function of the initial body fat and magnitude of weight loss. The resulting model predicts that a larger cumulative energy deficit is required per unit weight loss for people with greater initial body fat - a prediction supported by published weight loss data from obese and lean subjects. This may also explain why men can lose more weight than women for a given energy deficit since women typically have more body fat than men of similar body weight. Furthermore, additional weight loss is predicted to be associated with a lower average cumulative energy deficit since a greater proportion of the weight loss is predicted to result from loss of lean body mass which has a relatively low energy density in comparison with body fat. The rule of thumb approximately matches the predicted energy density of lost weight in obese subjects with an initial body fat above 30 kg but overestimates the cumulative energy deficit required per unit weight loss for people with lower initial body fat.
Keywords: Body Composition, Weight loss, Mathematical model, Energy Density, Diet
One of the most pervasive weight loss rules states that a cumulative energy deficit of 3500 kcal is required to lose 1 pound of body weight, or equivalently 32.2 MJ per kg. The origin of this rule can be traced back to a calculation that assumes exclusive loss of adipose tissue consisting of 87% fat (1, 2). However, Forbes has pointed out that lean body mass is lost in concert with body fat during weight loss (3, 4) and it is now generally acknowledged that this rule of thumb is an oversimplification (1). But under what conditions is this rule of thumb appropriate? In other words, what are the factors that determine the cumulative energy deficit required per unit weight loss?
When energy intake does not meet energy requirements, the deficit is accounted for by metabolism of stored energy in the form of body fat, protein, and glycogen. Since energy is conserved, the metabolizable energy content of the lost tissue is equivalent to the energy deficit required to produce that weight loss. The metabolizable energy density of the lost tissue is therefore determined by its chemical composition. Loss of body water results in a significant mass change, but contributes nothing to the metabolizable energy content. In contrast, the metabolizable energy densities of body glycogen, protein and fat are 17.6, 19.7, and 39.5 MJ/kg, respectively (5).
Recently, I developed a modification of the classic Forbes equation (3, 4) predicting the proportion of weight loss accounted for by loss of lean body mass as a nonlinear function of both the initial body fat content as well as the magnitude of weight loss (6). Based on this equation, the energy density of weight loss can be predicted once the metabolizable energy densities for the lean body mass loss, ΔL, and body fat mass loss, ΔF, have been determined.
The energy density of the body fat mass change, ρF = 39.5 MJ/kg is the same as the energy density of fat. It is important to note that the change of body fat,ΔF, is not equivalent to the loss adipose tissue, which includes a variable contribution of fluid and protein in addition to triglyceride (7, 8). Rather, ΔF is the amount of endogenous fat metabolized by the body to meet the energy deficit. Similarly, the energy density of the lean body mass change, ρL, is determined by the metabolism of tissue glycogen and protein as well as the loss of body water associated with their storage in body tissues. Note that ρL should not be identified with the energy density of the lean body mass as a whole, but rather the energy density of the change of lean body mass. These values are not equivalent. For example, extracellular fluid constitutes a significant fraction of the lean body mass, has a very low energy density, and changes relatively little with weight loss (9-12). Therefore, ρL has a higher value than the energy density of the lean body mass as a whole.
Both glycogen and protein have similar energy densities and are associated with similar amounts of water in the body tissues (13, 14). However, since glycogen constitutes such a small fraction of the lean body mass and its effects on weight loss typically occur within the first week, ρL is typically assumed to be derived exclusively from body protein changes and the associated water (2). The water content of the weight change is therefore determined by a protein hydration coefficient, h, which specifies the amount of water lost per gram of body protein change. It has been common to assume that the protein hydration coefficient is equivalent to the total body water divided by the body protein content resulting in a value of about h = 3.8 g H2O per g protein and a value of ρL = 4 MJ/kg. This assumption significantly overestimates the water changes associated with body protein changes since there is a relative increase of lean tissue hydration following weight loss (10). A somewhat better calculation would be the intracellular water divided by the intracellular protein content, resulting in a value of about h = 2.8 g H2O per g protein and a value for ρL = 5.6 MJ/kg. But this calculation also overestimates the protein hydration coefficient because it associates all of the intracellular water with cellular protein while we know that intracellular potassium, as well as other solutes, contributes to determining cellular water content. I propose that h = 1.6 g H2O per g protein is a reasonable estimate for the protein hydration coefficient because this value is in the middle of the range 1.1 to 2.2 g H2O per g measured as the osmotically unresponsive water compartment in a variety of cells (15) and corresponds to the monolayer hydration of a typical protein (16). This results in a calculated energy density for lean body mass changes of ρL = 7.6 MJ/kg.
Using ρL = 7.6 MJ/kg and ρF = 39.5 MJ/kg, the energy density of weight loss is simply determined by the relative proportion of lost body weight resulting from lean body mass loss as described by Hall (6). The resulting equation for the metabolizable energy density of the weight loss is:
The proportion of the lost weight from lean body mass loss was given by:
where Fi is the initial body fat and W is the Lambert W function (6, 17). The factor 10.4 arises from the parameterization of the best-fit logarithmic function used by Forbes to relate lean body mass to body fat mass in a cross-sectional dataset of 166 women of similar stature (3,4, 6).
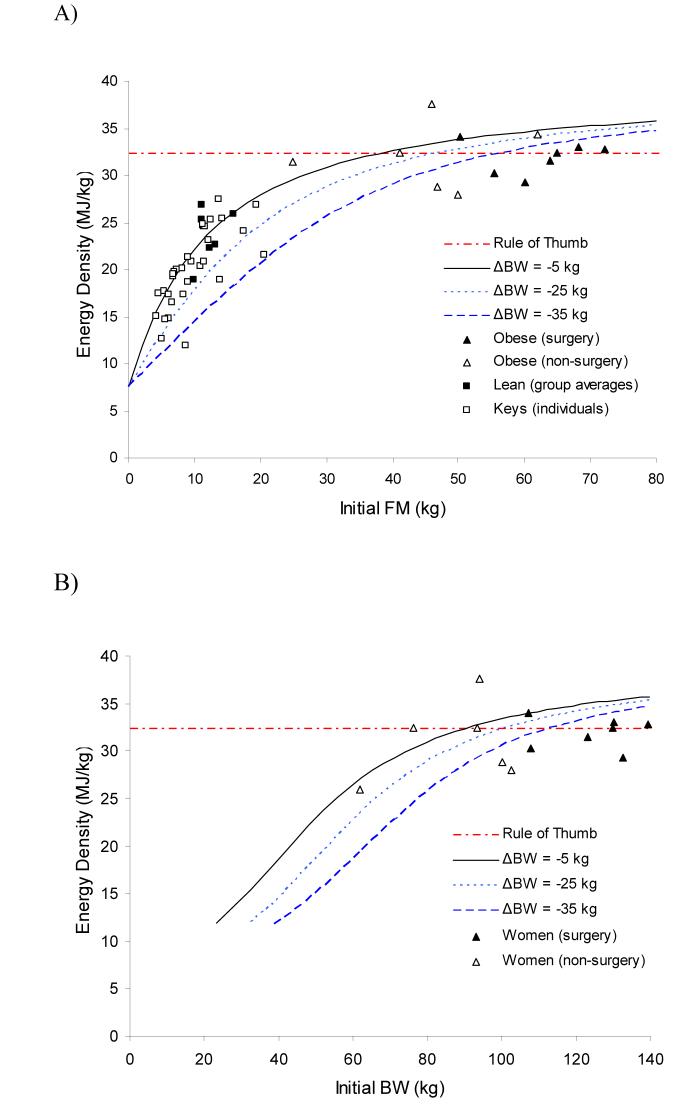
Figure 1A shows the predicted energy density of weight loss as a function of initial body fat mass for weight losses of 5, 25, and 35 kg. The higher the initial body fat, the greater the required cumulative energy deficit required to produce the same amount of weight loss. Greater weight loss is associated with a lower average cumulative energy deficit meaning that over time more weight is lost for the same degree of energy deficit. These curves are relatively insensitive to exact value of ρL, and the sensitivity decreases for increasing initial body fat (not shown). For example, a 15 kg weight loss for a person with an initial body fat of 20 kg gives a predicted energy density of 24.7 MJ per kg weight loss using the recommended h = 1.6 g H2O per g protein and this estimate decreases by only 6.5% to 23.1 MJ/kg when using the value h = 3.8 g H2O per g protein.
Figure 1.
The predicted energy density of weight loss expressed as a function of A)initial body fat content or B) initial body weight of women.Data points depict the calculated weight loss energy densities from several published studies in both obese and lean subjects.
For comparison purposes, Fig. 1A plots the calculated weight loss energy densities from several published weight loss studies. I plotted the group averages for each of seven bariatric surgery studies whose obese subjects lost an average of 40.5 kg (▲) (11, 18-23). Figure 1A also plots the group averages for four non-surgical weight loss studies whose obese subjects lost an average of 12.5 kgΔ(24-27). Since these data cluster around the curves predicted by the modified Forbes equation as well as the horizontal line in Figure 1, both the modified Forbes equation as well as the simple 32.2 MJ/kg rule of thumb approximate the data reasonably well for obese subjects.
However, the modified Forbes equation predicts deviations from the rule of thumb for weight loss in subjects with relatively low initial body fat. Figure 1A plots the data from individual subjects studied by Keys et al. whose lean male subjects lost an average of 16 kg after 6 months of semistarvation (□) (9). Also plotted are the group average data from three studies of lean subjects reported in references (28-30) where the average weight loss was 8 kg (■). These data indicate that the energy density of weight loss in people with less than 25 kg of initial body fat is indeed significantly lower than the 32.2 kJ/kg rule of thumb and approximately conforms to the predicted energy density of the modified Forbes model.
Since it is not common to measure initial body fat in a clinical setting, Figure 1B translates the curves as a function of initial body weight for the women originally studied by Forbes (3, 4). Since men typically have a higher lean body mass than women for the same amount of body fat, they would be better represented by a rightward shift of the curves in Figure 1B. This rightward shift for men may explain the sexual dimorphism observed in the energy content of weight change for men and women of similar initial body weight (31). In other words, since women typically have more body fat than men of similar body weight, this may explain why men tend to lose more weight than women for a given energy deficit.
The cumulative energy deficit required per unit weight loss is a statement about the metabolizable energy density of the lost body weight, but does not address how such an energy deficit is achieved or maintained. In particular, it is not appropriate to use Figure 1 to calculate the reduction of daily dietary energy required to lose a desired amount of weight over a prescribed period. The following is an example of such an inappropriate calculation: a 163 cm tall woman weighing 65 kg with 20 kg of body fat wants to lose 5 kg of body weight over 6 months. Using Figure 1, she determines that the cumulative energy deficit is 28 MJ per kg for a total cumulative energy deficit of 140 MJ over 6 months. Therefore, she cuts her energy intake by 780 kJ per day and maintains this diet. Such a calculation fails to account for how an individual’s energy expenditure adapts to the energy deficit and decreasing body weight and she will therefore not reach her weight loss goal. The magnitude of the expected energy expenditure adaptation during weight loss is a much-debated issue (32-36) and has not been addressed in the present study.
The modification of the classic Forbes equation predicts that a sustained energy deficit should result in an increasing rate of weight loss since an increasing proportion of the tissue loss will come from the relatively less energy dense lean body mass (3,4, 37). The fact that weight loss typically slows over time for a prescribed constant diet (9, 38) suggests that either the energy expenditure decreases with time, or the dietary intervention is relaxed over time, or both.
While the present study illustrates that the initial body fat as well as the magnitude of weight loss may influence the applicability of the simple 32.2 MJ/kg rule of thumb, it is likely that other factors play a role in the energy density of weight loss beyond what is accounted for by the modified Forbes model. For example, resistance exercise or high protein diets may modify the proportion of weight loss resulting from body fat versus lean tissue (39-41). A more comprehensive model of macronutrient metabolism and body composition change would be required to model such factors (42).
Acknowledgments
FUNDING INFORMATION: This work was supported by the Intramural Research Program of the NIH, NIDDK.
REFERENCES
- McArdle WD. Exercise physiology: energy, nutrition, and human performance. 4th edition edn. Williams & Wilkins; Baltimore: 1996. [Google Scholar]
- Wishnofsky M. Caloric equivalents of gained or lost weight. The American journal of clinical nutrition. 1958;6:542–546. doi: 10.1093/ajcn/6.5.542. [DOI] [PubMed] [Google Scholar]
- Forbes GB. Lean body mass-body fat interrelationships in humans. Nutrition reviews. 1987;45:225–231. doi: 10.1111/j.1753-4887.1987.tb02684.x. [DOI] [PubMed] [Google Scholar]
- Forbes GB. Body fat content influences the body composition response to nutrition and exercise. Annals of the New York Academy of Sciences. 2000;904:359–365. doi: 10.1111/j.1749-6632.2000.tb06482.x. [DOI] [PubMed] [Google Scholar]
- Livesey G, Elia M. Estimation of energy expenditure, net carbohydrate utilization, and net fat oxidation and synthesis by indirect calorimetry: evaluation of errors with special reference to the detailed composition of fuels. The American journal of clinical nutrition. 1988;47:608–628. doi: 10.1093/ajcn/47.4.608. [DOI] [PubMed] [Google Scholar]
- Hall KD. Body fat and fat-free mass inter-relationships: Forbes's theory revisited. The British journal of nutrition. 2007;97:1059–1063. doi: 10.1017/S0007114507691946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entenman C, Goldwater WH, Ayres NS, Behnke AR., Jr. Analysis of adipose tissue in relation to body weight loss in man. Journal of applied physiology. 1958;13:129–134. doi: 10.1152/jappl.1958.13.1.129. [DOI] [PubMed] [Google Scholar]
- Martin AD, Daniel MZ, Drinkwater DT, Clarys JP. Adipose tissue density, estimated adipose lipid fraction and whole body adiposity in male cadavers. Int J Obes Relat Metab Disord. 1994;18:79–83. [PubMed] [Google Scholar]
- Keys A. The biology of human starvation. University of Minnesota Press; Minneapolis: 1950. [Google Scholar]
- Leone PA, Gallagher D, Wang J, Heymsfield SB. Relative overhydration of fat-free mass in postobese versus never-obese subjects. Annals of the New York Academy of Sciences. 2000;904:514–519. doi: 10.1111/j.1749-6632.2000.tb06508.x. [DOI] [PubMed] [Google Scholar]
- Sergi G, Lupoli L, Busetto L, Volpato S, Coin A, Bertani R, et al. Changes in fluid compartments and body composition in obese women after weight loss induced by gastric banding. Annals of nutrition & metabolism. 2003;47:152–157. doi: 10.1159/000070038. [DOI] [PubMed] [Google Scholar]
- Zimmerman ME, Andersson H, Lundell L, Olbe L. Alterations in body composition after gastroplasty for morbid obesity. Scandinavian journal of gastroenterology. 1990;25:263–268. [PubMed] [Google Scholar]
- MacKay E, Bergman H. The amount of water stored with glycogen in the liver. Journal of Biological Chemistry. 1934;105:59–62. [Google Scholar]
- McBride J, Guest M, Scott E. The storage of the major liver components; emphasizing the relationship of glycogen to water in the liver and the hydration of glycogen. Journal of Biological Chemistry. 1941;139:943–952. [Google Scholar]
- Fullerton GD, Kanal KM, Cameron IL. On the osmotically unresponsive water compartment in cells. Cell biology international. 2006;30:74–77. doi: 10.1016/j.cellbi.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Fullerton GD, Amurao MR. Evidence that collagen and tendon have monolayer water coverage in the native state. Cell biology international. 2006;30:56–65. doi: 10.1016/j.cellbi.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Corless R, Gonnet G, Hare D, Jeffery D, Knuth D. On the Lambert W function. Adv Computational Maths. 1996;5:329–359. [Google Scholar]
- Benedetti G, Mingrone G, Marcoccia S, Benedetti M, Giancaterini A, Greco AV, et al. Body composition and energy expenditure after weight loss following bariatric surgery. Journal of the American College of Nutrition. 2000;19:270–274. doi: 10.1080/07315724.2000.10718926. [DOI] [PubMed] [Google Scholar]
- Das SK, Roberts SB, McCrory MA, Hsu LK, Shikora SA, Kehayias JJ, et al. Longterm changes in energy expenditure and body composition after massive weight loss induced by gastric bypass surgery. The American journal of clinical nutrition. 2003;78:22–30. doi: 10.1093/ajcn/78.1.22. [DOI] [PubMed] [Google Scholar]
- Strauss BJ, Marks SJ, Growcott JP, Stroud DB, Lo CS, Dixon JB, et al. Body composition changes following laparoscopic gastric banding for morbid obesity. Acta diabetologica. 2003;40(Suppl 1):S266–269. doi: 10.1007/s00592-003-0083-1. [DOI] [PubMed] [Google Scholar]
- Tacchino RM, Mancini A, Perrelli M, Bianchi A, Giampietro A, Milardi D, et al. Body composition and energy expenditure: relationship and changes in obese subjects before and after biliopancreatic diversion. Metabolism: clinical and experimental. 2003;52:552–558. doi: 10.1053/meta.2003.50109. [DOI] [PubMed] [Google Scholar]
- van Gemert WG, Westerterp KR, van Acker BA, Wagenmakers AJ, Halliday D, Greve JM, et al. Energy, substrate and protein metabolism in morbid obesity before, during and after massive weight loss. Int J Obes Relat Metab Disord. 2000;24:711–718. doi: 10.1038/sj.ijo.0801230. [DOI] [PubMed] [Google Scholar]
- Wadstrom C, Backman L, Forsberg AM, Nilsson E, Hultman E, Reizenstein P, et al. Body composition and muscle constituents during weight loss: studies in obese patients following gastroplasty. Obes Surg. 2000;10:203–213. doi: 10.1381/096089200321643313. [DOI] [PubMed] [Google Scholar]
- Carella MJ, Rodgers CD, Anderson D, Gossain VV. Serial measurements of body composition in obese subjects during a very-low-energy diet (VLED) comparing bioelectrical impedance with hydrodensitometry. Obesity research. 1997;5:250–256. doi: 10.1002/j.1550-8528.1997.tb00299.x. [DOI] [PubMed] [Google Scholar]
- de Boer JO, van Es AJ, Roovers LC, van Raaij JM, Hautvast JG. Adaptation of energy metabolism of overweight women to low-energy intake, studied with whole-body calorimeters. The American journal of clinical nutrition. 1986;44:585–595. doi: 10.1093/ajcn/44.5.585. [DOI] [PubMed] [Google Scholar]
- Stanko RT, Tietze DL, Arch JE. Body composition, nitrogen metabolism, and energy utilization with feeding of mildly restricted (4.2 MJ/d) and severely restricted (2.1 MJ/d) isonitrogenous diets. The American journal of clinical nutrition. 1992;56:636–640. doi: 10.1093/ajcn/56.4.636. [DOI] [PubMed] [Google Scholar]
- Webb P, Abrams T. Loss of fat stores and reduction in sedentary energy expenditure from undereating. Human nutrition. 1983;37:271–282. [PubMed] [Google Scholar]
- Friedl KE, Moore RJ, Martinez-Lopez LE, Vogel JA, Askew EW, Marchitelli LJ, et al. Lower limit of body fat in healthy active men. Journal of applied physiology. 1994;77:933–940. doi: 10.1152/jappl.1994.77.2.933. [DOI] [PubMed] [Google Scholar]
- Friedlander AL, Braun B, Pollack M, MacDonald JR, Fulco CS, Muza SR, et al. Three weeks of caloric restriction alters protein metabolism in normal-weight, young men. American journal of physiology. 2005;289:E446–455. doi: 10.1152/ajpendo.00001.2005. [DOI] [PubMed] [Google Scholar]
- Hoyt RW, Opstad PK, Haugen AH, DeLany JP, Cymerman A, Friedl KE. Negative energy balance in male and female rangers: effects of 7 d of sustained exercise and food deprivation. The American journal of clinical nutrition. 2006;83:1068–1075. doi: 10.1093/ajcn/83.5.1068. [DOI] [PubMed] [Google Scholar]
- Pietrobelli A, Allison DB, Heshka S, Heo M, Wang ZM, Bertkau A, et al. Sexual dimorphism in the energy content of weight change. Int J Obes Relat Metab Disord. 2002;26:1339–1348. doi: 10.1038/sj.ijo.0802065. [DOI] [PubMed] [Google Scholar]
- Doucet E, Imbeault P, St-Pierre S, Almeras N, Mauriege P, Despres JP, et al. Greater than predicted decrease in energy expenditure during exercise after body weight loss in obesemen. Clin Sci (Lond) 2003;105:89–95. doi: 10.1042/CS20020252. [DOI] [PubMed] [Google Scholar]
- Doucet E, St-Pierre S, Almeras N, Despres JP, Bouchard C, Tremblay A. Evidence for the existence of adaptive thermogenesis during weight loss. The British journal of nutrition. 2001;85:715–723. doi: 10.1079/bjn2001348. [DOI] [PubMed] [Google Scholar]
- Dulloo AG, Seydoux J, Jacquet J. Adaptive thermogenesis and uncoupling proteins: a reappraisal of their roles in fat metabolism and energy balance. Physiology & behavior. 2004;83:587–602. doi: 10.1016/j.physbeh.2004.07.028. [DOI] [PubMed] [Google Scholar]
- Leibel RL, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. The New England journal of medicine. 1995;332:621–628. doi: 10.1056/NEJM199503093321001. [DOI] [PubMed] [Google Scholar]
- Weinsier RL, Nagy TR, Hunter GR, Darnell BE, Hensrud DD, Weiss HL. Do adaptive changes in metabolic rate favor weight regain in weight-reduced individuals? An examination of the set-point theory. The American journal of clinical nutrition. 2000;72:1088–1094. doi: 10.1093/ajcn/72.5.1088. [DOI] [PubMed] [Google Scholar]
- Hall KD. Body fat and fat-free mass inter-relationships: Forbes's theory revisited. The British journal of nutrition. 2007:1–5. doi: 10.1017/S0007114507691946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heymsfield SB, Harp JB, Reitman ML, Beetsch JW, Schoeller DA, Erondu N, et al. Why do obese patients not lose more weight when treated with low-calorie diets? A mechanistic perspective. The American journal of clinical nutrition. 2007;85:346–354. doi: 10.1093/ajcn/85.2.346. [DOI] [PubMed] [Google Scholar]
- Hansen D, Dendale P, Berger J, van Loon LJ, Meeusen R. Sports medicine. Vol. 37. Auckland, NZ: 2007. The effects of exercise training on fatmass loss in obese patients during energy intake restriction; pp. 31–46. [DOI] [PubMed] [Google Scholar]
- Layman DK, Evans E, Baum JI, Seyler J, Erickson DJ, Boileau RA. Dietary protein and exercise have additive effects on body composition during weight loss in adult women. The Journal of nutrition. 2005;135:1903–1910. doi: 10.1093/jn/135.8.1903. [DOI] [PubMed] [Google Scholar]
- Stiegler P, Cunliffe A. Sports medicine. Vol. 36. Auckland, NZ: 2006. The role of diet and exercise for the maintenance of fat-free mass and resting metabolic rate during weight loss; pp. 239–262. [DOI] [PubMed] [Google Scholar]
- Hall KD. Computational model of in vivo human energy metabolism during semistarvation and refeeding. American journal of physiology. 2006;291:E23–37. doi: 10.1152/ajpendo.00523.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]