Abstract
A theoretical equation was developed by Forbes that quantifies the fat-free proportionof a weight change as a function of the initial body fat. However, Forbes’s equation was strictly valid only for infinitesimal weight changes. Here, I extended Forbes’s equation to account for the magnitude and direction of a macroscopic body weight changes. The new equation was also re-expressed in terms of an alternative representation of body composition change defined by an energy partitioning parameter called the P-ratio. The predictions of the resulting equations compared favorably with data from human under-feeding and over-feeding experiments and accounted for previously unexplained trends in the data. The magnitude of the body weight change had a relatively weak effect on the predicted body composition changes and the results were very similar to Forbes’s original equation for modest weight changes. However, for large weight changes, such as the massive weight losses found in patients following bariatric surgery, Forbes’s original equation consistently underestimated the fat-free mass loss, as expected. The new equation that accounts for the magnitude of the weight loss provides better predictions of body composition changes in such patients.
Keywords: Body Composition, Weight loss, Weight gain, Mathematical model
Introduction
Twenty years ago, Forbes remarked that body fat mass (FM) and fat-free mass (FFM) are, “in a sense companions: a change in one ... somehow induces a change in the other, and in the same direction.” (Forbes, 1987). Forbes described an empirical, non-linear relationship between FFM and FM using cross-sectional body composition data and theorized that longitudinal changes of body composition were described by movement along the cross-sectional curve (Forbes, 1987; Forbes, 2000). Based on this theory, Forbes derived a mathematical expression for the FFM proportion of a body weight (BW) change as a function of the initial FM (Forbes, 1987; Forbes, 2000). However, Forbes’s mathematical expression is strictly valid only for infinitesimal weight changes.
Here, I present an extension of Forbes’s theory to account for macroscopic weight changes. The new equation predicts that the composition of weight change depends on both the direction and magnitude of the weight change in addition to the initial FM. The new equation was compared with experimental data from under-feeding and over-feeding in humans. I also describe the relationship between Forbes’s theory and an alternative representation of body composition change that postulates the existence of an energy partitioning parameter, called the P-ratio (Dugdale & Payne, 1977; Payne & Dugdale, 1977b; Payne & Dugdale, 1977a). The P-ratio defines the fraction of an energy imbalance accounted for by changes of the body’s protein reserves. While the P-ratio was originally assumed to be a constant for each individual (Dugdale & Payne, 1977; Payne & Dugdale, 1977b; Payne & Dugdale, 1977a), here I show that the new expression of Forbes’s theory implies that the P-ratio depends on the initial body composition as well as the direction and magnitude of weight change.
Research Methods
In women of similar stature, Forbes found that FFM and FM were related according to the following empirical function, f:
| (1) |
with FFM, and FM in kg (Forbes, 1987; Forbes, 2000). Forbes theorized that longitudinal changes of body composition were described by movement along the cross-sectional curve. For infinitesimal BW changes, dBW, the following equation was derived (Forbes, 1987; Forbes, 2000):
| (2) |
Thus, Forbes’s theory predicted that the contribution of FFM to an infinitesimal weight change depended only on the FM.
For a macroscopic change of BW, ΔBW, I rewrote the equation ΔBW = ΔFFM + ΔFM as:
| (3) |
where FMi and FM f are the initial and final values of the FM, respectively. My goal was to find the equation for ΔFFM/ΔBW such that only FMi and ΔBW were on the right hand side. Such an equation predicts the macroscopic body composition change for a given change of BW and initial FM. To accomplish this goal, FM f was rewritten in terms of FMi and ΔBW as follows:
| (4) |
Since the final BW is equal to the initial BW plus ΔBW, then:
| (5) |
Solving equation 5 for FFM f, and substituting into equation 4 gave the following:
| (6) |
Equation 6 is a transcendental equation for FM f that was solved using the Lambert W function, W (Corless et al., 1996):
| (7) |
Therefore, the macroscopic body composition changes were given by:
| (8) |
Unlike Forbes’s original equation, equation 8 also depends on the sign and magnitude of the BW change. In the limit that ΔBW approaches zero, equation 8 reduces to Forbes’s original equation. I evaluated equation 8 using Mathematica™ software (Wolfram Research Inc.).
To examine the implications of Forbes’s modified equation for the P-ratio, I neglected the contribution of glycogen and assumed a constant proportion of FFM as protein (Dulloo et al., 1996; Dulloo & Jacquet, 1999). Therefore, the P-ratio was calculated as:
| (9) |
where α = 9.05 was the ratio of the energy densities of FM to FFM (Dulloo et al.,1996; Dulloo & Jacquet, 1999).
Results
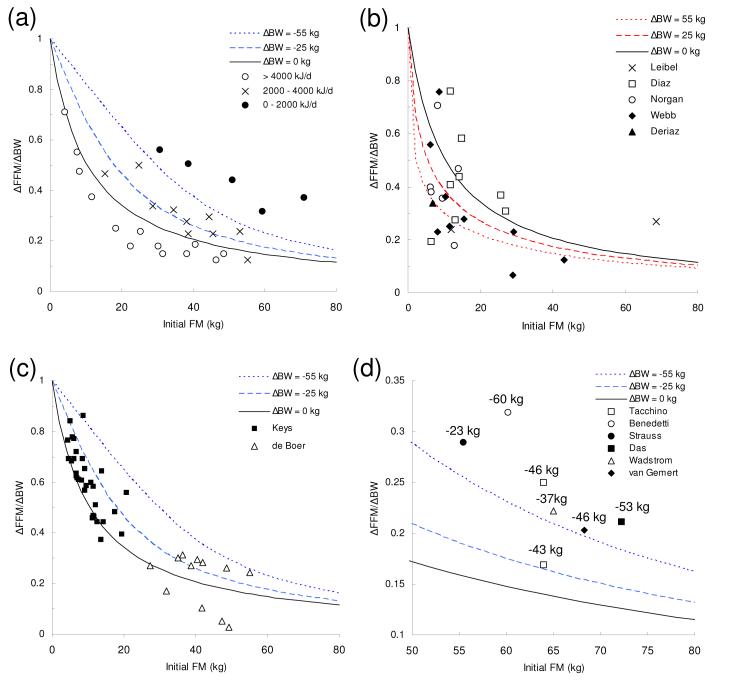
Figures 1(a) and 1(b) show the contribution of FFM to the overall weight change for different degrees of weight loss and weight gain, respectively. The solid curves correspond to Forbes’s original equation 2 for infinitesimal weight changes. Increased initial FM was associated with a smaller contribution of FFM to both weight gain and loss.
Figure 1.
The fat-free proportion of the body weight change (ΔFFM/ΔBW) as a function of initial fat mass (FM) during weight loss (a, c, d) and weight gain (b). The theoretical curves are presented for different degrees of body weight change along with data points from experimental feeding studies in humans. Panel d) shows data for body composition changes following bariatric surgery where the average body weight losses are indicated beside each data point. See the text for a detailed description.
The curves in Figure 1(a) show that larger BW losses resulted in a greater predicted contribution from FFM loss. While Forbes’s original theory did not account for such an effect, he noted that different degrees of energy intake appeared to impact the predicted body composition change (Forbes, 1987; Forbes, 2000). These data are reproduced in Figure 1(a) where the measured average body composition changes are grouped according to the level of energy intake. The subjects with less energy intake, and presumably greater weight loss, tended to have a higher proportion of FFM loss in accordance with the new equation but previously unexplained by Forbes’s equation 2.
Figure 1(b) shows the predicted effects of weight gain, where the curves illustrate that higher initial FM results in a greater the contribution of FM to the total weight gained. Furthermore, as BW increases a greater proportion of the weight gain is accounted for by increased FM. During the review of the present manuscript, a referee kindly pointed out that the weight gain data originally presented by Forbes in support of his theory included data from weight regain studies in anorexic patients (Forbes, 1987; Forbes, 2000). Consequently, after removing the data from the anorexic subjects with very low initial FM, there was insufficient evidence of a relationship between the composition of weight gain and the initial FM. To address this issue, I re-analyzed body composition data from several published weight gain experiments (Norgan & Durnin, 1980; Webb & Annis, 1983; Diaz et al., 1992; Leibel et al., 1995) and plotted the results in Figure 1(b). Data from the individual subjects are plotted with the exception of the studies by Deriaz et al. (Deriaz et al., 1993) and Leibel et al. (Leibel et al., 1995) where I plotted the average values. In accordance with Forbes’s theory, subjects with a higher initial FM tended to gain weight with an increased proportion of FM compared with the subjects with lower initial FM. The average weight gain in these studies was less than 10 kg.
Figure 1(c) plots the individual subject data from the classic Minnesota experiment (Keys, 1950) as well as body composition data from the weight loss study of de Boer et al. (de Boer et al., 1986). These data also appear to support Forbes’s theory regarding the relationship between initial FM and the composition of weight loss. The initially lean young men from the Minnesota experiment lost an average of 17 kg after 6 months of semi-starvation. Accordingly, most of the Minnesota experiment data points fall between Forbes’s original curve and the curve corresponding to ΔBW = -25 kg. The average weight loss from the de Boer study was 11 kg.
When large amounts of weight are lost, Forbes’s original equation 2 differs significantly from the new equation 8. Figure 1(d) plots the average body composition change as a function of initial FM resulting from massive weight loss following various bariatric surgery procedures (Benedetti et al., 2000; van Gemert et al., 2000; Wadstrom et al., 2000; Das et al., 2003; Strauss et al., 2003; Tacchino et al., 2003). The average body weight losses are indicated beside each data point. As expected, Forbes’s original equation consistently underestimated the proportion of FFM lost. For example, equation 2 predicted loss of 6.7 kg of FFM in the patients from the study of Das et al. (Das et al., 2003), whereas the average measured FFM loss was 11.3 kg and the new equation 8 predicted a loss of 9.9 kg.
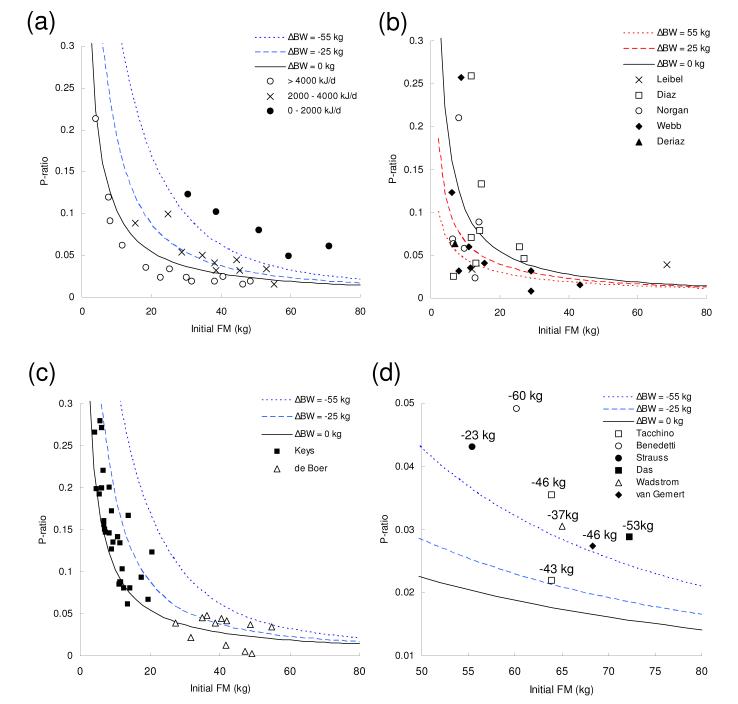
Figure 2 recasts the curves and data from Figure 1 in terms of the P-ratio. Forbes’s theory implies that the P-ratio during under-feeding is a decreasing function of the initial FM, an observation first pointed out by Dulloo et al. (Dulloo et al., 1996; Dulloo & Jacquet, 1999).
Figure 2.
The energy partitioning parameter (P-ratio) as a function of initial fat mass (FM) during weight loss (a, c, d) and weight gain (b).
Discussion
Forbes’s theory provides a framework for describing the interaction between the major determinants of body composition change: the initial FM as well as the direction and magnitude of weight change. Forbes’s original equation only accounted for the initial FM but the new equation 8 presented here now includes the contribution of the body weight change. Interestingly, the new equation demonstrated only a weak dependence on the magnitude of the body weight change as indicated in Figures 1 and 2 by the effect of large changes of body weight. This explains why Forbes’s original equation for infinitesimal weight changes (equation 2) worked so well for modest weight gain and loss.
However, large changes of body weight were predicted to have a significant influence on the composition of the weight change. Forbes’s original equation consistently underestimated the amount of FFM lost following bariatric surgery. Such surgical procedures are becoming increasingly popular for the treatment of obesity and it may be dangerous to erroneously assign a greater proportion of the observed weight loss to decreased body fat. The new equation 8 may provide the basis for better predictions of the relative loss of FFM versus FM following bariatric surgery. I have not adjusted Forbes’s original parameters to optimize the fit to the weight loss data, but such an optimized equation may be a valuable tool for the assessment of bariatric surgery patients.
The vast majority of the bariatric surgery patients depicted in Figures 1(d) and 2(d) were women, and the original parameterization of Forbes’s equation was derived from body composition studies of women. At first glance, it is surprising that the theory is at all applicable to men who typically have significantly higher FFM. However, the predicted body composition changes depend only on the shape of the logarithmic curve, but not its vertical position. If men have a similarly shaped curve, but shifted upwards corresponding to a higher FFM, then theory would hold for men as well as women.
Some investigators have assumed a constant composition of weight loss or gain (Dugdale & Payne, 1977; Payne & Dugdale, 1977b; Payne & Dugdale, 1977a; Kreitzman, 1992). The theory also provides the conditions for such an assumption to be valid. A linear relationship between FFM versus FM results in a body composition change that depends only on the slope of the line and is independent of the initial FM or the weight change (not shown). Thus, a group of subjects operating on an approximately linear part of the FFM versus FM curve would show very little dependence on initial FM or the weight change.
Forbes’s theory is a convenient model for body composition change in humans, but many questions still remain. For example, is it true that longitudinal changes follow the cross-sectional relationship of FFM versus FM? Furthermore, it is unclear whether the FFM versus FM curve would be followed over the entire time course of weight gain and loss, or only after the transients have dissipated and a new steady-state is achieved. Fortunately, these questions are amenable to both experimental as well as theoretical investigation and such studies will likely provide important new insights about how body composition is regulated in humans.
Acknowledgments
FUNDING INFORMATION: Kevin Hall is supported by the Intramural Research Program of the NIH, NIDDK.
REFERENCES
- Benedetti G, Mingrone G, Marcoccia S, Benedetti M, Giancaterini A, Greco AV, Castagneto M, Gasbarrini G. Body composition and energy expenditure after weight loss following bariatric surgery. J Am Coll Nutr. 2000;19:270–274. doi: 10.1080/07315724.2000.10718926. [DOI] [PubMed] [Google Scholar]
- Corless R, Gonnet G, Hare D, Jeffery D, Knuth D. On the Lambert W function. Adv. Computational Maths. 1996;5:329–359. [Google Scholar]
- Das SK, Roberts SB, McCrory MA, Hsu LK, Shikora SA, Kehayias JJ, Dallal GE, Saltzman E. Long-term changes in energy expenditure and body composition after massive weight loss induced by gastric bypass surgery. Am J Clin Nutr. 2003;78:22–30. doi: 10.1093/ajcn/78.1.22. [DOI] [PubMed] [Google Scholar]
- de Boer JO, van Es AJ, Roovers LC, van Raaij JM, Hautvast JG. Adaptation of energy metabolism of overweight women to low-energy intake, studied with whole-body calorimeters. Am J Clin Nutr. 1986;44:585–595. doi: 10.1093/ajcn/44.5.585. [DOI] [PubMed] [Google Scholar]
- Deriaz O, Tremblay A, Bouchard C. Non linear weight gain with long term overfeeding in man. Obes Res. 1993;1:179–185. doi: 10.1002/j.1550-8528.1993.tb00609.x. [DOI] [PubMed] [Google Scholar]
- Diaz EO, Prentice AM, Goldberg GR, Murgatroyd PR, Coward WA. Metabolic response to experimental overfeeding in lean and overweight healthy volunteers. Am J Clin Nutr. 1992;56:641–655. doi: 10.1093/ajcn/56.4.641. [DOI] [PubMed] [Google Scholar]
- Dugdale AE, Payne PR. Pattern of lean and fat deposition in adults. Nature. 1977;266:349–351. doi: 10.1038/266349a0. [DOI] [PubMed] [Google Scholar]
- Dulloo AG, Jacquet J. The control of partitioning between protein and fat during human starvation: its internal determinants and biological significance. Br J Nutr. 1999;82:339–356. doi: 10.1017/s0007114599001580. [DOI] [PubMed] [Google Scholar]
- Dulloo AG, Jacquet J, Girardier L. Autoregulation of body composition during weight recovery in human: the Minnesota Experiment revisited. Int J Obes Relat Metab Disord. 1996;20:393–405. [PubMed] [Google Scholar]
- Forbes GB. Lean body mass-body fat interrelationships in humans. Nutr Rev. 1987;45:225–231. doi: 10.1111/j.1753-4887.1987.tb02684.x. [DOI] [PubMed] [Google Scholar]
- Forbes GB. Body fat content influences the body composition response to nutrition and exercise. Ann N Y Acad Sci. 2000;904:359–365. doi: 10.1111/j.1749-6632.2000.tb06482.x. [DOI] [PubMed] [Google Scholar]
- Keys A. The biology of human starvation. University of Minnesota Press; Minneapolis: 1950. [Google Scholar]
- Kreitzman SN. Factors influencing body composition during very-low-calorie diets. Am J Clin Nutr. 1992;56:217S–223S. doi: 10.1093/ajcn/56.1.217S. [DOI] [PubMed] [Google Scholar]
- Leibel RL, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. N Engl J Med. 1995;332:621–628. doi: 10.1056/NEJM199503093321001. [DOI] [PubMed] [Google Scholar]
- Norgan NG, Durnin JV. The effect of 6 weeks of overfeeding on the body weight, body composition, and energy metabolism of young men. Am J Clin Nutr. 1980;33:978–988. doi: 10.1093/ajcn/33.5.978. [DOI] [PubMed] [Google Scholar]
- Payne PR, Dugdale AE. Mechanisms for the control of body-weight. Lancet. 1977a;1:583–586. doi: 10.1016/s0140-6736(77)92010-4. [DOI] [PubMed] [Google Scholar]
- Payne PR, Dugdale AE. A model for the prediction of energy balance and body weight. Ann Hum Biol. 1977b;4:525–535. doi: 10.1080/03014467700002521. [DOI] [PubMed] [Google Scholar]
- Strauss BJ, Marks SJ, Growcott JP, Stroud DB, Lo CS, Dixon JB, O’Brien PE. Body composition changes following laparoscopic gastric banding for morbid obesity. Acta Diabetol. 2003;40(Suppl 1):S266–269. doi: 10.1007/s00592-003-0083-1. [DOI] [PubMed] [Google Scholar]
- Tacchino RM, Mancini A, Perrelli M, Bianchi A, Giampietro A, Milardi D, Vezzosi C, Sacco E, De Marinis L. Body composition and energy expenditure: relationship and changes in obese subjects before and after biliopancreatic diversion. Metabolism. 2003;52:552–558. doi: 10.1053/meta.2003.50109. [DOI] [PubMed] [Google Scholar]
- van Gemert WG, Westerterp KR, van Acker BA, Wagenmakers AJ, Halliday D, Greve JM, Soeters PB. Energy, substrate and protein metabolism in morbid obesity before, during and after massive weight loss. Int J Obes Relat Metab Disord. 2000;24:711–718. doi: 10.1038/sj.ijo.0801230. [DOI] [PubMed] [Google Scholar]
- Wadstrom C, Backman L, Forsberg AM, Nilsson E, Hultman E, Reizenstein P, Ekman M. Body composition and muscle constituents during weight loss: studies in obese patients following gastroplasty. Obes Surg. 2000;10:203–213. doi: 10.1381/096089200321643313. [DOI] [PubMed] [Google Scholar]
- Webb P, Annis JF. Adaptation to overeating in lean and overweight men and women. Hum Nutr Clin Nutr. 1983;37:117–131. [PubMed] [Google Scholar]