Summary
Purpose
To examine antiepileptogenic, disease-modifying, and anticonvulsant effects of topiramate under conditions of rapid kindling at different stages of development.
Methods
Afterdischarge threshold (ADT) and duration (ADD) were examined in two-, three-, and five-week old Wistar rats before and after administration of topiramate (200 mg/kg). Animals underwent a rapid kindling protocol (sixty 10 second trains, bipolar 20 Hz square wave pulses delivered every five minutes). The progression of behavioral and electrographic seizures, and responses to test stimulations 24 hours after the protocol were compared between topiramate and vehicle treated control rats. In addition, rats that were previously given vehicle only prior to kindling, were then given topiramate to examine the effect on established kindled seizures.
Results
In two-week old animals, topiramate affected neither the baseline afterdischarge, nor the progression of kindled seizures. In three-week old rats, topiramate did not modify the baseline afterdischarge, but significantly delayed the occurrence of full motor seizures in response to repeated stimulations. Topiramate treatment of five-week old rats increased baseline ADT, shortened ADD, and delayed the progression of kindled seizures. Twenty four hours after the last kindling stimulation, animals of all ages exhibited a decreased ADT, an increase ADD, and developed behavioral seizures in response to threshold stimulation. Vehicle treated kindled rats that were then given topiramate displayed significantly attenuated behavioral seizures induced by the threshold stimulation.
Conclusions
Topiramate exhibited age-dependent disease-modifying effects under conditions of rapid kindling, but failed to block epileptogenesis. Topiramate also inhibited kindled seizures with equal efficacy across the three ages.
Keywords: Temporal lobe epilepsy, epileptogenesis, kindling, topiramate, antiepileptic drugs, development
INTRODUCTION
The successful entry of numerous new antiepileptic drugs (AEDs) during the 1990s has contributed to increased treatment options for patients with epilepsy. However, refractory epilepsy remains a significant problem and a third of patients with epilepsy may not achieve freedom from seizures with medical therapy (Kwan & Brodie, 2000). The limited impact of new AEDs on the fraction of patients considered to be medically refractory has prompted a reevaluation of our approach to drug development (Stables et al., 2002). The failure of the “gatekeeper screens” to identify potentially useful compounds was highlighted by the successful emergence of levetiracetam, which failed these screening tests involving acute seizures provoked by electroshock or pentylenetetrazol (Klitgaard, 2001). Further, there is increasing interest in developing drugs that not only block seizures, but may provide disease modification, that is alter the natural course of epilepsy (White, 2003). The concept of disease modification is especially important in those epilepsies that carry the highest burden of medical refractoriness.
A discouraging aspect of studying the disease modification potential of AEDs is the time required for such longitudinal studies. Application of such commonly used protocols as kindling and post-status epilepticus chronic epilepsy (Morimoto et al., 2004) are particularly challenging within the pediatric age range due to rapid maturation of experimental animals, which occurs faster than the progression of chronic epilepsy. In addition to the maturational issue, technical problems such as displaced electrodes can result from the physical growth of the brain and skull. A more rapid throughput is desirable to both extend the concept of disease modification to significant number of candidate AEDs, and to reproduce the whole continuum of epileptogenesis within a certain ontogenic window. A rapid kindling model, initially described by Lothman and coworkers (Lothman et al., 1985; Michelson and Lothman, 1991) might permit the study of the effect of an AED on disease progression much more rapidly than the traditional kindling model or post-status epilepticus epilepsy.
Rapid kindling affords a method by which to observe the epileptogenic process leading to enhanced limbic excitability, as well as the progression of altered cognitive changes and responses to AEDs (Löscher, 2002; Pitkänen and Sutula, 2002; Schmutz et al., 1988). Topiramate is a broad spectrum AED that is commonly used in pediatric patients (Glauser 1997; 1998). In addition to possessing multiple mechanisms of action that may be relevant to its clinical efficacy (Rho and Sankar, 1999; Sankar and Holmes, 2004; White et al., 1997), it also seems to demonstrate exceptional safety compared to traditional AEDs in the immature brain (Bittigau et al., 2002; Glier et al., 2004). Topiramate has also shown disease-modifying potential in developing rats after status epilepticus induced by lithium-pilocarpine treatment in terms of cognitive outcome (Cha et al., 2002) and the development of spontaneous recurrent seizures (Suchomelova et al., 2006). We examined the disease-modifying potential of topiramate under conditions of rapid kindling epileptogenesis in animals during different stages of ontogenic development.
MATERIALS AND METHODS
Animals
The experiments were performed on male Wistar rats (Charles River, Wilmington, MA), of postnatal days 13, 20, or 34 at the time of surgery. The experiments were done in accordance with the policies of the National Institutes of Health and the UCLA Office for the Protection of Research Subjects.
Surgery
Animals were anesthetized with Isoflurane and stereotaxically implanted with a twisted bipolar stimulating electrode (Plastics1 Inc., Roanoke, VA) in the left ventral hippocampus. The coordinates with respect to Bregma were: 13-day old- 3.0 mm posterior, 3.9 mm left, 4.2 mm ventral; 20 day old: 2.9 mm posterior, 3.7 mm left, 3.8 mm ventral; 34 day old- 3.6 mm posterior, 4.9 mm left, 5.0 mm ventral. A tripolar recording electrode (Plastics1 Inc.) was wrapped around skull screws using the nasal bone as the ground. Electrodes were fixed to the skull with Cerebond adhesive (MyNeurolab.com, St. Louis, MO). After surgery, animals were not returned to their respective dams as this pairing can lead to cannibalism or damage to the implants. Since the immature implanted animals were placed in cages rather than directly on the warming pads, the regulator was set at 39°C to insure the maintenance of normal core temperature, since some of the heat transfer was lost through the cages themselves. Further, animals received 2 daily injections of warmed lactated Ringer’s solution until euthanasia.
Kindling procedure and topiramate treatment
The rapid kindling protocol was originally developed for adult rats (Lothman et al., 1985), and was later adapted for immature animals (Michelson and Lothman et al., 1991). In contrast to conventional kindling which requires weeks for full motor seizures to develop, under conditions of rapid kindling, epileptogenesis is compressed to several hours, while still bearing key hallmarks of kindling: appearance and gradual progression of the severity of limbic seizures, and enhanced seizure susceptibility.
Twenty four hours after electrode implantation, the animals were connected to the DS8000 electrical stimulator via DSI100 stimulus isolators (World Precision Instruments) and to the MP100/EEG100B acquisition system (BIOPAC, Santa Barbara, CA). EEG was acquired using AcqKnowledge 3.8 software (BIOPAC) along with simultaneous digital video. Both EEG and behavioral responses were analyzed off-line in a blinded fashion. Throughout the procedure, animals were kept individually in the Plexiglas observation chambers, equipped with water bottles and feeder (Instech Laboratories, Plymouth Meeting, PA)
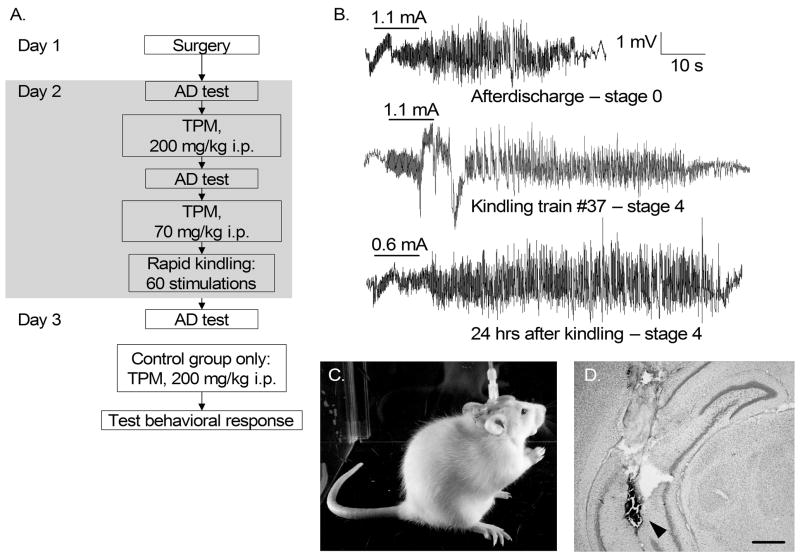
The experimental protocol is outlined in Fig. 1A. At the beginning of the experiment, afterdischarge threshold (ADT) and afterdischarge duration (ADD) were detected by applying electrical stimuli,- 10 s train duration, 20 Hz, one ms pulse duration, square wave monophasic stimuli, starting at 0.2 mA, with 0.1 mA increments, delivered every 10 minutes. After the detection of baseline afterdischarge, evident as a high-frequency response lasting five seconds or longer following the end of the train (Fig. 1B), animals were injected intraperitonealy (i.p.) with topiramate (200 mg/kg, Ortho-McNeil Pharmaceutical, Inc. Raritan, N.J.) dissolved in 25% dimethyl sulfoxide (DMSO, Sigma, St. Louis, MO). Thirty minutes after injection, afterdischarge properties were examined again, and animals received an additional injection of topiramate (70 mg/kg). Rapid kindling was started immediately after the second injection. Control animals received vehicle.
Figure 1. Experimental design.
A. All animals underwent surgery, topiramate (or vehicle) treatment + rapid kindling, and retesting over three consecutive days. Additionally, vehicle-treated kindled rats were given topiramate after Day 3 testing and examined for their behavioral seizure response. Abbreviations: AD- afterdischarge; TPM-topiramate. B. Examples of electrographic recordings obtained from a five-week old control (DMSO vehicle treated) rat in response to the threshold stimulation prior to kindling (top); during the kindling procedure (stimulus train #37, middle), and in response to the threshold stimulation 24 hrs after kindling (bottom). Horizontal bars on the top of each recording mark the stimulus train, and the numbers on the top of the bars reflect the applied current. C. A representative behavioral stage 4 seizure (indicated by rearing) in a five-week old rat. D. A cresyl violet-stained coronal section of the brain of a five-week old rat showing the track of the stimulating electrode (arrowhead) in the ventral hippocampus Scale bar: 500 μ.
Kindling consisted of 60 trains delivered every five minutes using the parameters described above using a current of 100 μA over the ADT (total procedure duration was 5 hours). Behavioral seizures were scored using the following scale: 1- Motor arrest and twitching vibrissae; 2 – chewing, head bobbing; 3- forelimb clonus; 4- forelimb clonus and rearing (Fig. 1C); 5- rearing and falling. Kindling progression was analyzed by calculating the number of stimulations required to reach each consecutive seizure score; the number of stage 4–5 seizures; and the duration of electrographic correlates of stage 4–5 convulsions. Twenty four hours after the end of the rapid kindling procedure, animals were reconnected to the stimulating/recording system and afterdischarge properties were studied again.
Rats from the control (DMSO-treated) groups were further studied to examine the effects of topiramate on the seizure response of kindled animals. Twenty four hours after kindling, the control rats were injected with topiramate (200 mg/kg) and behavioral seizure response was examined 30 min after drug injection using their respective threshold stimulus.
After the end of the experiments, animals were anesthetized with pentobarbital (100 mg/kg), and underwent intracardiac perfusion with saline followed by paraformaldehyde. The placement of the stimulating electrode was verified in 40 micron thick coronal sections that were cut using a sliding microtome and stained with cresyl violet (Fig. 1D).
Statistical analysis
Data were analyzed using Prizm 4 software (GraphPad, San Diego, CA). First we examined whether the values for each parameter fit a Gaussian distribution, using the Kolmogorov-Smirnov test; since the distribution for all the examined data was normal, further analyses were performed using parametric tests. The following tests were applied, as indicated in the table and figure legends: one-way ANOVA followed by Bonferroni post hoc test for across-the-age comparisons of the same parameters; unpaired Student t-test for the same age comparison between control and topiramate-treated animals; paired Student t-test for comparisons of (a) same parameters before and after topiramate treatment for each age, and (b) same parameters before and after kindling for each age. Each group included six animals.
RESULTS
Kindling in naïve animals
Afterdischarge properties and kindling progression in naive animals of different ages are shown in Table 1. While no statistically significant differences were observed in the ADT among the three age groups, the duration of afterdischarge was longer in both three- and five-week old rats, as compared to two-week old pups. Administration of vehicle did not modify baseline afterdischarge properties (Figures 2A–4A).
Table 1.
Afterdischarge properties and parameters of rapid kindling in rat pups of different ages.
| Age | Two-week old | Three-week old | Five-week old |
|---|---|---|---|
| Afterdischarge properties before kindling | |||
| Threshold, mA | 1.2±0.1 | 1.1±0.1 | 1.2±0.2 |
| Duration, s | 21.5±1.7 | 31.5±2.9 * | 37.9±2.9 * |
| Kindling parameters | |||
| Number of stimulations to the 1st stage 1 | 6.2±0.8 | 5.5±0.96 | 5.4±1.4 |
| Number of stimulations to the first stage 4 | 21.3±2.1 | 17.5±2.9 | 12.2±1.3 * |
| Total number of stage 4–5 seizures | 19.3±2.6 | 24.0±3.1 | 26.6±1.3 |
| EEG seizure duration, s | 45.7±4.5 | 56.6±8.6 | 60.2±5.7 |
| Responses 24 hours after kindling | |||
| Afterdischarge threshold, mA | 0.7±0.1 † | 0.5±0.05 † | 0.5±0.05 † |
| Afterdischarge duration, s | 55.7±6.5 † | 72.5±9.0 † | 79.4±9.1 † |
| Behavioral seizure score in response to the threshold stimulation | 2.7±0.3 | 3.5±0.3 | 3.43±0.4 |
Each group included 6 animals.
p<0.05 vs. Two-week old animals (one-way ANOVA+Bonferroni post hoc test).
- p<0.05 vs. the same parameter for the same age before kindling (paired t-Student test).
Figure 2. Afterdischarge (AD) properties and kindling progression in two-week old rats.
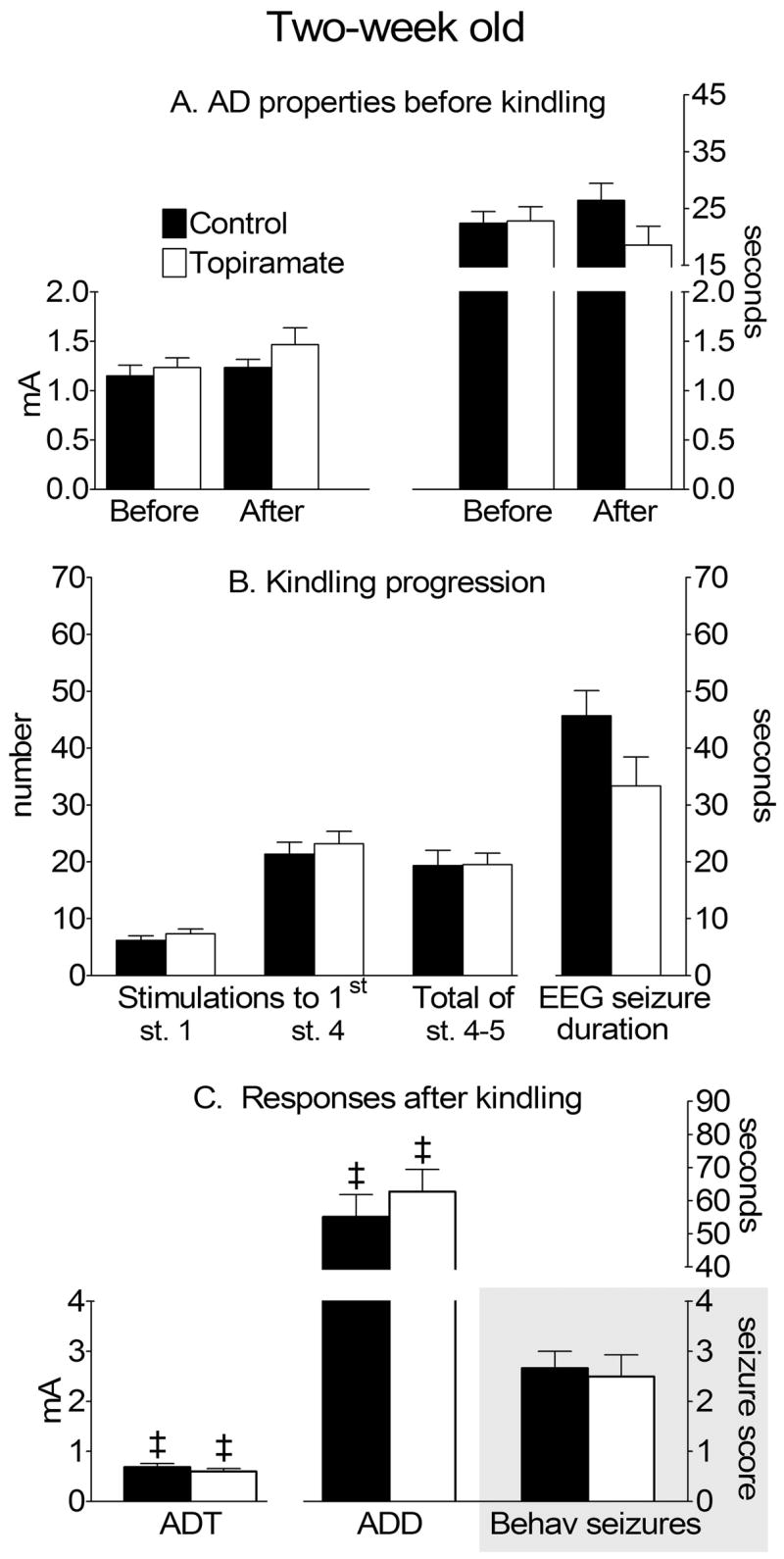
A AD properties before kindling. Afterdischarge threshold (ADT) is on the left ordinate, and duration (ADD)- is on the top right ordinate. “Before” and “After” refers to either DMSO (Control) or topiramate treatment. B. Kindling progression. Number of stimulations and seizure count are on the left, and EEG seizure duration is on the right ordinates. C. Electrographic and behavioral responses 24 hours after kindling, 29 hours after topiramate treatment. ADT is on the left, and ADD is on the top right ordinate. Behavioral seizure score is on the lower right ordinate, shaded area. ‡- p<0.05 vs. similar parameters in the same groups before kindling (paired Student t-test).
Figure 4. Afterdischarge (AD) properties and kindling progression in five-week old rats.
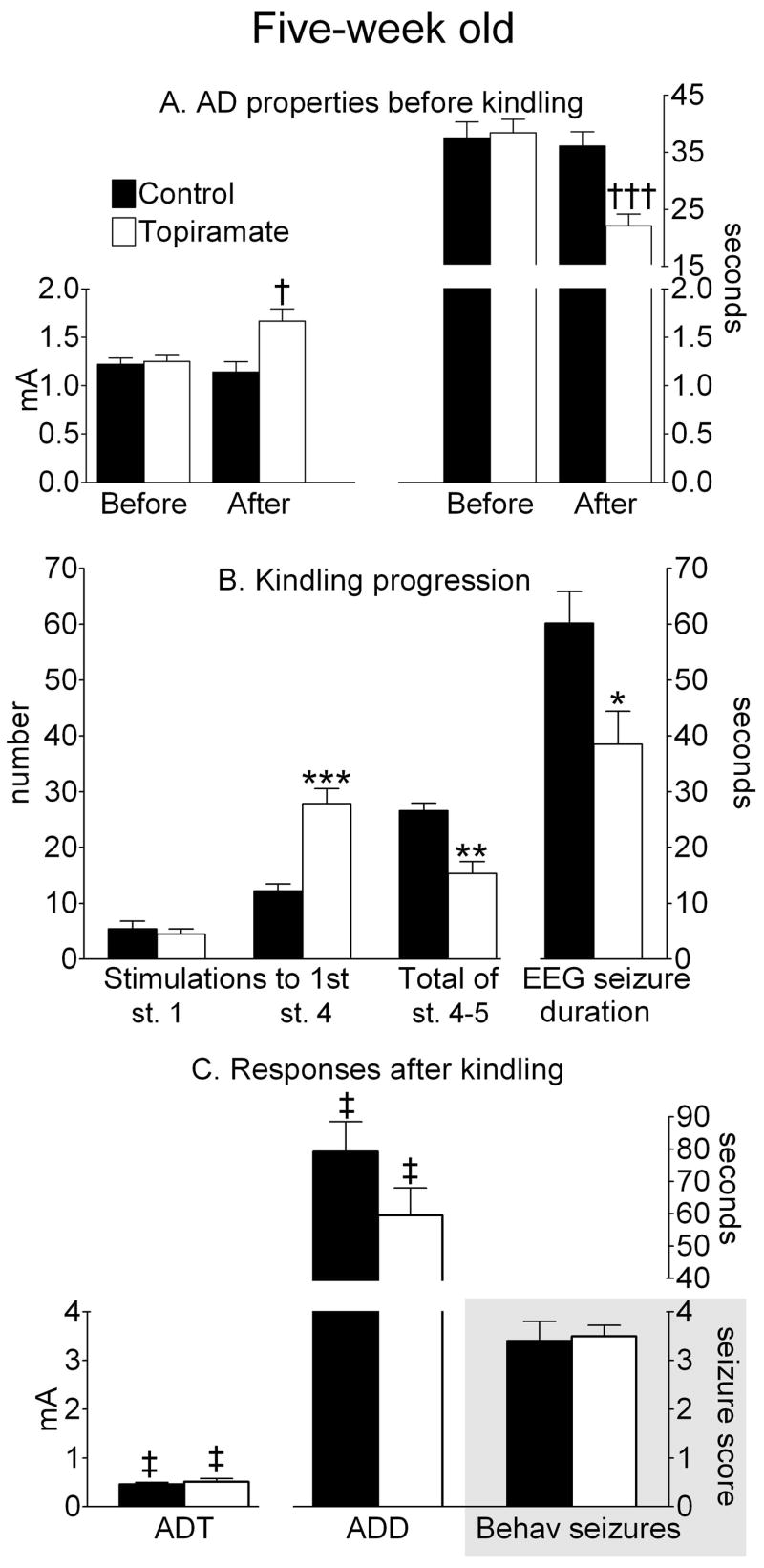
A AD properties before kindling. Afterdischarge threshold (ADT) is on the left ordinate, and duration (ADD)- is on the right ordinates. “Before” and “After” refers to either DMSO (Control) or topiramate treatment. †-p<0.01; †† dagger;- p<0.001 vs. Before treatment (paired Student t-test). B. Kindling progression. Number of stimulations and seizure count are on the left, and EEG seizure duration is on the right ordinates.; *- p<0.05; **- p<0.01 vs. ***- p<0.001 Control (Student T-test). C. Electrographic and behavioral responses 24 hours after kindling (29 hours after topiramate treatment). ADT is on the left, and ADD is on the right ordinate. Behavioral seizure score is on the lower right ordinate, shaded area. ‡- p<0.05 vs. similar parameters in the same groups before kindling (paired Student t-test).
During kindling procedure, average number of stimulations required to develop first stage 1 seizure was not different among the three ages. However, five-week old animals required fewer stimulations to develop first full motor seizure (stage 4), than both two- and three-week old pups. Despite this fact, no age-dependent significant differences were recorded for the number of full motor seizures (stage 4–5) developed during the course of kindling procedure, although a trend towards an increase of the number of such responses was observed as the function of age. Similar non-significant trend was detected for the duration of electrographic seizure responses. Upon the completion of full motor seizures, animals exhibited brief (10–15 s) periods of wet dog shakes, after which they quickly returned to normal behavior, such as exploration, grooming, drinking and food consumption. Animals did not exhibit visible signs of postictal depression (inhibition of motor function, posture, exophtalmus etc).
Twenty four hours after kindling procedure, the reduction of the ADT and the increase of the ADD were recorded in the animals of all three ages. The extent of the changes in the afterdischarge properties was not different across the ages. Furthermore, in response to threshold stimulation all animals developed behavioral convulsions, the severity of which varied between stages 2 and 4; in two-week old group, full motor seizure was observed in one rat, and in three- and five-week old in 3 rats.
Effects of topiramate
Administration of topiramate (200+70 mg/kg) induced no visible changes in animals’ behavior.
Baseline afterdischarge properties
Treatment with topiramate (200 mg/kg) affected neither ADT, nor ADD in two- and three-week old animals (Figure 2A, 3A). However, in five-week old rats topiramate significantly increased the threshold (1.7±0.13 mA, p<0.05 vs. Before topiramate), and shortened the duration of afterdischarge (21.8±2.1 s, p<0.0001 vs. Before topiramate, Fig. 4A).
Figure 3. Afterdischarge (AD) properties and kindling progression in three-week old rats.
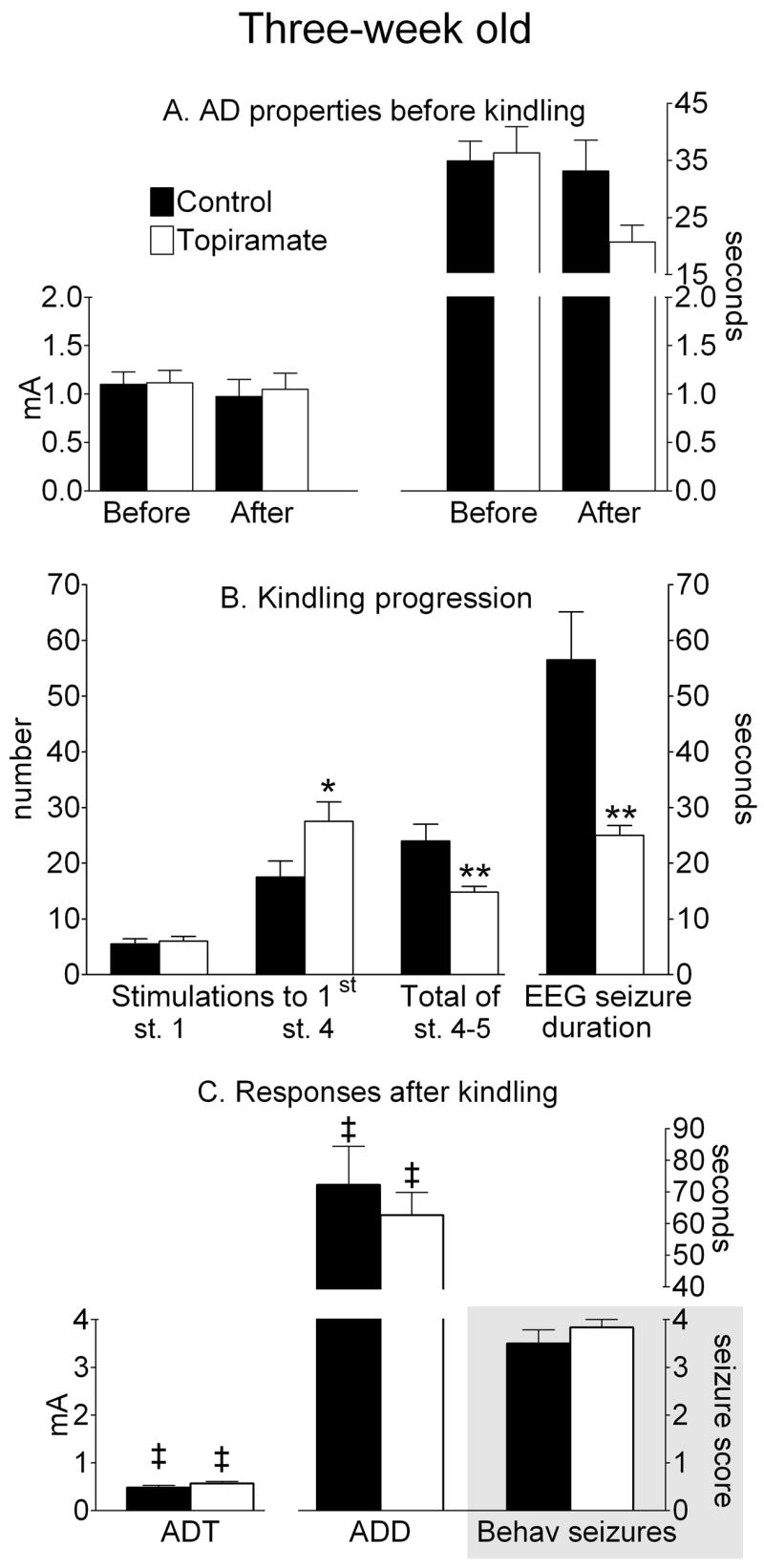
A AD properties before kindling. Afterdischarge threshold (ADT) is on the left ordinate, and duration (ADD)- is on the right ordinate. “Before” and “After” refers to either DMSO (Control) or topiramate treatment. B. Kindling progression. Number of stimulations and seizure count are on the left, and EEG seizure duration is on the right ordinates.; *- p<0.05; **- p<0.01 vs. Control (Student T-test). C. Electrographic and behavioral responses 24 hours after kindling 29 hours after topiramate treatment. ADT is on the left, and ADD is on the top right ordinate. Behavioral seizure score is on the lower right ordinate, shaded area. ‡- p<0.05 vs. similar parameters in the same groups before kindling (paired Student t-test).
Kindling progression
In two-week old rats, treatment with topiramate affected neither of the kindling parameters (Fig. 2B). At the age of three weeks, topiramate treatment did not affect the occurrence of the stage 1 seizure response (Figure 3B). However, the development of full motor seizures was delayed (27.5±3.5 stimulations, p<0.05 vs. control). Furthermore, EEG correlates of full motor seizures were shorter (27±1.8 s, p<0.01 vs. control), and the total number of full motor seizures was lower (14.8±1.1, p<0.01 vs. control, Fig. 3B).
In five-week old rats, topiramate treatment delayed the progression of full motor seizures (27.8±2.7 stimulations, p<0.001 vs. control), reduced the number of stage 4–5 convulsions (15.3±2.1, p<0.01 vs. control) and shortened an average seizure duration to 38.5±5.9 s (p<0.05 vs. control, Fig. 4B).
Topiramate treatment did not prevent the occurrence of full motor seizures in any animal of any age tested.
Responses after kindling
Twenty four hours after kindling procedure, all topiramate-treated animals at all ages exhibited the reduction of ADT and the increase of ADD, similar to those observed in control rats (p>0.05); the changes were statistically different as compared to the pre-kindling values (Figures 2–4). Threshold electrical stimulation induced behavioral seizures of stage 2–4 regardless of age or treatment (Panel C on Figures 2–4).
Kindled seizures
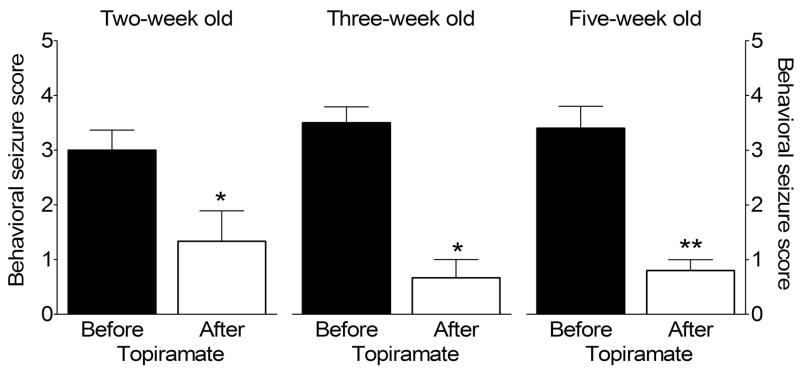
We examined whether treatment with topiramate would modify seizure responses in already kindled animals. Using the control animals from each of the three ages which had been treated with vehicle only and were then kindled, administration of topiramate to these rats significantly attenuated the severity of behavioral seizures in response to the threshold stimulations (Fig. 5) However, the extent of anticonvulsant effect was more pronounced at five weeks of age (p<0.01 vs. before topiramate injection), than in both two- and three-week old rats (p<0.05 vs. before topiramate treatment).
Figure 5. Effect of topiramate on kindled seizures.
These data are obtained from control animals that had been treated with DMSO prior to kindling. After detection of afterdischarge properties, the animals received topiramate (200 mg/kg i.p.), followed by test electrical stimulation with the threshold current 30 minutes after injection. *- p<0.05, **- p<0.01, vs. before topiramate for the same age (paired Student t-test).
DISCUSSION
Our experiments showed that topiramate exhibited age-dependent anticonvulsant effects under conditions of rapid kindling. Comparison of the effects of topiramate on baseline afterdischarge properties and the rate of kindling reveals that the inhibitory and antiepileptic efficacy of topiramate increases as a function of age. The term “disease modifications” is employed to convey the concept that while a treatment may not prevent the occurrence of a disease, it may nevertheless modify the natural course of the disease. The ability to retard the progression of a neurologically debilitating disease is therapeutically valuable.
The present study demonstrates the value of the rapid kindling model to study disease modification in terms of retardation of the development of motor seizures in response to repeated stimulations. The dose of topiramate employed by us was derived from a reasonable estimate of appropriate dose for these studies based on the literature. Amano et al (1998) found only doses of 100 mg/kg and 200 mg/kg to retard amygdaloid kindling. They began to see some sedation at the higher dose in mature rats. Our animals received 200 mg/kg plus an additional 70 mg/kg dose to help maintain adequate levels of topiramate through the rapid kindling procedure which took five hours. Shank et al (1994) reported a peak effect of topiramate fours after oral administration in its ability to protect rats against maximal electroshock-induced convulsions and reported duration of action of at least sixteen hours.
We performed our experiments on two-, three- and five-week old animals. Our previous studies (Sankar et al., 1998) involving lithium-pilocarpine status epilepticus (SE) showed a dramatic increase in SE-induced epileptogenesis from two-week old to three week-old animals and these ages were logical choices for our laboratory. It has been observed that boys may sometimes experience increased frequency and severity of seizures during adolescence (Morrell, 1992). In male rats, testosterone and its metabolites enhanced the development of amygdala-kindled seizures (Edwards et al., 1999). These clinical and laboratory observations prompted as to verify if the test AED remains effective at that stage of development, and include the five-week old animals.
Our results involving both three- and five-week old animals are remarkably similar to those of Amano et al. (1998). We have been able to demonstrate a significant increase in the number of stimulations required to elicit the first motor seizure at the age of three weeks, and an even greater increase in five-week old rats treated with topiramate compared to controls, very much like in the study of Amano et al. (1998), but in a much shorter experiment involving only 5 hours of stimulation. To facilitate rapid kindling, we applied lower frequency (20 Hz), longer duration (10 s) trains to the ventral hippocampus in contrast to the 60 Hz, 1 s trains applied to the amygdala by Amano et al. The parameters that subserve rapid kindling have been studied in detail by Lothman and Williamson (1993; 1994). Other investigators have explored these parameters in developing animals (Moshé et al., 1981; Holmes and Thompson, 1987). To our knowledge, this method has not been exploited previously to study disease modification in the treatment of epilepsy. The striking similarity of our results to those of Amano et al. (1998) encourages further development of our approach.
As initially observed by Lothman and Williamson (1994), the rapid kindling paradigm does not produce a lasting or stable kindled state. Our primary aim was to evaluate the effect of an AED on the rate of acquisition of kindling, rather than to produce permanent epilepsy. We focused on the “journey” representing the epileptogenic process, rather than the “destination” of lasting spontaneous seizures. Recently, De Smedt et al. (2005) employed a variation on the rapid kindling model (rapid kindling with recurrent hippocampal seizures) to study levetiracetam. That study evaluated the modification of the seizure stage and ADD by levetiracetam after three days of stimulations, but did not evaluate the rate of kindling acquisition under levetiracetam treatment. Similarly, Lado et al. (2001) used a rapid kindling to study the anticonvulsant effects of gabapentin in developing rats. Their study also involved the effect of gabapentin on kindled seizures rather than on the kindling acquisition process. Moshé et al (1992) have characterized kindling in immature rats and had observed that immature rats have very short periods of postictal refractoriness. Although Löscher and Honack (1990) found that the reduction of interstimulus intervals may modify the effectiveness of anticonvulsant therapy, the discussed studies prove generally validity of rapid kindling in evaluating the efficacy of AED therapy. Furthermore, in our experiments animals of all ages underwent the same stimulation protocol, and while it can be argued that the pharmacological profile of topiramate may be somewhat different when applied to conventional kindling, our studies were designed to reveal age-dependent effects of AED therapy.
We could not demonstrate an inhibitory effect of topiramate as measured by the ADT or ADD using our stimulation parameters and stimulation site in two- and three-week old animals. A modest effect on these parameters could be demonstrated at the age of five weeks. The variability of ADT as a function of train duration and stimulus frequency has been studied by Lothman and Williamson (1992), who found that the variability was highest at low train durations and the ADT became unvariate at higher train durations. Those data, taken with our results, suggest that the baseline afterdischarge properties, which likely reflect ambient excitability of the non-epileptic hippocampus, may not be the ideal parameters for evaluating the therapeutic potential of AEDs. At the same time, this observation might be age-dependent, as Reissmuller et al. (2000) found that topiramate increased ADT in adult rats.
The evolution of limbic seizures appeared to be sensitive to AED intervention. As rapid kindling progressed, topiramate treated three- and five-week old rats displayed fewer stage 4/5 seizures as compared to their respective DMSO-treated controls, as well as diminished EEG seizure durations. That focal afterdischarges may be much more resistant to anticonvulsants than are stage 4 or 5 seizures has been observed in earlier studies by other investigators (Albright & Burnham, 1980; McNamara et al, 1989) involving traditional kindling and several classic AEDs. Wauquier and Zhou (1996) have reported on the potent anticonvulsant activity of topiramate on a variety of kindling properties including afterdischarge properties.
Despite the demonstrated retardation of rapid kindling acquisition, topiramate-treated animals in all age groups exhibited enhanced hippocampal excitability (as determined by ADT) and seizure response 24 hours after kindling. This finding suggests that the increased seizure susceptibility, which represents a hallmark of the kindling process, was not modified by topiramate. This observation is not surprising since topiramate treatment did not completely abolish the occurrence of full motor seizures, which are a prerequisite for the maintenance of the kindled state (Racine, 1972). However, topiramate application to already kindled rats (previously given vehicle only) did suppress behavioral seizures, similar to the efficacy in kindled animals reported by others (Wauquier and Zhou, 1996; Amano et al., 1998).
In the present study, topiramate retarded kindling acquisition robustly in more mature, but not in two-week old animals. This finding contrasts with the results seen in a status epilepticus model where a single treatment with topiramate in postnatal day 14 rat pups early during the course of SE completely blocked the subsequent development of spontaneous recurrent seizures (Suchomelova et al., 2006), but the disease-modifying effect was less pronounced in older animals (four-week old). The influence of models on the outcome of SE has been noted by us (Sankar et al., 2000) and others (Cilio et al., 2003). Treatment with topiramate after lithium-pilocarpine status epilepticus at postnatal day 21 (Cha et al., 2002) or after repetitive neonatal seizures (Zhao et al., 2005) resulted in improved cognitive function as determined by water maze performance. In the model involving perinatal hypoxia-induced seizures, Koh et al. found both acute (2001) and chronic disease modifying (2004) results with topiramate treatment. These findings, taken with those of Bittigau et al. (2002) and Glier et al. (2004) which demonstrated a superior safety profile for topiramate compared to many traditional AEDs in the immature brain, suggest that topiramate is a distinctive and important AED for pediatric applications, limited to some extent by the lack of availability of a parenteral dosage form for application in neonates after hypoxic-ischemic insults (Sankar and Painter, 2005).
In conclusion, topiramate did not modify ambient excitability of the hippocampus of the immature rats. Interestingly, the inability of topiramate to modify ambient hippocampal excitability might be a desirable property because an AED that possesses such an effect may have a greater adverse impact on the execution of normal brain functions. Furthermore, topiramate delayed the evolution of epileptogenesis in three- and five-week old rats, but did not prevent the establishment of the kindled state. Finally, topiramate was effective in suppressing acute kindled seizures. Thus, in the rapid kindling model, topiramate exhibited age-dependent disease-modifying abilities and age-independent anticonvulsant effects.
The utility of rapid kindling as a model of “compressed” epileptogenesis remains to be validated more completely. It is not advanced as a replacement for studies involving epileptogenesis that follows status epilepticus. The advantages and disadvantages of kindling in immature animals have been discussed in detail (Moshé et al., 1993) and rapid kindling overcomes one of the major disadvantages, namely the duration involved. Our goal was to explore a method in which candidate AEDs can be compared for antiepileptogenic activity with a more rapid throughput than is possible with traditional kindling, and also accommodate young animals during a stage of rapid brain growth. Rapid kindling is likely better suited to studying the early molecular and cellular changes than the long-term circuit rearrangements seen in chronic TLE. It remains to be demonstrated if intervention with the early molecular and cellular processes can prove a useful strategy to minimize the long-term consequences. However, if the protocol is proven to be adequate, it can become an excellent tool for studying antiepileptogenic/disease-modifying effects of emerging AEDs with high throughput and efficiency. An important advantage of the model is the ability to examine pediatric epileptogenesis without concerns about physical growth of the brain and skull. Additionally, the model is distinctive in that it allows studying the whole epileptogenic process from the onset to the end, within a defined ontogenic window.
Acknowledgments
This work was supported by NS046516 and the DAPA Foundation. The authors thank Ortho-McNeil Pharmaceuticals for a gift of topiramate. We are grateful for the critical reading of the manuscript by Dr. H. Steve White, and his suggestions.
References
- Albright PS, Burnham WM. Development of a new pharmacological seizure model: effects of anticonvulsants on cortical- and amygdala-kindled seizures in the rat. Epilepsia. 1980;21:681–689. doi: 10.1111/j.1528-1157.1980.tb04321.x. [DOI] [PubMed] [Google Scholar]
- Amano K, Hamada K, Yagi K, Seino M. Antiepileptic effects of topiramate on amygdaloid kindling in rats. Epilepsy Research. 1998;31:123–128. doi: 10.1016/s0920-1211(98)00021-7. [DOI] [PubMed] [Google Scholar]
- Bittigau P, Sifringer M, Genz K, Reith E, Pospischil D, Govindarajalu S, Dzietko M, Pesditschek S, Mai I, Dikranian K, Olney JW, Ikonomidou C. Antiepileptic drugs and apoptotic neurodegeneration in the developing brain. The Proceedings of the National Academy of Sciences, USA. 2002;99:15089–15094. doi: 10.1073/pnas.222550499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha BH, Silveira DC, Liu X, Hu Y, Holmes GL. Effect of topiramate following recurrent and prolonged seizures during early development. Epilepsy Research. 2002;51:217–232. doi: 10.1016/s0920-1211(02)00157-2. [DOI] [PubMed] [Google Scholar]
- Cilio MR, Sogawa Y, Cha BH, Liu X, Huang LT, Holmes GL. Long-term effects of status epilepticus in the immature brain are specific for age and model. Epilepsia. 2003;44:518–528. doi: 10.1046/j.1528-1157.2003.48802.x. [DOI] [PubMed] [Google Scholar]
- De Smedt T, Vonck K, Raedt R, Dedeurwaerdere S, Claeys P, Legros B, Wyckhuys T, Wadman W, Boon P. Rapid kindling in preclinical anti-epileptic drug development: the effect of levetiracetam. Epilepsy Research. 2005;67:109–116. doi: 10.1016/j.eplepsyres.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Edwards HE, Burnham WM, MacLusky NJ. Testosterone and its metabolites affect afterdischarge thresholds and the development of amygdala kindled seizures. Brain Research. 1999;838:151–157. doi: 10.1016/s0006-8993(99)01620-0. [DOI] [PubMed] [Google Scholar]
- Glauser TA. Topiramate. Seminars in Pediatric Neurology. 1997;4:34–42. doi: 10.1016/s1071-9091(97)80007-1. [DOI] [PubMed] [Google Scholar]
- Glauser TA. Topiramate use in pediatric patients. The Canadian Journal of Neurological Sciences. 1998;25:S8–12. doi: 10.1017/s0317167100034843. [DOI] [PubMed] [Google Scholar]
- Glier C, Dzietko M, Bittigau P, Jarosz B, Korobowicz E, Ikonomidou C. Therapeutic doses of topiramate are not toxic to the developing rat brain. Experimental Neurology. 2004;187:403–409. doi: 10.1016/j.expneurol.2004.01.025. [DOI] [PubMed] [Google Scholar]
- Holmes GL, Thompson JL. Rapid kindling in the prepubescent rat. Brain Research. 1987;433:281–284. doi: 10.1016/0165-3806(87)90032-0. [DOI] [PubMed] [Google Scholar]
- Klitgaard H. Levetiracetam: the preclinical profile of a new class of antiepileptic drugs? Epilepsia. 2001;42(Suppl 4):13–18. [PubMed] [Google Scholar]
- Koh S, Jensen FE. Topiramate blocks perinatal hypoxia-induced seizures in rat pups. Annals of Neurology. 2001;50:366–372. doi: 10.1002/ana.1122. [DOI] [PubMed] [Google Scholar]
- Koh S, Tibayan FD, Simpson JN, Jensen FE. NBQX or topiramate treatment after perinatal hypoxia-induced seizures prevents later increases in seizure-induced neuronal injury. Epilepsia. 2004;45:569–575. doi: 10.1111/j.0013-9580.2004.69103.x. [DOI] [PubMed] [Google Scholar]
- Kwan P, Brodie MJ. Early identification of refractory epilepsy. The New England Journal of Medicine. 2000;342:314–319. doi: 10.1056/NEJM200002033420503. [DOI] [PubMed] [Google Scholar]
- Lado FA, Sperber EF, Moshé SL. Anticonvulsant efficacy of gabapentin on kindling in the immature brain. Epilepsia. 2001;42:458–463. doi: 10.1046/j.1528-1157.2001.30900.x. [DOI] [PubMed] [Google Scholar]
- Löscher W. Animal models of epilepsy for the development of antiepileptogenic and disease-modifying drugs. A comparison of the pharmacology of kindling and post-status epilepticus models of temporal lobe epilepsy. Epilepsy Research. 2002;50:105–123. doi: 10.1016/s0920-1211(02)00073-6. [DOI] [PubMed] [Google Scholar]
- Löscher W, Honack D. The effect of interstimulation interval on the assessment of anticonvulsant drug potency in fully kindled rats. Epilepsy Res. 1990;7:182–196. doi: 10.1016/0920-1211(90)90014-m. [DOI] [PubMed] [Google Scholar]
- Lothman EW, Hatlelid JM, Zorumski CF, Conry JA, Moon PF, Perlin JB. Kindling with rapidly recurring hippocampal seizures. Brain Research. 1985;360:83–91. doi: 10.1016/0006-8993(85)91223-5. [DOI] [PubMed] [Google Scholar]
- Lothman EW, Williamson JM. Influence of electrical stimulus parameters on afterdischarge thresholds in the rat hippocampus. Epilepsy Research. 1992;13:205–213. doi: 10.1016/0920-1211(92)90054-w. [DOI] [PubMed] [Google Scholar]
- Lothman EW, Williamson JM. Rapid kindling with recurrent hippocampal seizures. Epilepsy Research. 1993;14:209–220. doi: 10.1016/0920-1211(93)90045-9. [DOI] [PubMed] [Google Scholar]
- Lothman EW, Williamson JM. Closely spaced recurrent hippocampal seizures elicit two types of heightened epileptogenesis: a rapidly developing, transient kindling and a slowly developing, enduring kindling. Brain Research. 1994;649:71–84. doi: 10.1016/0006-8993(94)91050-2. [DOI] [PubMed] [Google Scholar]
- McNamara JO, Rigsbee LC, Butler LS, Shin C. Intravenous phenytoin is an effective anticonvulsant in the kindling model. Annals of Neurology. 1989;26:675–678. doi: 10.1002/ana.410260514. [DOI] [PubMed] [Google Scholar]
- Michelson HB, Lothman EW. An ontogenetic study of kindling using rapidly recurring hippocampal seizures. Developmental Brain Research. 1991;61:79–85. doi: 10.1016/0165-3806(91)90116-z. [DOI] [PubMed] [Google Scholar]
- Morimoto K, Fahnestock M, Racine RJ. Kindling and status epilepticus models of epilepsy: rewiring the brain. Progress in Neurobiology. 2004;73:1–60. doi: 10.1016/j.pneurobio.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Morrell MJ. Hormones and epilepsy through the lifetime. Epilepsia. 1992;33:S49–S61. doi: 10.1111/j.1528-1157.1992.tb06227.x. [DOI] [PubMed] [Google Scholar]
- Moshé SL, Sharpless NS, Kaplan J. Kindling in developing rats: variability of afterdischarge thresholds with age. Brain Research. 1981;211:190–195. doi: 10.1016/0006-8993(81)90082-2. [DOI] [PubMed] [Google Scholar]
- Moshé SL, Sperber EF, Haas K, Xu S, Shinnar S. Effects of the maturational process on epileptogenesis. In: Lüders H, editor. Epilepsy Surgery. Raven Press; New York: 1992. pp. 741–8. [Google Scholar]
- Moshé SL, Stanton PK, Sperber EF. Sensitivity of the immature central nervous system to epileptogenic stimuli. In: Schwartzkroin PA, editor. Epilepsy: Models, mechanisms, and concepts. Cambridge University Press; Cambridge: 1993. pp. 171–198. [Google Scholar]
- Pitkänen A, Sutula TP. Is epilepsy a progressive disorder? Prospects for new therapeutic approaches in temporal-lobe epilepsy. The Lancet Neurology. 2002;1:173–181. doi: 10.1016/s1474-4422(02)00073-x. [DOI] [PubMed] [Google Scholar]
- Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalography and Clinical Neurophysiology. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- Reissmuller E, Ebert U, Löscher W. Anticonvulsant efficacy of topiramate in phenytoin-resistant kindled rats. Epilepsia. 2000;41:372–379. doi: 10.1111/j.1528-1157.2000.tb00176.x. [DOI] [PubMed] [Google Scholar]
- Rho JM, Sankar R. The pharmacologic basis of antiepileptic drug action. Epilepsia. 1999;40:1471–1483. doi: 10.1111/j.1528-1157.1999.tb02029.x. [DOI] [PubMed] [Google Scholar]
- Sankar R, Holmes GL. Mechanisms of action for the commonly used antiepileptic drugs: relevance to antiepileptic drug-associated neurobehavioral adverse effects. Journal of Child Neurology. 2004;19(Suppl 1):S6–14. doi: 10.1177/088307380401900102. [DOI] [PubMed] [Google Scholar]
- Sankar R, Painter MJ. Neonatal seizures: after all these years we still love what doesn’t work. Neurology. 2005;64:776–777. doi: 10.1212/01.WNL.0000157320.78071.6D. [DOI] [PubMed] [Google Scholar]
- Sankar R, Shin DH, Liu H, Mazarati A, Pereira de Vasconcelos A, Wasterlain CG. Patterns of status epilepticus-induced neuronal injury during development and long-term consequences. J Neurosci. 1998;18:8382–8393. doi: 10.1523/JNEUROSCI.18-20-08382.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankar R, Shin D, Mazarati AM, Liu H, Katsumori H, Lezama R, Wasterlain CG. Epileptogenesis after status epilepticus reflects age- and model-dependent plasticity. Annals of Neurology. 2000;48:580–589. [PubMed] [Google Scholar]
- Schmutz M, Klebs K, Baltzer V. Inhibition or enhancement of kindling evolution by antiepileptics. Journal of Neural Transmission. 1988;72:245–257. doi: 10.1007/BF01243423. [DOI] [PubMed] [Google Scholar]
- Shank RP, Gardocki JF, Vaught JL, Davis CB, Schupsky JJ, Raffa RB, Dodgson SJ, Nortey SO, Maryanoff BE. Topiramate: preclinical evaluation of structurally novel anticonvulsant. Epilepsia. 1994;35:450–460. doi: 10.1111/j.1528-1157.1994.tb02459.x. [DOI] [PubMed] [Google Scholar]
- Stables JP, Bertram EH, White HS, Coulter DA, Dichter MA, Jacobs MP, Löscher W, Lowenstein DH, Moshe SL, Noebels JL, Davis M. Models for epilepsy and epileptogenesis: report from the NIH workshop, Bethesda, Maryland. Epilepsia. 2002;43:1410–1420. doi: 10.1046/j.1528-1157.2002.06702.x. [DOI] [PubMed] [Google Scholar]
- Suchomelova L, Baldwin RA, Kubova H, Thompson KW, Sankar R, Wasterlain CG. Treatment of experimental status epilepticus in immature rats: dissociation between anticonvulsant and antiepileptogenic effects. Pediatric Research. 2006;59:237–243. doi: 10.1203/01.pdr.0000196333.16608.30. [DOI] [PubMed] [Google Scholar]
- Wauquier A, Zhou S. Topiramate: a potent anticonvulsant in the amygdala-kindled rat. Epilepsy Research. 1996;24:73–77. doi: 10.1016/0920-1211(95)00105-0. [DOI] [PubMed] [Google Scholar]
- White HS. Preclinical development of antiepileptic drugs: past, present, and future directions. Epilepsia. 2003;44(Suppl 7):2–8. doi: 10.1046/j.1528-1157.44.s7.10.x. [DOI] [PubMed] [Google Scholar]
- White HS, Brown SD, Woodhead JH, Skeen GA, Wolf HH. Topiramate enhances GABA-mediated chloride flux and GABA-evoked chloride currents in murine brain neurons and increases seizure threshold. Epilepsy Research. 1997;28:167–179. doi: 10.1016/s0920-1211(97)00045-4. [DOI] [PubMed] [Google Scholar]
- Zhao Q, Hu Y, Holmes GL. Effect of topiramate on cognitive function and activity level following neonatal seizures. Epilepsy& Behavior. 2005;6:529–536. doi: 10.1016/j.yebeh.2005.03.001. [DOI] [PubMed] [Google Scholar]