Abstract
Circadian photoentrainment is the process by which the brain’s internal clock becomes synchronized with the daily external cycle of light and dark. In mammals, this process is mediated exclusively by a novel class of retinal ganglion cells that send axonal projections to the suprachiasmatic nuclei (SCN), the region of the brain that houses the circadian pacemaker. In contrast to their counterparts that mediate image-forming vision, SCN-projecting RGCs are intrinsically sensitive to light, independent of synaptic input from rod and cone photoreceptors. The recent discovery of these photosensitive RGCs has challenged the long-standing dogma of retinal physiology that rod and cone photoreceptors are the only retinal cells that respond directly to light and has explained the perplexing finding that mice lacking rod and cone photoreceptors can still reliably entrain their circadian rhythms to light. These SCN-projecting RGCs selectively express melanopsin, a novel opsin-like protein that has been proposed as a likely candidate for the photopigment in these cells. Research in the past three years has revealed that disruption of the melanopsin gene impairs circadian photoentrainment, as well as other nonvisual responses to light such as the pupillary light reflex. Until recently, however, there was no direct demonstration that melanopsin formed a functional photopigment capable of catalyzing G-protein activation in a light-dependent manner. Our laboratory has recently succeeded in expressing melanopsin in a heterologous tissue culture system and reconstituting a pigment with the 11-cis-retinal chromophore. In a reconstituted biochemical system, the reconstituted melanopsin was capable of activating transducin, the G-protein of rod photoreceptors, in a light-dependent manner. The absorbance spectrum of this heterologously expressed melanopsin, however, does not match that predicted by previous behavioral and electophysiological studies. Although melanopsin is clearly the leading candidate for the elusive photopigment of the circadian system, further research is needed to resolve the mystery posed by its absorbance spectrum and to fully elucidate its role in circadian photoentrainment.
Keywords: Melanopsin, Circadian rhythm, Photoentrainment, Opsin
SUNRISE, SUNSET, SUNRISE, SUNSET, SWIFTLY FLY THE YEARS
Even as Tevyes laments the inexorable passage of time in these lyrics from Fiddler on the Roof, he concedes that the solar cycle of day and night has been a fact of life on Earth since the dawn of evolution. In fact, most living organisms—from cyanobacteria to man—have adapted their physiology and behavior to this daily cycle (Devlin and Kay, 2001; Dunlap, 1999; Harmer et al., 2001; Young and Kay, 2001). In humans, many physiological and behavioral rhythms, including the sleep-wake cycle, body temperature, and hormone levels, oscillate with a period of approximately 24 h. In mammals, these circadian rhythms (circa—about; dian—day) are driven by an autonomous master pacemaker located in the pear-shaped suprachiasmatic nuclei (SCN) of the hypothalamus (Moore and Eichler, 1972; Ralph et al., 1990). Like an old watch, however, this internal clock does not keep perfect time, and it must be reset each morning by dawn’s early light, a process termed circadian photoentrainment. This process also allows the clock to be reset following travel across time zones and to adjust to seasonal differences in the length of day. Proper synchronization of our internal clock with the external environment is essential for optimal physical and mental performance. When our internal clock is out-of-synch with the external environment, it results in the malaise experienced as a result of jet-lag and shift work.
Over the past several years, researchers around the world have made great strides in understanding how the mammalian retina generates the neuronal signals that reset the circadian clock, and their findings have challenged long-standing dogmas in retinal physiology. Perhaps the most surprising finding was the discovery of a novel class of retinal ganglion cells (RGCs) that are intrinsically sensitive to light. These photosensitive RGCs selectively express a novel opsin-like protein, known as melanopsin, which has been proposed to be the photopigment that triggers the intrinsic light response. In this review, we have taken a historical perspective on the research that has led to these breakthroughs, and we critically evaluate the evidence for melanopsin as the elusive circadian photopigment.
CIRCADIAN ENTRAINMENT IN BLIND MICE
In mammals, light entrainment of the circadian clock requires input from the retina, which arises from the axonal projections of a small subset (1–2%) of RGCs. These projections form the retinohypothalamic tract (RHT), a dedicated monosynaptic pathway between the retina and the suprachiasmatic nuclei (SCN) (Moore et al., 1995). In contrast to the circadian system of lower vertebrates that possess extraocular circadian photoreceptors (Menaker et al., 1970; Underwood and Menaker, 1990), the mammalian clock can no longer be light entrained if the eyes of the animal are removed (Foster et al., 1991; Freedman et al., 1999; Yamazaki et al., 1999). Surprisingly, the rod and cone photoreceptors that mediate image-forming vision are not required for circadian photoentrainment. The pioneering behavioral work of Foster and colleagues demonstrated almost a decade ago that mice harboring the rd mutation, which causes nearly complete degeneration of rods and cones, effectively entrained their circadian clock, despite being totally blind in terms of visual perception (Foster et al., 1991; Freedman et al., 1999). These rodless-coneless mice also retain several other physiological and behavioral measures of nonvisual illuminance detection, including the pupillary light reflex (PLR) and suppression of pineal melatonin production (Lucas et al., 1999, 2001). Furthermore, a subset of blind people can suppress melatonin synthesis and reliably entrain their circadian clock in response to light despite a total lack of image-forming visual perception (Czeisler et al., 1995; Klerman et al., 2002). These findings began to dispel the long-standing dogma of retinal physiology that in vertebrate retinas only rods and cones were directly photosensitive, and suggested that a population of neurons in the inner retina might be photoreceptive as well.
ANATOMY AND PHYSIOLOGY OF SCN-PROJECTING RETINAL GANGLION CELLS
Following several years of intense speculation about the possibility of an inner retinal photoreceptor, Berson et al. (2002) conclusively demonstrated that SCN-projecting RGCs function as autonomous photoreceptors (Berson et al., 2002). These cells were identified by retrograde labeling with fluorescent microspheres following stereotaxic injection of the SCN, and light-evoked changes in membrane potential were recorded using whole-cell patch clamp techniques. These SCN-projecting RGCs exhibited a slow depolarization and spiking activity in response to light, which persisted in the presence of a formidable cocktail of synaptic blockers and when synaptic connections were physically severed. The action spectrum for the light-dependent depolarization of these cells peaked at 480 nm, and the shape was characteristic of a retinal-based visual pigment (Berson et al., 2002). Unlike the light response of rod and cone photoreceptors, the intrinsic light response of SCN-projecting RGCs persisted following exposure to bright illumination, and it can be elicited for hours even in the absence of exogenously supplied chromophore, suggesting that the photopigment does not bleach following illumination. This characteristic is most unusual for vertebrate photopigments, where the isomerized chromophore is typically hydrolyzed and dissociates from the opsin following excitation. In contrast, most invertebrate photopigments retain their chromophores after photoisomerization, and the inactive pigment is regenerated by photoconversion following absorption of a second photon of light of different wavelength (Hillman et al., 1983). Immunohistochemical experiments revealed that these photosensitive RGCs selectively express melanopsin, a novel opsin-like protein, which has been proposed to be the photopigment in these cells (Hattar et al., 2002). At present, the intracellular signaling pathway that generates the intrinsic light response in these cells and the molecular identity of the light-activated channel (LAC) remain unknown. However, Warren et al. (2003) have reported that the voltage dependence and permeation properties of the LAC resemble those of some transient receptor potential (TRP) ion channels, which mediate the light response in invertebrate photoreceptors (Warren et al., 2003).
The morphology of the melanopsin-containing, SCN-projecting cells in the rat resembles that of Type III RGCs with a small (12 μm) cell body and two to three long, sparsely branched dendrites, which often extend for > 300 μm (Fig. 1A) (Berson et al., 2002; Hattar et al., 2002; Warren et al., 2003). The dendritic projections from individual cells have been found to stratify in either the outer or inner lamina of the inner plexiform layer (IPL). These layers correspond, respectively, to synaptic connections that are inhibitory in response to light and those that are excitatory in response to light. Warren et al. (2003) have also observed a population of cells that is bistratified with projections in both laminae. Similar findings have been reported in the cat (Pu, 1999). As predicted from the anatomical pattern of dendritic stratification, these SCN-projecting cells receive both inhibitory and excitatory synaptic inputs as measured with electrophysiological recordings (Dunn and Berson, 2002; and Erin J. Warren, Charles N. Allen, David W. Robinson, and RLB, unpublished results) or observed with electron microscopy (Belenky et al., 2003).
Figure 1.
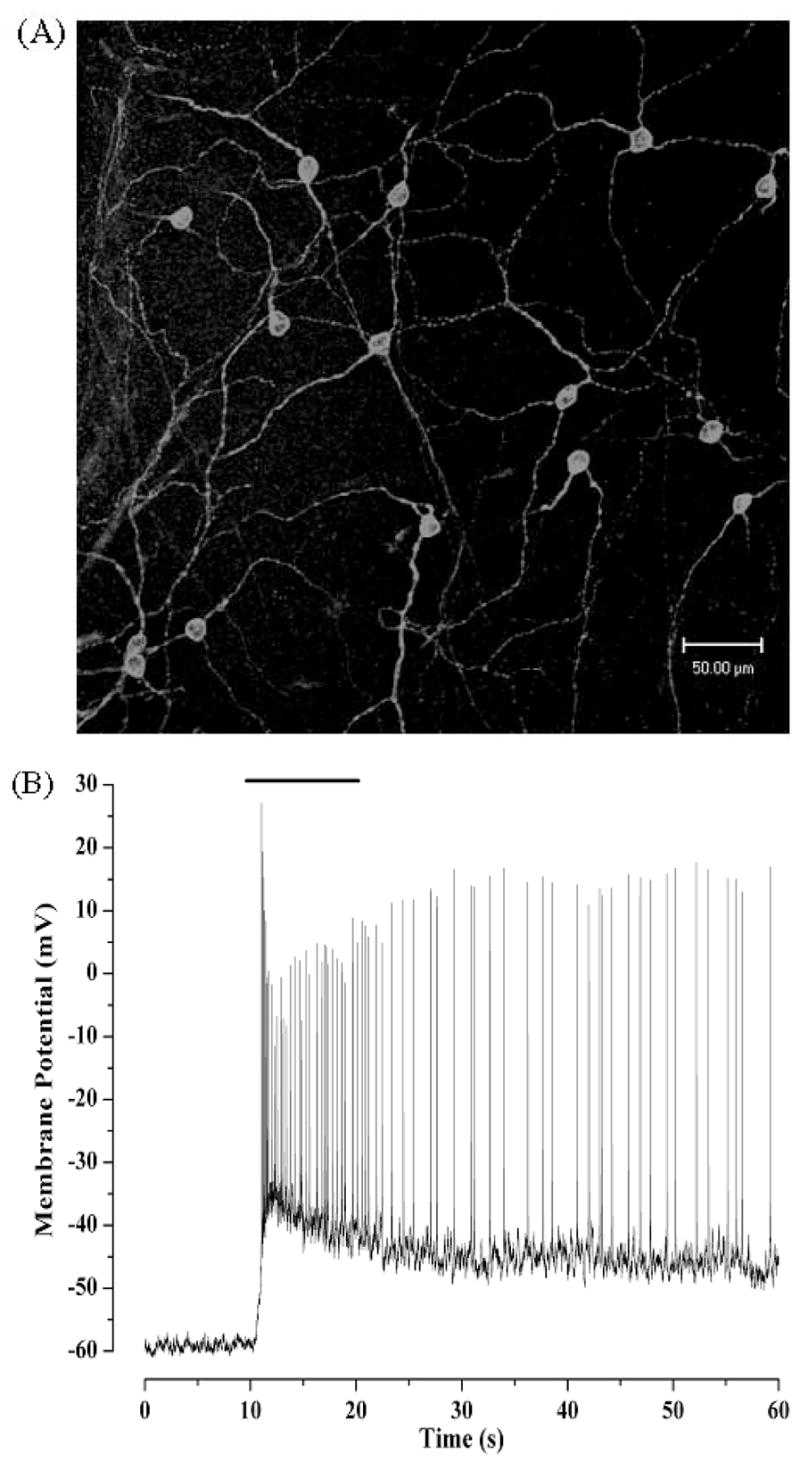
Melanopsin-containing retinal ganglion cells form a meshwork of cells that are intrinsically sensitive to light. (A) Approximately 1–2% of retinal ganglion cells express melanopsin. Shown is a confocal projection of a rat retinal wholemount immunostained with affinity-purified rabbit polyclonal antibodies raised against the amino-terminal peptide of melanopsin. (B) SCN-projecting RGCs are intrinsically sensitive to light. The light response shown was recorded in whole-cell perforated-patch clamp mode from a rat RGC that was labeled following stereotaxic injection of a fluorescent retrograde tracer into the SCN. All rod-and cone-driven synaptic inputs were blunted by prior exposure of the isolated retina to bright light, which bleaches the photopigments in traditional photoreceptors, rendering them unresponsive to light. The bar at the top indicates the duration of the light stimulus.
Melanopsin-containing RGCs are involved in numerous nonvisual photic responses, besides their critical role in circadian entrainment. In addition to their direct projection to the SCN, these cells also project to the intergeniculate leaflet and olivary pretectal nucleus, brain regions involved in modulation of circadian rhythms and the pupillary light reflex (Hattar et al., 2002). More recently, several groups have shown that melanopsin-containing RGCs also project to the ventral subparaventricular zone, the ventrolateral preoptic nucleus, regions of the brain involved in sleep and circadian locomotion (Gooley et al., 2003; Morin et al., 2003).
IN SEARCH OF THE ELUSIVE CIRCADIAN PHOTOPIGMENT
The identity of the visual pigment that mediates circadian photoentrainment has been a topic of speculation and controversy for several decades. In 1984, Takahashi and colleagues first measured the action spectrum for photic entrainment of the circadian clock and found that wavelengths of light near 500 nm were most effective (Takahashi et al., 1984). Based on the similarity of spectral characteristics, the opsin-based visual pigments found in rod and cone photoreceptors were originally proposed as the photopigments of the circadian system. However, as described above, transgenic mice missing rods and cones can still reliably entrain their circadian clocks. For a time in the late 1990s the spotlight was focused on the flavin-based cryptochrome pigments, which appear to be the circadian photoreceptor in Drosophila (Ceriani et al., 1999). In mammals, however, cryptochromes function as circadian clock components, but they do not demonstrate any light-dependent functions (Griffin et al., 1999). At the present time, melanopsin is the most promising candidate for the photopigment driving the intrinsic light responses of SCN-projecting RGCs. Since the discovery of melanopsin in the mammalian retina, a variety of experiments have been performed to clarifying its role in circadian entrainment and other nonvisual illuminance detection.
MELANOPSIN—HUMBLE BEGINNINGS FOR A RISING STAR
Melanopsin was discovered by Provencio and colleagues in 1998 in the photosensitive melanophores of Xenopus skin (Provencio et al., 1998). These melanophores allow the frog to alter its coloration in response to light by reorganizing pigment granules within the cells. In situ hybridization studies demonstrated that mRNA encoding melanopsin is also expressed in other photosensitive tissues, including the retina, iris, pigment epithelium, as well as the magnocellular preoptic nucleus and the SCN in the brain. Based on its amino acid sequence, melanopsin appeared to be a novel opsin-like molecule that most likely contains a retinal-based chromophore. The sequence of melanopsin reveals a striking evolutionary relationship to squid rhodopsin, sharing 39% identity over the entire sequence. Like all G-protein-coupled receptors (GPCRs), melanopsin is predicted to have seven transmembrane helices (Fig. 2). Melanopsin also contains a lysine residue in the seventh transmembrane helix, which is characteristic of all opsins and functions as the site of attachment for the retinal chromophore via a protonated Schiff ’s base linkage. Surprisingly for a vertebrate opsin, however, melanopsin displays several hallmarks of invertebrate opsins, including a tyrosine residue in place of the glutamate counterion for the retinal Schiff ’s base, which is characteristic of vertebrate opsins. Unlike vertebrate opsins that release their retinal chromophore following photoisomerization, invertebrate opsins retain their chromophore, and their activity is terminated by photoconversion by a second photon of a different wavelength. This property might explain melanopsin’s ability to resist bleaching in electrophysiological experiments. Another interesting feature of the melanopsin sequence is that the second and third cytoplasmic loops exhibit no apparent homology to the analogous regions of other known GPCRs. Because these loops are involved in the interaction of a GPCR and its cognate G-protein, it is difficult to predict which G-protein is activated by melanopsin in vivo. Finally, an abundance of serine and threonine residues in the carboxy-terminus suggests the possibility of regulatory phosphorylation.
Figure 2.
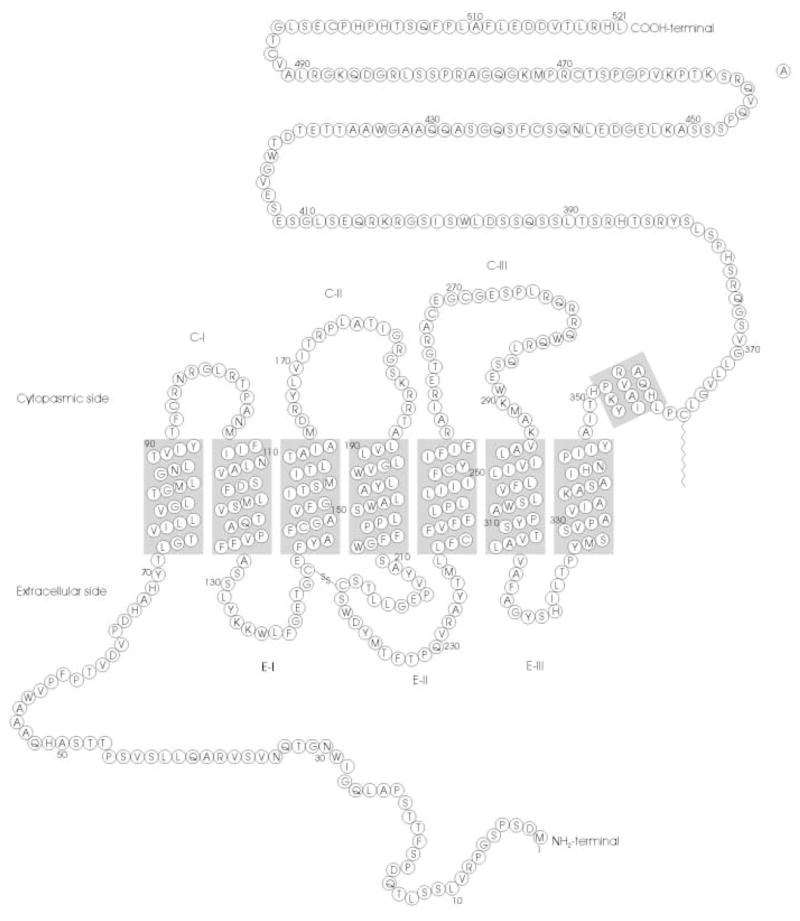
Deduced amino acid sequence and predicted secondary structure of mouse melanopsin. Predicted transmembrane regions [NORSp algorithm (Liu and Burkhard, 2002)] are indicated by shaded rectangles.
In 2000, melanopsin burst into the international spotlight, when Provencio et al. discovered that it is expressed in a small subset of RGCs in the mammalian retina (Provencio et al., 2002). This finding generated immediate interest in a number of laboratories, as the position and number of these RGCs was suspiciously similar to that of the RGCs whose axons formed the RHT. Using a combination of retrograde labeling and in situ hybridization, Gooley et al. demonstrated that melanopsin RNA is selectively expressed in RGCs of the RHT (Gooley et al., 2001). Hannibal et al. extended this result using an immunohistochemical approach, demonstrating that the melanopsin protein was expressed in the soma and processes of RGCs that contain pituitary adenylate cyclase-activating peptide (PACAP), a known marker and transmitter for neurons of the RHT (Hannibal et al., 2002). Recent findings indicate that melanopsin is expressed in RGCs that project to the SCN, as well as the RGCs projecting to the ventral lateral geniculate nucleus, intergeniculate leaflet, and olivary pretectal nucleus, brain regions that control other nonvisual photic responses (Gooley et al., 2003). These findings make melanopsin a most attractive candidate for the circadian photopigment and other nonvisual photic responses.
To test the role of melanopsin in circadian entrainment and other nonvisual photoresponses, several research groups have now generated lines of transgenic knockout mice in which the melanopsin gene has been disrupted (opn4−/− mice) (Lucas et al., 2003; Panda et al., 2002; Ruby et al., 1993). Although circadian entrainment is somewhat impaired in these mice, and they exhibit a reduced pupillary light reflex, their deficiencies are relatively minor. These confounding findings led to the speculation that melanopsin was not the photopigment responsible for the intrinsic light response in SCN-projecting neurons (Van Gelder, 2003). However, this speculation was put to rest when it was subsequently discovered that the melanopsin-containing RGCs also receive rod- and cone-driven synaptic inputs (Belenky et al., 2003; Dunn and Berson, 2002), and that melanopsin was required for nonvisual light responses in blind mice. In the first set of experiments, Hattar et al. (2003) created a triple-knockout mouse by combining the melanopsin knockout with the disruption of the gene encoding rod transducin and that encoding the cone cyclic nucleotide-gated ion channel (Hattar et al., 2003). Thus, these investigators disabled both the intrinsic light response in the melanopsin-containing RGCs, as well as the phototransduction pathways in traditional rod and cone photoreceptors. In these triple-knockout mice, the pupillary light response was nonexistant, and the mice failed to entrain their circadian locomotory rhythms to a light–dark cycle. In a second set of experiments, Panda et al. placed the melanopsin knockout in the background of an rd mouse (Panda et al., 2003). Subsequent experiments revealed that circadian entrainment and masking responses were abolished in these mice.
In summary, melanopsin has been proposed to be the elusive circadian photopigment based on several lines of indirect evidence: (1) the primary structure of melanopsin resembles that of an invertebrate-type opsin protein (Provencio et al., 2000), (2) melanopsin is selectively expressed in the intrinsically photosensitive RGCs whose axons form the RHT (Hattar et al., 2002), and (3) disruption of the melanopsin gene eliminates the intrinsic photosensitivity of SCN-projecting RGCs (Lucas et al., 2003). However, even these elegant experiments do not directly address the question of whether melanopsin is a photopigment responsible for generating the light response, or an isomerase required to regenerate the retinal chromophore (Bellingham and Foster, 2002).
MELANOPSIN AS A PHOTOPIGMENT
At present, the evidence implicating melanopsin as the photopigment responsible for generating the intrinsic light response in SCN-projecting RGC, though compelling, is largely circumstantial—melanopsin was found in the right place at the right time. This preponderance of evidence might suffice for conviction in a civil court, but it does not constitute evidence beyond a reasonable doubt that is required in the criminal court, or in the scientific court. A smoking gun is needed—in this case, melanopsin needs to be expressed in a heterologous system, and the absorbance of the reconstituted pigment must match the action spectrum of the native responses. Alternatively, melanopsin needs to be extracted from a mammalian retina, purified to homogeneity, and analyzed spectrophotometrically. Given the small number of cells expressing melanopsin in the retina, we chose to pursue an in vitro approach. Until recently, a direct demonstration that melanopsin could form a functional photopigment capable of G-protein activation had been lacking, making firm conclusions about melanopsin’s role in circadian entrainment impossible. However, work from our laboratories has provided the first direct evidence that melanopsin does indeed form a functional photopigment (Newman et al., 2003). We have expressed melanopsin in COS-7 tissue culture cells, reconstituted the heterologously expressed opsin with 11-cis-retinal, and assayed the visual pigment in a spectrophotometer and in a functional G-protein assay. We found that melanopsin formed a functional short-wavelength photopigment that activated the rod photoreceptor G-protein, transducin, in a light-dependent manner. However, its in vitro absorbance spectrum and the wavelength dependence of G-protein activation do not match the spectrum predicted by previous work.
Recent behavioral studies on rodless, coneless mice, and electrophysiological studies of SCN-projecting RGCs have allowed us to infer the photochemical properties of the dedicated circadian photopigment, without the confounding contribution of synaptic input from rod and cone photoreceptors. The action spectrum of the light-evoked depolarization of these photosensitive RGCs is well described by templates for an A1 retinal-based photopigment with a λmax of 484 nm (Berson et al., 2002). This action spectrum is similar to those measured for the pupillary light reflex in rodless, coneless mice (Lucas et al., 2001). These results suggest that the photopigment mediating these nonvisual photic responses would have an absorbance spectrum with a similar shape, peaking near 480 nm. In our heterologous expression system, melanopsin was reconstituted with 11-cis-retinal, solubilized in detergent, and purified by immunoaffinity chromatography. When an absorbance spectrum was measured prior to light exposure, the measured wavelength of peak absorbance was near 420 nm (Fig. 3) (Newman et al., 2003). This λmax for the dark pigment is further emphasized by measuring a difference spectrum after bleaching the pigment by treatment with hydroxylamine which cleaves the Schiff ’s base linkage and releases the retinal chromophore. The difference spectum also peaks at 424 nm. Because of the unexpected blue-shift in the melanopsin spectrum, we performed several control experiments to establish that the pigment was formed properly, with the retinal chromophore attached to Lys337 in the seventh transmembrane helix of the mouse protein, and was not the result of spurious attachment between 11-cis-retinal and a surface lysine. When Lys337 was mutated to an alanine, the mutant protein did not form a photopigment with a absorbance peak in the 420 nm range, and did not display any light-dependent shift in its absorbance profile (Newman et al., 2003).
Figure 3.
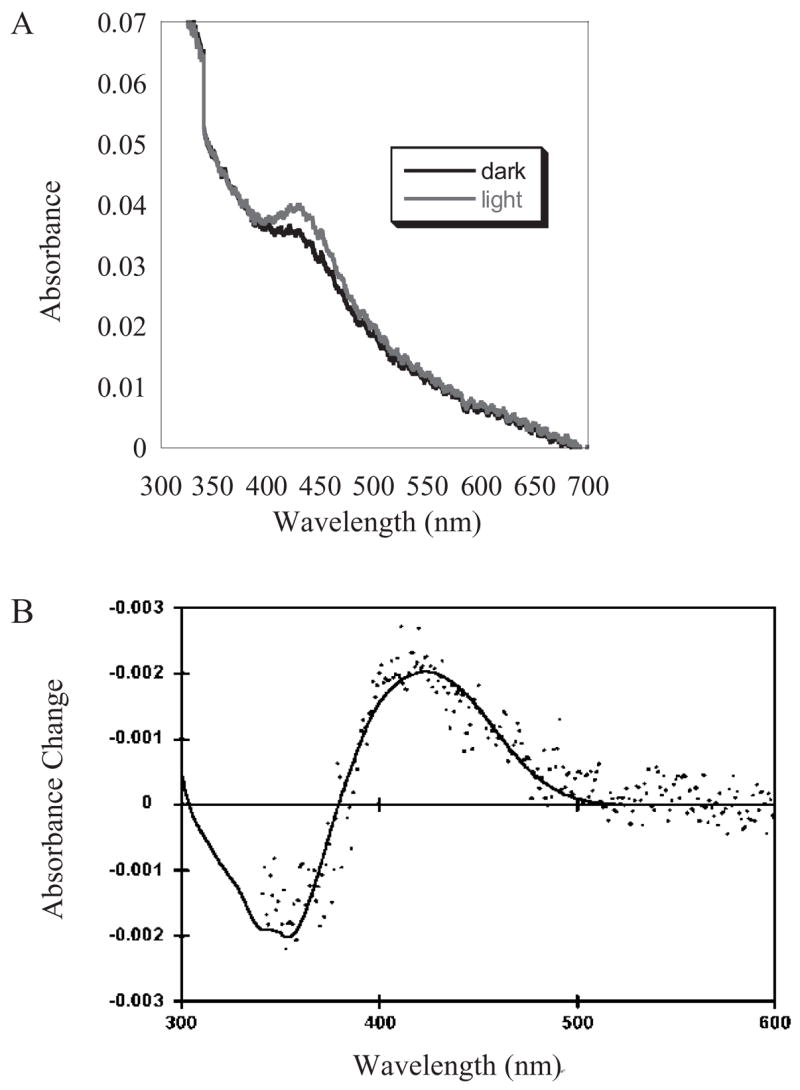
Spectrum of purified expressed melanopsin reconstituted with 11-cis-retinal. Melanopsin was expressed in COS cells reconstituted with 11-cis-retinal and purified by immunoaffinty chromatography. Purified melanopsin was solubilized in a PBS buffer containing 0.1% dodecyl maltoside. (A) Raw absorbance spectra of dark and illuminated melanopsin pigment. (B) Difference spectrum of purified expressed melanopsin. The spectrum (shown as filled circles) was obtained by subtracting the spectrum obtained following hydroxylamine treatment (50 mM hydroxylamine, pH 7.0) from that of melanopsin in the dark prior to treatment with hydroxylamine. The positive component of the spectrum reveals the dark pigment with a peak at 420 nm, which is susceptible to hydroxylamine attack. The smooth curve is a fit based upon the combination of a rhodopsin template (λmax of 421 nm) with the spectrum of ll-cis-retinaldehyde oxime.
Using a reconstituted, in vitro biochemical assay, we found that melanopsin forms a functional photopigment that is capable of catalyzing GTP uptake by transducin, the G-protein found in rod photoreceptors. COS cell membranes containing melanopsin were reconstituted in the dark with 11-cis-retinal, and mixed with purified bovine rod transducin and GTP-γ-35S. As an indication of G-protein activation, GTP-γ-S binding was measured using a filter-binding assay. As depicted in Fig. 4A, melanopsin catalyzed transducin activation in a light-dependent manner. This is indicated by a light-dependent increase in the rate of GTP-γ-35S binding. The activation of transducin is dependent on the presence of melanopsin as control COS membranes lacking any heterologously expressed G-protein coupled receptor failed to increase the rate of GTP-γ-S binding. These experiments only demonstrate that reconstituted melanopsin can activate a G-protein. They do not at all identify the cognate G-protein that melanopsin activates in vivo. It has been previously demonstrated that transducin can be activated by non-opsin G-protein receptors (Weng et al., 1997). Furthermore, we examined the wavelength dependence of transducin activation using this assay system (Fig. 4B). In doing so, we found that the melanopsin was activated most efficiently by blue light between 420 and 440 nm, rather than the 480 nm green light predicted from the electrophysiological and behavioral experiments (Berson et al., 2002; Lucas et al., 2001). This result suggests that the blue-shift in the melanopsin absorbance spectrum is also not a result of detergent solubilization.
Figure 4.

Melanopsin stimulates GTP-γ-35S uptake by transducin. (A) Melanopsin-mediated GTP-γ-35S binding in bright white light (□) and in the dark (■). Reaction rates were 0.04 pmol GTP-γ-35S bound/s/pmol receptor for the light reaction and 0.02 pmol GTP-γ-35S bound/s/pmol receptor for the dark reaction. In this assay, reaction mixtures contained 2.1 μM transducin, 3 μM GTP-γ-35S, and 9.14 nM melanopsin in a buffer containing 10 mM Tris-Cl (pH 7.4), 150 mM NaCl, 1 mM MgCl2, and 1 mM DTT. Circles represent GTP-γ-35S binding by untransfected COS-1 membranes in bright white light (○), and in the dark (●). Reaction mixtures contained 2.1 μM transducin, 3 μM GTP-γ-35S and untransfected COS-1 membranes in the Tris buffer described above. (B) Melanopsin is activated most efficiently by blue light. Mean rates of melanopsin-catalyzed GTP-γ-35S binding following illumination with UV (381 ±10 nm), blue (443 ±19 nm), green (516 ±30 nm), and red (> 700 nm) light. Illumination was provided by a calibrated light source. The samples were illuminated for 10 s. and the rate of transducin activation was determined. The irradiance (photons/s/cm2) was: UV—1.3 ×1013; Blue—1.54 ×1013; Green—2.72 ×1013; Red—1.9 ×1015. Wavelengths were selected using bandpass or longpass filters. Intensities were chosen to provide clear discrimination amongst action spectra for candidate pigments that have absorption maxima in the spectral range from the UV to the green. The intensity of red light was 100-fold greater than the other stimuli to rule out any contribution of photoactivation by light outside the intended spectral bands. The GTP-γ-35S binding rate in the dark (4.8 ×10−4 pmol/s) was subtracted from raw rate values to generate the data shown (Mean ± SD). Reaction mixtures contained 2.1 μM transducin, 3 μM GTP-γ-35S, and 0.3 nM melanopsin.
An investigation into the spectral and functional properties of melanopsin is also important in order to clarify its functional relationship to ancient VA opsins and the retinal G-protein-coupled receptor (RGR), two recently discovered class of opsin proteins (Bellingham and Foster, 2002; Shen et al., 1994). These novel opsins are expressed in retinal cells other than rod and cone photoreceptors, as well as nonocular tissue and appear to function as photopigments, and may mediate light signals involved in nonvisual photic responses, such as circadian photoentrainment (Bellingham and Foster, 2002). On the other hand, the retinal G protein-coupled receptor (RGR) is not involved in sensory transduction, but rather acts as a photoisomerase in the retinal pigment epithelium (RPE) (Chen et al., 2001). By analogy, it is not known if melanopsin could also act either in sensory transduction or as a photoisomerase. However, the possibility that melanopsin acts as an isomerase is particularly intriguing for the light-sensitive RGCs because these cells are located in the inner retina where they are quite distant from the RPE (Bellingham and Foster, 2002).
CONCLUSIONS, FLIES IN THE OINTMENT, AND FUTURE DIRECTIONS
Although the results described above seem to make melanopsin a very strong candidate for the photopigment driving the intrinsic light response in SCN-projecting RGCs, there are still a few confounding results. First, we need to reconcile the absorbance spectrum measured using heterologously expressed melanopsin with the action spectrum measured for the behavioral and electrophysiological responses driven by the melanopsin-containing cells. This raises several intriguing possibilities: first, melanopsin may form a pigment with an unusual chromophore, rather than the typical A1 11-cis-retinal chromophores found in all mammalian rod and cone opsins. For instance, the opsins of several fish and amphibian species are known to use the dihydro A2 derivative of retinal. Although this seems highly unlikely as there is no evidence that mammals can synthesize the A2 retinal derivative, pigments formed with the A2 retinal display a red shift in their absorbance when compared to the same opsin formed with A1 retinal. Furthermore, trace amounts of 9-cis-retinal have recently been detected in the mammalian retina (Fan et al., 2003), and these may be used to form the melanopsin photopigment. However, because the mechanism of spectral tuning in melanopsin remains totally unknown, it is difficult to predict the magnitude and/or direction of the spectral shift that would be caused by reconstitution with alternative chromophores. The melanopsin spectrum in vivo might also be altered by interaction with endogenous proteins in the RGCs. For instance, the spectrum of mammalian rhodopsin is altered by the stabilization of the catalytically active meta-II state by binding of transducin or arrestin (Hofmann, 1999). A similar phenomenon occurs in invertebrate photoreceptors, in which binding of arrestin stabilizes the metastable state (Kiselev and Subramaniam, 1997).
A second perplexing result is that severe dietary depletion of 11-cis-retinal, thought to be essential for formation of opsin pigments, does not reduce the light-dependent induction of per expression in the SCN, a presumptive measure of circadian photoentrainment (Thompson et al., 2001). These results may be explained if melanopsin retains its pigment for extended periods of time, making it resistant to dietary depletion. Alternatively, the retinas of these animal may contain trace amounts of other retinal derivatives, as discussed above. A third compelling question that is sure to be experimentally addressed in the near future is the identity of the cognate G-protein for melanopsin in the RGCs. Although we have shown that melanopsin can catalyze the activation to transducin, a readily purified and rather promiscuous G-protein, melanopsin’s cognate G-protein in RGCs remains unknown. The nature of the light-activated intracellular signaling pathway also remains elusive.
Until these remaining questions are answered, melanopsin will remain an intriguing object of inquiry for chronobiologists interested in the retinal mechanisms that generate the signals for circadian entrainment. Only when we have addressed the questions listed here, as well as the others that will certainly arise, will we have a definitive answer. Until then, however, our money is on melanopsin.
Acknowledgments
The authors would like to thank Erin J. Warren, who produced the data shown in Fig. 1, and Thomas W. Cronin, Lucy A. Newman, and Marquis T. Walker for the data depicted in Figs. 3 and 4. RLB is supported by grants from the National Institute of Mental Health (MH63364 and MH67094); PRR is supported by a grant from NSF (0119102).
References
- Belenky MA, Smeraski CA, Provencio I, Sollars PJ, Pickard GE. Melanopsin retinal ganglion cells receive bipolar and amacrine cell synapses. Journal of Comparative Neurology. 2003;460:380–393. doi: 10.1002/cne.10652. [DOI] [PubMed] [Google Scholar]
- Bellingham J, Foster RG. Opsins and mammalian photoentrainment. Cell and Tissue Research. 2002;309:57–71. doi: 10.1007/s00441-002-0573-4. [DOI] [PubMed] [Google Scholar]
- Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295:1070–1073. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- Ceriani MF, Darlington TK, Staknis D, Mas P, Petti AA, Weitz CJ, Kay SA. Light-dependent sequestration of TIMELESS by CRYPTOCHROME. Science. 1999;285:553–556. doi: 10.1126/science.285.5427.553. [DOI] [PubMed] [Google Scholar]
- Chen P, Hao W, Rife L, Wang XP, Shen D, Chen J, Ogden T, Van Boemel GB, Wu L, Yang M, Fong HK. A photic visual cycle of rhodopsin regeneration is dependent on Rgr. Nauret Geneicst. 2001;28:256–260. doi: 10.1038/90089. [DOI] [PubMed] [Google Scholar]
- Czeisler CA, Shanahan TL, Klerman EB, Martens H, Brotman DJ, Emens JS, Klein T, Rizzo JF. Suppression of melatonin secretion in some blind patients by exposure to bright light. New England Journal of Medicine. 1995;332:6–11. doi: 10.1056/NEJM199501053320102. [DOI] [PubMed] [Google Scholar]
- Devlin PF, Kay SA. Circadian photoperception. Annual Review of Physiology. 2001;63:677–694. doi: 10.1146/annurev.physiol.63.1.677. [DOI] [PubMed] [Google Scholar]
- Dunlap JC. Molecular bases for circadian clocks. Cell. 1999;96:271–290. doi: 10.1016/s0092-8674(00)80566-8. [DOI] [PubMed] [Google Scholar]
- Dunn FA, Berson DM. Are intrinsically photosensitive retinal ganglion cells influenced by rods or cones. Investigative Ophthalmology & Visual Science. 2002;43:U839. [Google Scholar]
- Fan J, Rohrer B, Moiseyev G, Ma JX, Crouch RK. Isorhodopsin rather than rhodopsin mediates rod function in RPE65 knock-out mice. Proc Natl Acad Sci USA. 2003;100:13662–13667. doi: 10.1073/pnas.2234461100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster RG, Provencio I, Hudson D, Fiske S, Degrip W, Menaker M. Circadian photoreception in the retinally degenerate mouse (Rd/Rd) Journal of Comparative Physiology. 1991;169:39–50. doi: 10.1007/BF00198171. [DOI] [PubMed] [Google Scholar]
- Freedman MS, Lucas RJ, Soni B, von Schantz M, Munoz M, David-Gray Z, Foster R. Regulation of mammalian circadian behavior by nonrod, non-cone, ocular photoreceptors. Science. 1999;284:502–504. doi: 10.1126/science.284.5413.502. [DOI] [PubMed] [Google Scholar]
- Gooley JJ, Lu J, Chou TC, Scammell TE, Saper CB. Melanopsin in cells of origin of the retinohypothalamic tract. Nature Neuroscience. 2001;4:1165. doi: 10.1038/nn768. [DOI] [PubMed] [Google Scholar]
- Gooley JJ, Lu J, Fischer D, Saper CB. Broad role for melanopsin in nonvisual photoreception. Journal of Neuroscience. 2003;23:7093–7106. doi: 10.1523/JNEUROSCI.23-18-07093.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin EAJ, Staknis D, Weitz CJ. Light-independent role of CRY1 and CRY2 in the mammalian circadian clock. Science. 1999;286:768–771. doi: 10.1126/science.286.5440.768. [DOI] [PubMed] [Google Scholar]
- Hannibal J, Hindersson P, Knudsen SM, Georg B, Fahrenkrug J. The photopigment melanopsin is exclusively present in pituitary adenylate cyclaseactivating polypeptide-containing retinal ganglion cells of the retinohypothalamic tract. Journal of Neuroscience. 2002:22. doi: 10.1523/JNEUROSCI.22-01-j0002.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmer SL, Panda S, Kay SA. Molecular bases of circadian rhythms. Annual Review of Cell and Developmental Biology. 2001;17:215–253. doi: 10.1146/annurev.cellbio.17.1.215. [DOI] [PubMed] [Google Scholar]
- Hattar S, Liao HW, Takao M, Berson DM, Yau KW. Melanopsincontaining retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science. 2002;295:1065–1070. doi: 10.1126/science.1069609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattar S, Lucas RJ, Mrosovsky N, Thompson S, Douglas RH, Hankins MW, Lem J, Biel M, Hofmann F, Foster RG, Yau KW. Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice. Nature. 2003;424:76–81. doi: 10.1038/nature01761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman P, Hochstein S, Minke B. Transduction in invertebrate photoreceptors: role of pigment bistability. Physiol Rev. 1983;63:668–772. doi: 10.1152/physrev.1983.63.2.668. [DOI] [PubMed] [Google Scholar]
- Hofmann KP. Signalling states of photoactivated rhodopsin. Novartis Found Symp. 1999;224:158–175. doi: 10.1002/9780470515693.ch10. [DOI] [PubMed] [Google Scholar]
- Kiselev A, Subramaniam S. Studies of Rh1 metarhodopsin stabilization in wild-type Drosophila and in mutants lacking one or both arrestins. Biochemistry. 1997;36:2188–2196. doi: 10.1021/bi9621268. [DOI] [PubMed] [Google Scholar]
- Klerman EB, Shanahan TL, Brotman DJ, Rimmer DW, Emens JS, Rizzo JF, Czeisler CA. Photic resetting of the human circadian pacemaker in the absence of conscious vision. Journal of Biological Rhythms. 2002;17:548–555. doi: 10.1177/0748730402238237. [DOI] [PubMed] [Google Scholar]
- Liu JT, Burkhard R. Loopy proteins appear conserved in evolution. J Mol Biol. 2002;322:53–64. doi: 10.1016/s0022-2836(02)00736-2. [DOI] [PubMed] [Google Scholar]
- Lucas RJ, Freedman MS, Munoz M, Garcia-Fernandez JM, Foster RG. Regulation of the mammalian pineal by non-rod, non-cone, ocular photoreceptors. Science. 1999;284:505–507. doi: 10.1126/science.284.5413.505. [DOI] [PubMed] [Google Scholar]
- Lucas RJ, Douglas RH, Foster RG. Characterization of an ocular photopigment capable of driving pupillary constriction in mice. Nature Neuroscience. 2001;4:621–626. doi: 10.1038/88443. [DOI] [PubMed] [Google Scholar]
- Lucas RJ, Hattar S, Takao M, Berson DM, Foster RG, Yau KW. Diminished pupillary light reflex at high irradiances in melanopsinknockout mice. Science. 2003;299:245–247. doi: 10.1126/science.1077293. [DOI] [PubMed] [Google Scholar]
- Menaker M, Roberts R, Elliott J, Underwood H. Extraretinal light perception in the sparrow 3. The eyes do not participate in photoperiodic photoreception. Proc Natl Acad Sci USA. 1970;67:320–325. doi: 10.1073/pnas.67.1.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore RY, Eichler VB. Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Research. 1972;42:201–206. doi: 10.1016/0006-8993(72)90054-6. [DOI] [PubMed] [Google Scholar]
- Moore RY, Speh JC, Card JP. The retinohypothalamic tract originates from a distinct subset of retinal ganglion-cells. Journal of Comparative Neurology. 1995;352:351–366. doi: 10.1002/cne.903520304. [DOI] [PubMed] [Google Scholar]
- Morin LP, Blanchard JH, Provencio I. Retinal ganglion cell projections to the hamster suprachiasmatic nucleus, intergeniculate leaflet, and visual midbrain: Bifurcation and melanopsin immunoreactivity. Journal of Comparative Neurology. 2003;465:401–416. doi: 10.1002/cne.10881. [DOI] [PubMed] [Google Scholar]
- Newman LA, Walker MT, Brown RL, Cronin TW, Robinson PR. Melanopsin forms a functional short-wavelength photopigment. Biochemistry. 2003;42:12734–12738. doi: 10.1021/bi035418z. [DOI] [PubMed] [Google Scholar]
- Panda S, Provencio I, Tu DC, Pires SS, Rollag MD, Castrucci AM, Pletcher MT, Sato TK, Wiltshire T, Andahazy M, Kay SA, Van Gelder RN, Hogenesch JB. Melanopsin is required for nonimage- forming photic responses in blind mice. Science. 2003;301:525–527. doi: 10.1126/science.1086179. [DOI] [PubMed] [Google Scholar]
- Panda S, Sato TK, Castrucci AM, Rollag MD, Degrip WJ, Hogenesch JB, Provencio I, Kay SA. Melanopsin (Opn4) requirement for normal light-induced circadian phase shifting. Science. 2002;298:2213–2216. doi: 10.1126/science.1076848. [DOI] [PubMed] [Google Scholar]
- Provencio I, Jiang GS, De Grip WJ, Hayes WP, Rollag MD. Melanopsin: an opsin in melanophores, brain, and eye. Proc Natl Acad Sci USA. 1998;95:340–345. doi: 10.1073/pnas.95.1.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provencio I, Rodriguez IR, Jiang GS, Hayes WP, Moreira EF, Rollag MD. A novel human opsin in the inner retina. Journal of Neuroscience. 2000;20:600–605. doi: 10.1523/JNEUROSCI.20-02-00600.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pu ML. Dendritic morphology of cat retinal ganglion cells projecting to suprachiasmatic nucleus. Journal of Comparative Neurology. 1999;414:267–274. [PubMed] [Google Scholar]
- Ralph MR, Foster RG, Davis FC, Menaker M. Transplanted suprachiasmatic nucleus determines circadian period. Science. 1990;247:975–978. doi: 10.1126/science.2305266. [DOI] [PubMed] [Google Scholar]
- Ruby NF, Brennan TJ, Xie XM, Cao V, Franken P, Heller HC, O’Hara BF. Role of melanopsin in circadian responses to light. Science. 2002;298:2211–2213. doi: 10.1126/science.1076701. [DOI] [PubMed] [Google Scholar]
- Shen D, Jiang M, Hao W, Tao L, Salazar M, Fong HK. A human opsin-related gene that encodes a retinaldehyde-binding protein. Biochemistry. 1994;33:13117–13125. doi: 10.1021/bi00248a022. [DOI] [PubMed] [Google Scholar]
- Takahashi JS, DeCoursey PJ, Bauman L, Menaker M. Spectral sensitivity of a novel photoreceptive system mediating entrainment of mammalian circadian rhythms. Nature. 1984;308:186–188. doi: 10.1038/308186a0. [DOI] [PubMed] [Google Scholar]
- Thompson CL, Blaner WS, Van Gelder RN, Lai K, Quadro L, Colantuoni V, Gottesman ME, Sancar A. Preservation of light signaling to the suprachiasmatic nucleus in vitamin A-deficient mice. Proc Natl Acad Sci USA. 2001;98:11708–11713. doi: 10.1073/pnas.201301498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underwood H, Menaker M. Extraretinal light perception: entrainment of the biological clock controlling lizard locomotor activity. Science. 1970;170:190–193. doi: 10.1126/science.170.3954.190. [DOI] [PubMed] [Google Scholar]
- Van Gelder RN. Making (a) sense of non-visual ocular photoreception. Trends in Neurosciences. 2003;26:458–461. doi: 10.1016/S0166-2236(03)00211-X. [DOI] [PubMed] [Google Scholar]
- Warren EJ, Allen CN, Brown RL, Robinson DW. Intrinsic light responses of retinal ganglion cells projecting to the circadian system. European Journal of Neuroscience. 2003;17:1727–1735. doi: 10.1046/j.1460-9568.2003.02594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng K, Lu C, Daggett LP, Kuhn R, Flor PJ, Johnson EC, Robinson PR. Functional coupling of a human retinal metabotropic glutamate receptor (hmGluR6) to bovine rod transducin and rat Go in an in vitro reconstitution system. J Biol Chem. 1997;272:33100–33104. doi: 10.1074/jbc.272.52.33100. [DOI] [PubMed] [Google Scholar]
- Yamazaki S, Goto M, Menaker M. No evidence for extraocular photoreceptors in the circadian system of the Syrian hamster. Journal of Biological Rhythms. 1999;14:197–201. doi: 10.1177/074873099129000605. [DOI] [PubMed] [Google Scholar]
- Young MW, Kay SA. Time zones: a comparative genetics of circadian clocks. Nature Reviews Genetics. 2001;2:702–715. doi: 10.1038/35088576. [DOI] [PubMed] [Google Scholar]


