Abstract
Activation of nuclear factor-κB (NF-κB) can interfere with induction of apoptosis triggered by the tumour necrosis factor-related apoptosis-inducing ligand (TRAIL; Apo2L). Therefore, agents that suppress NF-κB activation may sensitise cells to TRAIL-dependent apoptosis. Exposure of Jurkat cells to TRAIL resulted in massive and saturable apoptosis induction, following an initial lag time. This lag was abolished by pretreatment of the cells with subapoptotic doses of α-tocopheryl succinate (α-TOS) or the proteasome inhibitor MG132. Exposure of the cells to TRAIL led to a rapid, transient activation of NF-κB, a process that was suppressed by cell pretreatment with α-TOS or MG132. Activation of NF-κB by TNF-α prior to TRAIL exposure increased resistance of the cells to TRAIL-mediated apoptosis. We conclude that α-TOS sensitises cells to TRAIL killing, at least in some cases, through inhibition of NF-κB activation. This further supports the possibility that this semisynthetic analogue of vitamin E is a potential adjuvant in cancer treatment, such as in the case of TRAIL-mediated inhibition of cancer.
Keywords: apoptosis, vitamin E succinate, TRAIL, NF-κB, signalling
The tumour necrosis factor-related apoptosis-inducing ligand (TRAIL, Apo2L), a member of the TNF superfamily, is a recently discovered potent inducer of apoptosis produced by cells of the immune system (Wiley et al, 1995). TRAIL transmits its proapoptosis signal via crosslinking its cognate receptors, death receptor-4 (DR4), also called TRAIL receptor-1 (TRAIL-R1) and DR5 (TRAIL-R2) (Pan et al, 1997b; Schneider et al, 1997a; Sheridan et al, 1997). These receptors recruit and activate the proximal caspase-8, which in turn activates the effector caspases, an event culminating in cell death (Muhlenbeck et al, 1998; Bodmer et al, 2000; Hopkins-Donaldson et al, 2000). The interaction between TRAIL receptors and the proximal caspase is likely mediated by a protein containing the Fas-associated death domain (FADD) (Kischkel et al, 2000; Sprick et al, 2000) and/or via a GTP-binding adaptor protein (Miyazaki and Reed, 2001). While mitochondrial signalling in TRAIL-induced apoptosis has been postulated in some reports (Thomas et al, 2000; Munshi et al, 2001), it may not be involved at all in some cases (Walczak et al, 2000; Keogh et al, 2000), or may act rather as an amplification loop (Suliman et al, 2001; Alleva et al, 2001).
Two additional receptors for TRAIL have been identified, decoy receptor-1 (DcR-1) (also known as TRAIL-R3) and DcR-2 (TRAIL-R4) (Pan et al, 1997a; Schneider et al, 1997a,1997b; Sheridan et al, 1997; Ashkenazi and Dixit, 1999). These receptors bind TRAIL but fail to transmit its apoptosis-inducing signal downstream, thereby acting as competitive inhibitors of TRAIL apoptosis (Ashkenazi and Dixit, 1999). While the decoy receptors appear unique to the TRAIL system, apoptosis induced by this ligand can also be suppressed by inhibition of caspase-8 (FLICE) activity, via induction of the FLICE-inhibitory protein (FLIP) (Schneider et al, 1997b). The fact that normal cells, compared to malignant cells, appear to overexpress DcR-1 and DcR-2 and/or FLIP suggest that TRAIL-induced apoptosis may be selective for cancer cells (Bonavida et al, 1999; Kim et al, 2000), making TRAIL attractive as a potential anticancer agent (Nagane et al, 2001). Furthermore, recent studies indicate that anticancer chemotherapeutics can sensitise cells to killing by immunological agents, including TRAIL, by upregulating the cognate death receptors and/or overcoming TRAIL resistance (Bonavida et al, 1999; Nagane et al, 2001; Nimmanapalli et al, 2001; Matsuzaki et al, 2001).
We and others have found that certain analogues of vitamin E, in particular α-tocopheryl succinate (α-TOS), are potent inducers of apoptosis in a variety of cells (Fariss et al, 1994; Neuzil et al, 1999,2001d; Yu et al, 1999,2001), and that this action appears to be specific for malignant cells (Neuzil et al, 2001c). Several reports suggest that vitamin E analogues sensitise cancer cells to killing by agents like Fas (Yu et al, 1999) or 5-fluorouracil (Chinery et al, 1997). As α-TOS is an inhibitor of activation of the nuclear factor-κB (NF-αB) (Suzuki and Packer, 1993; Erl et al, 1997; Neuzil et al, 2001b) and because activation of NF-κB has been shown to negatively modulate TRAIL-dependent apoptosis in multiple cancer cells (Bernard et al, 2001; Franco et al, 2001; Oya et al, 2001; Kreuz et al, 2001), we have investigated whether this vitamin E analogue might sensitise malignant cells to TRAIL killing via an NF-κB inhibitory activity. In this report, we show that α-TOS inhibits NF-κB activation in Jurkat T lymphoma cells and that this amplifies their susceptibility to TRAIL.
Materials and methods
Cell culture and treatment
Jurkat T lymphoma cells were maintained in RPMI-1640 medium supplemented with 10% FCS and antibiotics. The cells were regularly split when reaching a density of 1.5×106 ml−1, and used for experiments at 0.5×106 ml−1. Cells were treated with α-tocopherol (α-TOH) or α-tocopheryl succinate (α-TOS) (both from Sigma) at 25 or 50 μM, 10 μM hydrogen peroxide (Fluka) or 40 ng ml−1 recombinant human TNF-related apoptosis-inducing ligand (rhTRAIL) prepared as described elsewhere (Alleva et al, 2001; Plasilova et al, 2002). In brief, the extracellular part of human TRAIL (AA 95-281), obtained by PCR from the HPB T cell line cDNA library, was subcloned into pBSK, sequenced and further subcloned into the His-tagged reading frame of pET15b. The protein was expressed in Escherichia coli and purified using the TALON (Clontech) and SP-Sepharose columns. In some cases, cells were treated with the proteasome inhibitor MG132 (Calbiochem) at 0.5 or 1 μM, or tumour necrosis factor-α (TNF-α; PharMingen) at 100 U ml–1.
Apoptosis assessment
Apoptosis was routinely assessed by the annexin V-binding method, which is based on the affinity of annexin V for phosphatidylserine externalised to the outer leaflet of the plasma membrane early in the course of opoptosis. In brief, cells were harvested by centrifugation, washed with PBS, spun down again, and resuspended in the binding buffer (10 mM Hepes/NaOH, 140 mM NaCl and 25 mM CaCl2, pH 7.4). Cells were then incubated with 2 μl of annexin V-FITC (PharMingen) for 20 min at room temperature, and analysed by flourescence-assisted cell sorting (FACS; Becton Dickinson). Activation of caspase-3 was estimated by incubating cells with an anticaspase-3 IgG (PharMingen) that recognises the activated form, followed by incubation with FITC-conjugated secondary antibody. Fluorescence intensity of the cells was assessed by FACS (Neuzil et al, 2001b).
Assessment of NF-κB activation
Activation of NF-κB was estimated using the Trans-AM kit (Active Motif, Carlsbad, CA, USA) according to the manufacturer's protocol. In brief 5–10×106 cells were treated as specified, and lysed using the buffer provided by the manufacturer. The lysates were then transferred into wells containing the immobilised NF-κB (p65) consensus sequence, and incubated for 1 h at 37°C. The wells were washed and the bound p65 protein was detected by horseradish peroxidase (HRP)-dependent staining following incubation with anti-p65 lgG and secondary HRP-conjugated secondary lgG. The level of absorbance at 450 nm, assessed in a microplate reader, reflected the level of bound p65.
Transmission electron microscopy (TEM)
For TEM, Jurkat cells were grown in complete RPMI medium at 0.5×106 ml−1, and treated for 12 h with 40 ng ml−1 rhTRAIL or buffer alone (control cells). The cells (107) were briefly rinsed with PBS, centrifuged, fixed overnight in 2% glutaraldehyde, and postfixed with 1% OsO4 for 1 h. Both fixatives were made up in 0.1 M cacodylate buffer supplemented with 0.1 M sucrose (pH 7.2, 300 mOsmol) and applied at room temperature. After standard dehydration in ascending concentrations of ethanol, the cells were embedded in Epon-812 monomer and polymerised. Ultrathin sections were cut with a diamond knife mounted in a Reichart ultramicrotome, contrasted with uranyl acetate and lead citrate, and examined in a Jeol 1200 EX transmission electron microscope operated at 80 kV (Brunk et al, 1995).
Results and Discussion
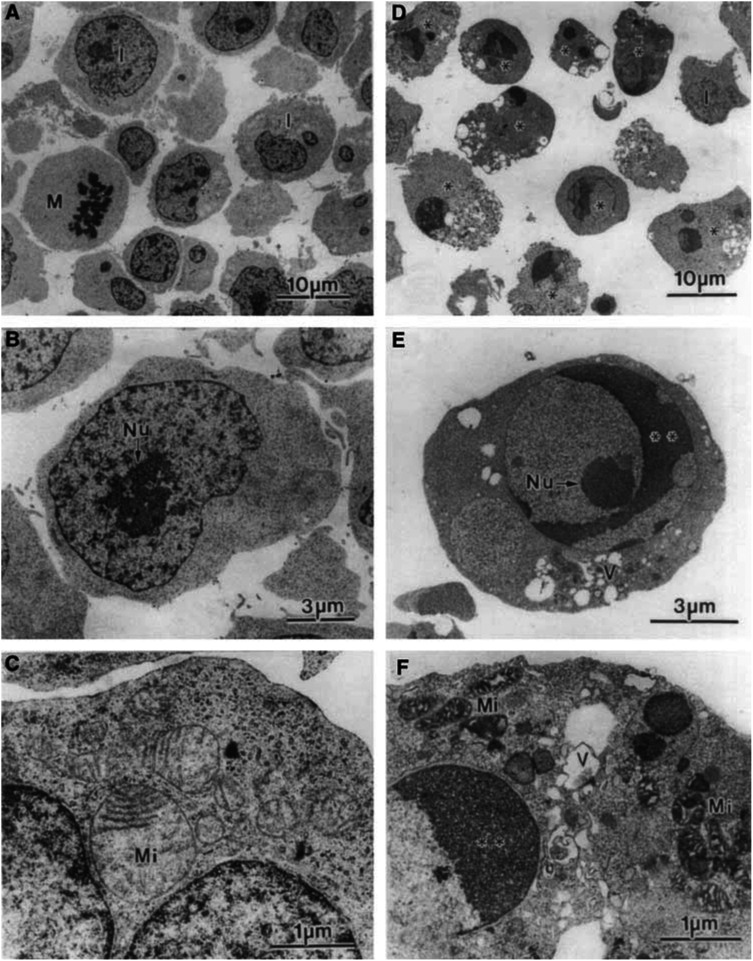
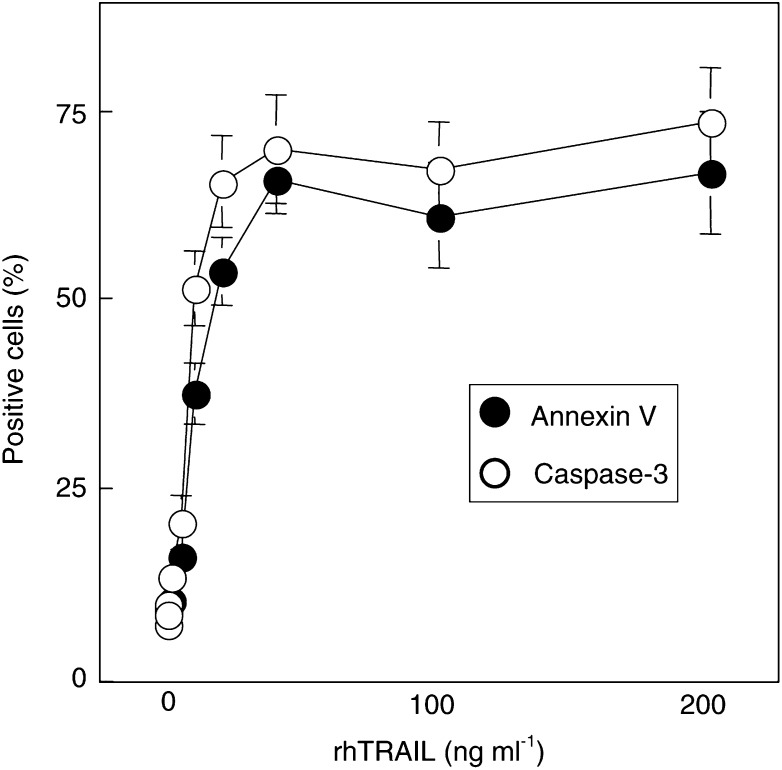
The aim of the present study was to determine whether the semisynthetic vitamin E analogue, α-TOS, could enhance the sensitivity of Jurkat T lymphoma cells to the induction of apoptosis by the immunological agent TRAIL. For initiation of apoptosis, we used rhTRAIL that was expressed in bacterial cells. As shown in Figures 1 and 2, our rhTRAIL preparation caused massive apoptosis in Jurkat cells, as documented by both morphological changes evaluated by TEM, and by PS externalisation and caspase-3 activation. Figure 2 also demonstrates that apoptosis induction was saturable with regard to the rhTRAIL used, with rhTRAIL being maximally effective at ca 20 ng ml−1.
Figure 1.
TRAIL is a potent inducer of apoptosis in Jurkat cells. Jurkat T lymphoma cells (0.5×106 ml−1) were exposed to the vehicle (A–C) or 40 ng ml−1 rhTRAIL (D–F), processed for TEM, observed, and images taken at the following magnifications: A, D–1800×; B–5000×; C, F–18 000×; and E–7000×; M-mitotic cell; I–cell in the interphase; Mi–mitochondrium; Nu–nucleolus; V–vacuole; *apoptotic cell; **nucleus with condensed chromatin.
Figure 2.
Apoptotic effect of rhTRAIL on Jurkat cells is saturable. Jurkat cells at 0.5×106 ml−1 were exposed to increasing concentrations of rhTRAIL for 12 h, and the extent of apoptosis (annexin V-FITC staining) and caspase-3 activation was assessed.
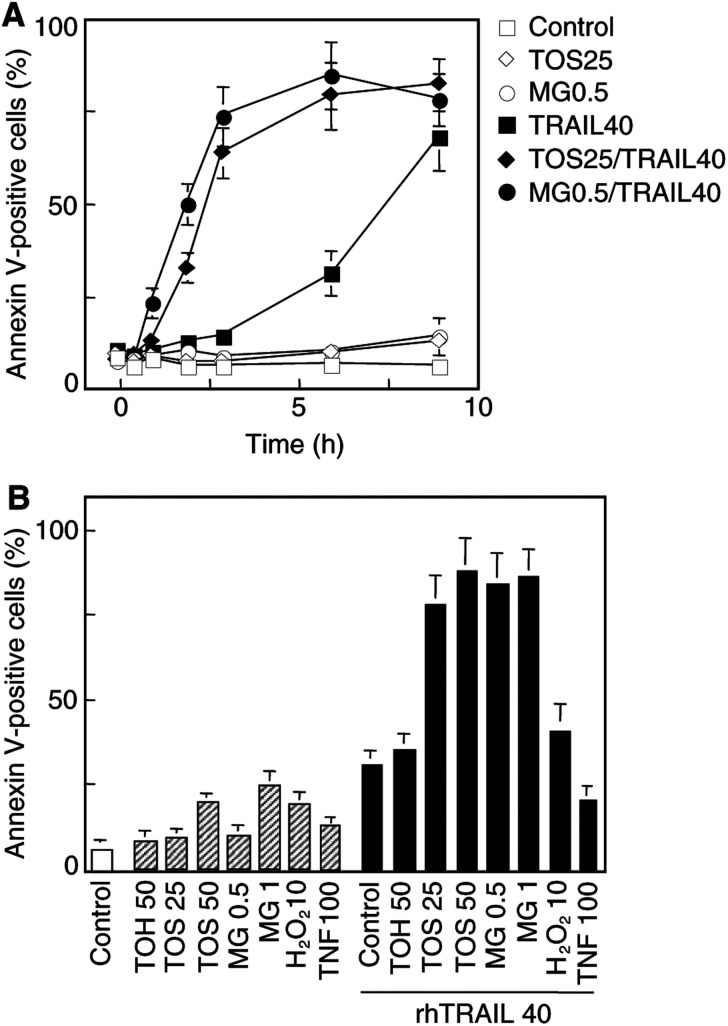
We next investigated whether preincubation with α-TOS might sensitise Jurkat cells to TRAIL. As shown in Figure 3, α-TOS–but not α-TOH–rendered the cells more susceptible to TRAIL-induced apoptosis at a concentration at which the vitamin E analogue itself did not cause substantial cell death. To determine whether this potentiation of TRAIL killing might invlove inhibition, by α-TOS, NF-κB activation, the cells were pretreated with the proteasome inhibitor, MG132, or with TNF-α, a potent activator of NF-κB. Figure 3 shows that preincubation with MG132 sensitised cells to TRAIL, as did α-TOS, and that MG132 itself did not cause apoptosis. On the contrary, pretreatment with TNF-α increased resistance of the cells to TRAIL, consistent with the idea that activation of NF-κB may be antiapoptotic (Bernard et al, 2001; Franco et al, 2001). Finally, we used hydrogen peroxide as a negative control. At a low concentration (10 μM) that does not interfere with NF-κB activation (see below), hydrogen peroxide did not induce substantial apoptosis nor did it sensitise cells to TRAIL (Figure 3).
Figure 3.
α-TOS sensitises Jurkat cells to TRAIL killing. Jurkat cells (0.5×106 ml−1) were pretreated with α-TOH, α-TOS, MG132 or hydrogen peroxide at the concentrations indicated (μM) for 4 h, or to TNF-α at 100 U ml−1 for 1 h, after which they were exposed to rhTRAIL at 40 ng ml−1. At time points indicated (A) or at 6 h (B) following TRAIL addition, cells were evaluated for apoptosis.
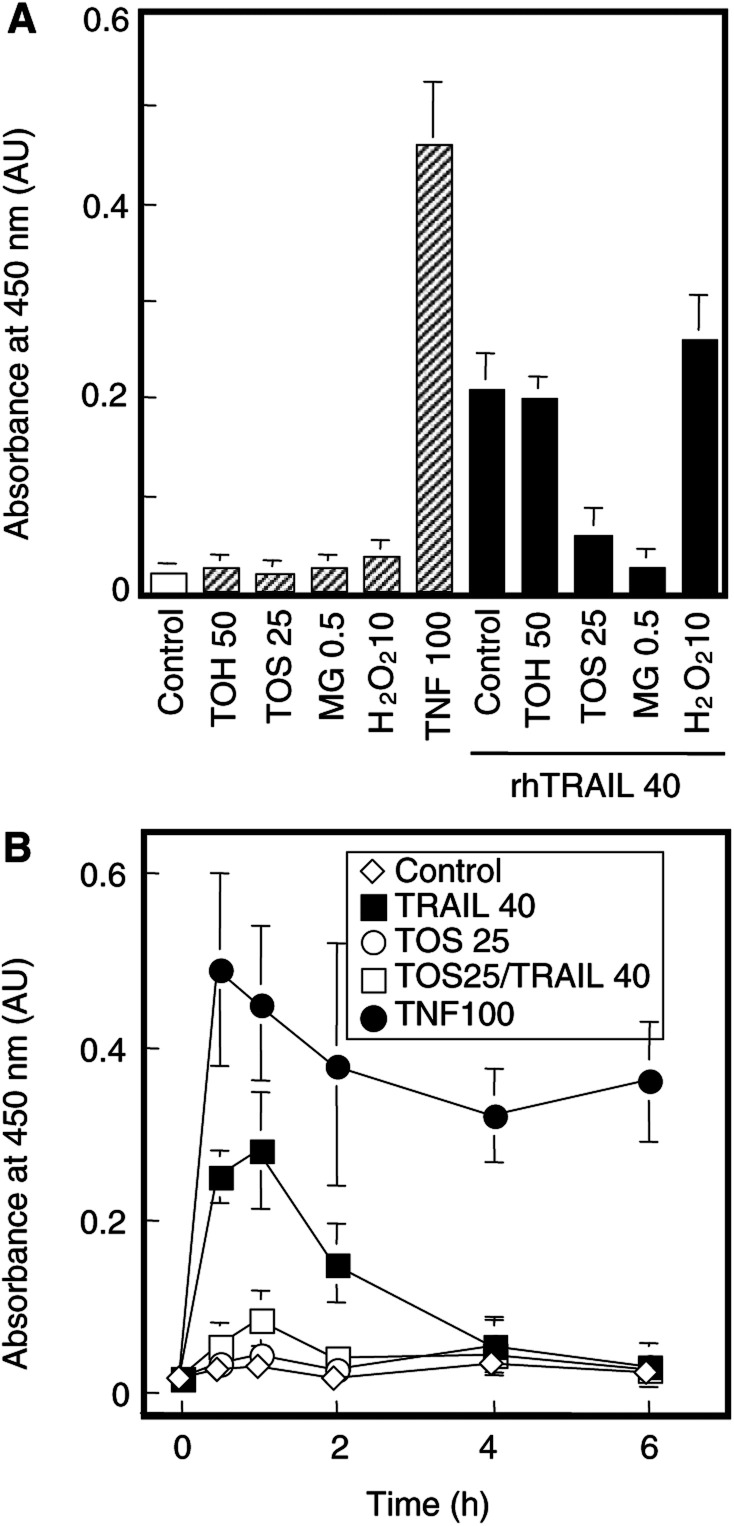
More direct studies of NF-κB activation revealed that Jurkat cells exposed to rhTRAIL did activate NF-κB. Figure 4 shows a substantial activation of NF-κB, 30 min following addition of rhTRAIL to the cells, although this activation was less pronounced than that caused by treatment with the strong NF-κB activator, TNF-α. This activation was transient and lasted for about 1 h, after which it declined. Pretreatment with α-TOS or MG132 abolished the initial NF-κB activation observed with TRAIL alone. Once again, hydrogen peroxide at 10 μM had no effect on NF-κB, either alone or in combination with TRAIL (Figure 4).
Figure 4.
α-TOS abolishes transient activation of NF-κB by TRAIL. Jurkat cells (0.5×106 ml−1, 107 total) were treated as specified in the legend to Figure 3 (concentrations in μM except U ml−1 for TNF-α, rhTRAIL at 40 ng 1−1). At a 2-h time point (A) or as specified (B), cells were washed with PBS, spun down, the pellet resuspended in the lysis buffer, and the lysate probed for NF-κB activation using the TRANS-AM kit as detailed in Materials and Methods. The level of NF-κB activation (p65 binding to its cognate DNA sequence) is expressed as a relative absorbance at 450 nm.
We demonstrate in this communication that vitamin E succinate, but not vitamin E itself, potentiates killing of Jurkat T lymphoma cells by the immunological inducer of apoptosis, TRAIL. These data are consistent with, and further extend, the earlier observations that α-TOS promotes apoptosis caused by a variety of agonists. This is true, for example, of Fas-dependent killing of breast (Yu et al, 1999) and prostate cancer cells (Israel et al, 2000). In these instances, the vitamin E analogue sensitised the cells to Fas ligand by causing plasma membrane translocation of Fas. Furthermore, α-TOS also promotes TRAIL-induced apoptosis in colon cancer cells, apparently by modulating different, converging signalling pathways, thereby maximising the apoptotic potential of the cells (Weber et al, 2002). Importantly, this cooperation was also reflected in the inhibition of colon cancer in an animal model (Weber et al, 2002).
There are several possible mechanisms by which α-TOS may sensitise leukemic cells towards TRAIL killing. TRAIL crosslinks two cognate, death-signalling receptors. One of these, DR4 (TRAIL-R1), has been shown to transiently activate NF-κB. This leads to an initial expression of survival signals including the inhibitor of apoptosis protein (IAP) family members (Degli-Esposti et al, 1997; Schneider et al, 1997b; Bernard et al, 2001). Perhaps by this mechanism, activation of NF-κB can protect leukemic cells from apoptotic killing (Jeremias et al, 1998). Activation of NF-κB also leads to upregulation of the caspase-8 inhibitor, cFLIP (Kreuz et al, 2001). Jurkat cells express both DR4 and DR5, although the level of expression of the former receptor is lower than that of the latter (JN et al, unpublished). In spite of this, the level of DR4 expression appears to be sufficient to activate NF-κB upon exposure of the cells to TRAIL (this report). We hypothesised that inhibition of NF-κB activation–likely responsible for the lag phase in apoptosis induction by TRAIL in Jurkat cells– could be inhibited by α-TOS. In support of this, preincubation of the cells with α-TOS suppressed TNF-α-dependent NF-κB activation (cf. Fig. 4). The exact mode of suppression of NF-κB activation by α-TOS is not yet clear but there are several possibilities. For example, activation of NF-κB might be suppressed by α-TOS by affecting degradation of the inhibitory subunit, IκB. Indeed, cleavage of, or mutations in, IκB can accentuate apoptosis (Jeremias et al, 1998; Keane et al, 2000) and a recent report documents a caspase-dependent cleavage of IκB in TRAIL-resistant cells, thereby sensitising them to killing by TRAIL (Kim et al, 2002).
The concept that α-TOS can inhibit NF-κB activation is not new (cf. Erl et al, 1997), but the precise structural requirements are not fully known. It is clear, however, that α-TOH, the redox-active counterpart of α-TOS, fails to exert such activity (Erl et al, 1997; Neuzil et al, 2001b). One possibility is suggested by the observation that α-TOS activates caspases that cleave the NF-κB subunit p65, while not killing the cells (Neuzil et al, 2001b), probably via a mitochondria-dependent pathway (Neuzil et al, 2001d; Weber et al, 2002). We have earlier suggested that under certain circumstances, α-TOS can cause ‘subapoptotic’ signalling that may lead to activation of early apoptotic events while not bringing the cell into the execution phase of apoptosis, a possibility also suggested by others (Harvey et al, 2000). Such a mechanism may underlie the inhibitory activity of α-TOS towards activation of NF-κB in Jurkat cells, thereby sensitsing the cells to killing by TRAIL.
α-TOS is not just another example of an inducer of apoptosis that senstises cells to TRAIL killing, a principle that has been published in multiple reports (see, e.g. Bonavida et al, 1999; Nagane et al, 2001). Unlike many chemotherapeutic agents, α-TOS appears to be selective for malignant cells (Neuzil et al, 2001c,2001d; Weber et al, 2001). α-TOS, which has proapoptotic activity in vitro and antineoplastic effects in vivo (Neuzil et al, 2001d; Malafa et al, 2002; Weber et al, 2002), is carried within the bloodstream by circulating lipoproteins (Pussinen et al, 2000), which are cleared in the liver. Here, α-TOS is hydrolysed to α-TOH, at least some of which is released into the circulation, thereby boosting the antioxidant defence system (Neuzil et al, 2001a). Because both α-TOS and TRAIL are relatively nontoxic to normal cells (Bonavida et al, 1999; Jo et al, 2000; Kim et al, 2000; Nagane et al, 2001; Neuzil et al, 2001c,2001d; Nesterov et al, 2002; Weber et al, 2002), the two agents, that is, α-TOS and TRAIL, would seem to represent an exciting partnership of potentially high therapeutic relevance.
In conclusion, we have shown that α-TOS potentiates TRAIL-induced apoptosis in Jurkat T lymphoma cells by inhibiting transient activation of the transcription factor NF-κB. In practical terms, this finding could be utilised for devising strategies of treatment for potentially fatal disorders like lymphomas or carcinomas on two levels: first, by coadministration of α-TOS and TRAIL; second, by administration of α-TOS alone, as the agent could be expected to sensitise cancer cells to endogenously produced TRAIL, thereby potentiating the immune defences against neoplasia. This principle may be especially useful for suppressing cancer involving malignant cells with a high expression of DR4. Further exploration of these possibilities may lead to an effective approach to the treatment of malignancies.
Acknowledgments
We thank Dr L Andera for providing rhTRAIL, and Drs L Andera, UT Brunk and JW Eaton for a critical reading of the manuscript. The technical assistance of AMS Austarheim and TF Gulbransen is highly appreciated. JN was partially supported by the University of Linköping Grant 83081030 and a grant from the Dust Diseases Board of Australia. HD was supported by the Norwegian Research Council Grant 129557/310.
References
- Alleva R, Tomasetti M, Andera L, Gellert N, Borghi B, Weber C, Murphy MP, Neuzil J (2001) Coenzyme Q blocks biochemical but not receptor-mediated apoptosis by increasing mitochondrial antioxidant protection. FEBS Lett 503: 46–50 [DOI] [PubMed] [Google Scholar]
- Ashkenazi A, Dixit VM (1999) Apoptosis control by death and decoy receptors. Curr Opin Cell Biol 11: 255–260 [DOI] [PubMed] [Google Scholar]
- Bernard D, Quatannens B, Vandenbunder B, Abbadie C (2001) Rel/NF-κB transcription factors protect against tumour necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL)-induced apoptosis by up-regulating the TRAIL decoy receptor DcR1. J Biol Chem 276: 27322–27328 [DOI] [PubMed] [Google Scholar]
- Bodmer JL, Holler N, Reynard S, Vinciguerra P, Schneider P, Juo P, Blenis J, Tschopp J (2000) TRAIL receptor-2 signals apoptosis through FADD and caspase-8. Nat Cell Biol 2: 241–243 [DOI] [PubMed] [Google Scholar]
- Bonavida B, Ng CP, Jazirehi A, Schiller G, Mizutani Y (1999) Selectivity of TRAIL-mediated apoptosis of cancer cells and synergy with drugs: the trail to non-toxic cancer therapeutics (review). Int J Oncol 15: 793–802 [DOI] [PubMed] [Google Scholar]
- Brunk UT, Zhang H, Dalen H, Öllinger K (1995) Exposure of cells to nonlethal concentrations of hydrogen peroxide induces degeneration–repair mechanisms invloving lysosomal destabilization. Free Radic Biol Med 19: 813–822 [DOI] [PubMed] [Google Scholar]
- Chinery R, Brockman JA, Peeler MO, Shyr Y, Beauchamp RD, Coffey RJ (1997) Antioxidants enhance the cytotoxicity of chemotherapeutic agents in colorectal cancer: a p53-independent induction of p21WAF1/CIP1 via C/EBPbeta. Nat Med 3: 1233–1241 [DOI] [PubMed] [Google Scholar]
- Degli-Esposti MA, Dougall WC, Smolak PJ, Waugh JY, Smith CA, Goodwin RG (1997) The novel receptor TRAIL-R4 induces NF-kappaB and protects against TRAIL-mediated apoptosis, yet retains an incomplete death domain. Immunity 7: 813–820 [DOI] [PubMed] [Google Scholar]
- Erl W, Weber C, Wardemann C, Weber PC (1997) α-Tocopheryl succinate inhibits monocytic cell adhesion to endothelial cells by suppressing NF-κB mobilization. Am J Physiol 273: H634–H640 [DOI] [PubMed] [Google Scholar]
- Fariss MW, Fortuna MB, Everett CK, Smith JD, Trent DF, Djuric Z (1994) The selective antiproliferative effects of α-tocopheryl hemisuccinate and cholesteryl hemisuccinate on murine leukemia cells result from the action of the intact compounds. Cancer Res 54: 3346–3351 [PubMed] [Google Scholar]
- Franco AV, Zhang XD, Van Berkel E, Sanders JE, Zhang XY, Thomas WD, Nguyen T, Hersey P (2001) The role of NF-κB in TNF-related apoptosis-inducing ligand (TRAIL)-induced apoptosis of melanoma cells. J Immunol 166: 5337–5345 [DOI] [PubMed] [Google Scholar]
- Harvey KJ, Lukovic D, Ucker DS (2000) Caspase-dependent Cdk activity is a requisite effector of apoptotic death events. J Cell Biol 148: 59–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins-Donaldson S, Bodmer JL, Bourloud KB, Brognara CB, Tschopp J, Gross N (2000) Loss of caspase-8 expression in highly malignant human neuroblastoma cells correlates with resistance to tumour necrosis factor-related apoptosis-inducing ligand-induced apoptosis. Cancer Res 60: 4315–4319 [PubMed] [Google Scholar]
- Israel K, Yu W, Sanders BG, Kline K (2000) Vitamin E succinate induces apoptosis in human prostate cancer cells: role for Fas in vitamin E succinate-triggered apoptosis. Nutr Cancer 36: 90–100 [DOI] [PubMed] [Google Scholar]
- Jeremias E, Kupatt C, Bauman B, Herr I, Wirth T, Debatin KM (1998) Inhibition of nuclear factor kappaB activation attenuates apoptosis resistance in lymphoid cells. Blood 91: 4624–4631 [PubMed] [Google Scholar]
- Jo M, Kim TH, Seol DW, Esplen JE, Dorko K, Billiar TR, Strom SC (2000) Apoptosis induced in normal human hepatocytes by tumour necrosis factor-related apoptosis-inducing ligand. Nat Med 6: 564–567 [DOI] [PubMed] [Google Scholar]
- Keane MM, Rubinstein Y, Cuello M, Ettenberg SA, Banerjee P, Nau MM, Lipkowitz S (2000) Inhibition of NF-κB activity enhances TRAIL mediated apoptosis in breast cancer cell lines. Breast Cancer Res Treat 64: 211–219 [DOI] [PubMed] [Google Scholar]
- Keogh SA, Walczak H, Bouchier-Hayes L, Martin SJ (2000) Failure of Bcl-2 to block cytochrome c redistribution during TRAIL-induced apoptosis. FEBS Lett 471: 93–98 [DOI] [PubMed] [Google Scholar]
- Kim K, Fisher MJ, Xu SQ, el-Deiry WS (2000) Molecular determinants of response to TRAIL in killing of normal and cancer cells. Clin Cancer Res 6: 335–346 [PubMed] [Google Scholar]
- Kim KW, Kim BJ, Chung CW, Jo DG, Kim IK, Song YH, Kwon YK, Woo HN, Jung YK (2002) Caspase cleavage product lacking amino-terminus of IκBα sensitizes resistant cells to TNF-alpha and TRAIL-induced apoptosis. J Cell Biochem 85: 334–345 [DOI] [PubMed] [Google Scholar]
- Kischkel FC, Lawrence DA, Chuntharapai A, Schow P, Kim KJ, Ashkenazi A (2000) Apo2L/TRAIL-dependent recruitment of endogenous FADD and caspase-8 to death receptors 4 and 5. Immunity 12: 611–620 [DOI] [PubMed] [Google Scholar]
- Kreuz S, Siegmund D, Scheurich P, Wajant H (2001) NF-κB inducers upregulate cFLIP, a cycloheximide-sensitive inhibitor of death receptor signalling. Mol Cell Biol 21: 3964–3973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malafa MP, Fokum FD, Mowlavi A, Abusief M, King M (2002) Vitamin E inhibits melanoma growth in mice. Surgery 31: 85–91 [DOI] [PubMed] [Google Scholar]
- Matsuzaki H, Schmied BM, Ulrich A, Standop J, Scheider MB, Batra SK, Picha KS, Pour PM (2001) Combination of tumour necrosis factor-related apoptosis-inducing ligand (TRAIL) and actinomycin D induces apoptosis even in TRAIL-resistant human pancreatic cancer cells. Clin Cancer Res 7: 407–414 [PubMed] [Google Scholar]
- Miyazaki T, Reed JC (2001) A GTP-binding adapter protein couples TRAIL receptors to apoptosis-inducing proteins. Nat Immunol 2: 493–500 [DOI] [PubMed] [Google Scholar]
- Muhlenbeck F, Haas E, Schwenzer R, Schubert G, Grell M, Smith C, Scheurich P, Wajant H (1998) TRAIL/Apo2L activates c-Jun NH2-terminal kinase (JNK) via caspase-dependent and caspase-independent pathways. J Biol Chem 273: 33091–33098 [DOI] [PubMed] [Google Scholar]
- Munshi A, Pappas G, Honda T, McDonnell TJ, Younes A, Li Y, Meyn RE (2001) TRAIL (APO-2L) induces apoptosis in human prostate cancer cells that is inhibitable by Bcl-2. Oncogene 20: 3757–3765 [DOI] [PubMed] [Google Scholar]
- Nagane M, Huang HJ, Cavenee WK (2001) The potential of TRAIL for cancer chemotherapy. Apoptosis 6: 191–197 [DOI] [PubMed] [Google Scholar]
- Nesterov A, Ivashchenko Y, Kraft AS (2002) Tumour necrosis factor-related apoptosis-inducing ligand (TRAIL) triggers apoptosis in normal prostate epithelial cells. Oncogene 21: 1135–1140 [DOI] [PubMed] [Google Scholar]
- Neuzil J, Kågedal K, Andera L, Weber C, Brunk UT (2001a) Vitamin E analogs: a new class of multiple action agents with anti-neoplastic and anti-atherogenic activity. Apoptosis 7: 177–185 [DOI] [PubMed] [Google Scholar]
- Neuzil J, Schroder A, von Hundelshausen P, Zernecke A, Weber T, Gellert N, Weber C (2001b) Inhibition of inflammatory endothelial responses by a pathway involving caspase activation and p65 cleavage. Biochemistry 40: 4686–4692 [DOI] [PubMed] [Google Scholar]
- Neuzil J, Svensson I, Weber T, Weber C, Brunk UT (1999) α-Tocopheryl succinate-induced apoptosis in Jurkat T cells involves caspase-3 activation, and both lysosomal and mitochondrial destabilisation. FEBS Lett 445: 295–300 [DOI] [PubMed] [Google Scholar]
- Neuzil J, Weber T, Gellert N, Weber C (2001c) Selective cancer cell killing by α-tocopheryl succinate. Br J Cancer 84: 87–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuzil J, Weber T, Schröder A, Lu M, Ostermann G, Gellert N, Mayne GC, Olejnicka B, Negre-Salvayre A, Sticha M, Coffey RJ, Weber C (2001d) Induction of cancer cell apoptosis by α-tocopheryl succinate: molecular pathways and structural requirements. FASEB J 15: 403–415 [DOI] [PubMed] [Google Scholar]
- Nimmanapalli R, Perkins CL, Orlando M, O'Bryan E, Nguyen D, Bhalla KN (2001) Pretreatment with paclitaxel enhances apo-2 ligand/tumour necrosis factor-related apoptosis-inducing ligand-induced apoptosis of prostate cancer cells by inducing death receptors 4 and 5 protein levels. Cancer Res 61: 759–763 [PubMed] [Google Scholar]
- Oya M, Ohtsubo M, Takayanagi A, Tachibana M, Shimizu N, Murai M (2001) Constitutive activation of nuclear factor-αB prevents TRAIL-induced apoptosis in renal cancer cells. Oncogene 20: 3888–3896 [DOI] [PubMed] [Google Scholar]
- Pan G, Ni J, Wei YF, Yu G, Gentz R, Dixit VM (1997a) An antagonist decoy receptor and a death domain-containing receptor for TRAIL. Science 277: 815–818 [DOI] [PubMed] [Google Scholar]
- Pan G, O'Rourke K, Chinnaiyan AM, Gentz R, Ebner R, Ni J, Dixit VM (1997b) The receptor for the cytotoxic ligand TRAIL. Science 276: 111–113 [DOI] [PubMed] [Google Scholar]
- Plasilova M, Zivny J, Jelinek J, Neuwirthova R, Cermak J, Necas E, Andera L, Stopka T (2002) TRAIL (Apo2L) suppresses growth of primary human leukemia and myelodysplasia progenitors. Lukemia 16: 67–73 [DOI] [PubMed] [Google Scholar]
- Pussinen PJ, Lindner H, Glatter O, Reicher H, Kostner GM, Winter-sperger A, Malle E, Sattler W (2000) Lipoprotein-associated α-tocopheryl-succinate inhibits cell growth and induces apoptosis in human MCF-7 and HBL-100 breast cancer cells. Biochim Biophys Acta 1485: 129–144 [DOI] [PubMed] [Google Scholar]
- Schneider P, Bodmer JL, Thome M, Hofmann K, Holler N, Tschopp J (1997a) Characterization of two receptors for TRAIL. FEBS Lett 416: 329–334 [DOI] [PubMed] [Google Scholar]
- Schneider P, Thome M, Burns K, Bodmer JL, Hofmann K, Kataoka T, Holler N, Tschopp J (1997b) TRAIL receptors 1 (DR4) and 2 (DR5) signal FADD-dependent apoptosis and activate NF-κB. Immunity 7: 831–836 [DOI] [PubMed] [Google Scholar]
- Sheridan JP, Marsters SA, Pitti RM, Gurney A, Skubatch M, Baldwin D, Ramakrishnan L, Gray CL, Baker K, Wood WI, Goddard AD, Godowski P, Ashkenazi A. (1997) Control of TRAIL-induced apoptosis by a family of signaling and decoy receptors. Science 277: 818–821 [DOI] [PubMed] [Google Scholar]
- Sprick MR, Weigand MA, Rieser E, Rauch CT, Juo P, Blenis J, Krammer PH, Walczak H (2000) FADD/MORT1 and caspase-8 are recruited to TRAIL receptors 1 and 2 and are essential for apoptosis mediated by TRAIL receptor 2. Immunity 12: 599–609 [DOI] [PubMed] [Google Scholar]
- Suliman A, Lam A, Datta R, Srivastava RK (2001) Intracellular mechanisms of TRAIL: apoptosis through mitochondrial-dependent and -independent pathways. Oncogene 20: 2122–2133 [DOI] [PubMed] [Google Scholar]
- Suzuki YJ, Packer L (1993) Inhibition of NF-κB DNA binding activity by α-tocopheryl succinate. Biochem Mol Biol Int 31: 693–700 [PubMed] [Google Scholar]
- Thomas WD, Zhang XD, Franco AV, Nguyen T, Hersey P (2000) TNF-related apoptosis-inducing ligand-induced apoptosis of melanoma is associated with changes in mitochondrial membrane potential and perinuclear clustering of mitochondria. J Immunol 165: 5612–5620 [DOI] [PubMed] [Google Scholar]
- Walczak H, Bouchon A, Stahl H, Krammer PH (2000) Tumour necrosis factor-related apoptosis-inducing ligand retains its apoptosis-inducing capacity on Bcl-2- or Bcl-xL-overexpressing 2-chemotherapy-resistant tumour cells. Cancer Res 60: 3051–3057 [PubMed] [Google Scholar]
- Weber T, Lu M, Andera L, Lahm H, Gellert N, Fariss MW, Korinek V, Sattler W, Ucker DS, Terman A, Schröder A, Erl W, Brunk U, Coffey RJ, Weber C, Neuzil J (2002) Vitamin E succinate is a potent novel antineoplastic agent with high selectivity and cooperativity with tumour necrosis factor-related apoptosis-inducing ligand (Apo2 ligand) in vivo. Clin Cancer Res 8: 863–869 [PubMed] [Google Scholar]
- Wiley SR, Schooley K, Smolak PJ, Din WS, Huang CP, Nicholl JK, Sutherland GR, Smith TD, Rauch C, Smith CA (1995) Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity 3: 673–678 [DOI] [PubMed] [Google Scholar]
- Yu W, Israel K, Liao QY, Aldaz CM, Sanders BG, Kline K (1999) Vitamin E succinate (VES) induces Fas sensitivity in human breast cancer cells: role for Mr 43,000 Fas in VES-triggered apoptosis. Cancer Res 59: 953–961 [PubMed] [Google Scholar]
- Yu W, Liao QY, Hantash FM, Sanders BG, Kline K (2001) Activation of extracellular signal-regulated kinase and c-Jun-NH(2)-terminal kinase but not p38 mitogen-activated protein kinases is required for RRR-α-tocopheryl succinate-induced apoptosis of human breast cancer cells. Cancer Res 61: 6569–6576 [PubMed] [Google Scholar]