Abstract
The effects of castration and hormone treatment on cognitive performance were evaluated in male rats. Castrated animals received either testosterone or estradiol and were compared with gonadally intact animals and with castrated controls. Results revealed a dissociation between the effects of testosterone and estradiol on cognitive performance in male rats. Specifically, estradiol enhanced acquisition of a delayed matching-to-position spatial task, similar to previously published observations in females. In contrast, neither castration nor testosterone treatment had any significant effect on acquisition of the delayed matching-to-position task, but did appear to affect delay-dependent working memory. None of the treatments had any significant effect on acquisition of a configural association negative patterning task, suggesting that effects on the delayed matching-to-position task were not due to effects on motivational factors. These data demonstrate that, as in females, gonadal hormones influence cognitive performance in males and suggest that estradiol and testosterone affect distinct cognitive domains.
Keywords: Castration, Hormone therapy, Learning and memory
Introduction
Numerous studies have demonstrated that, in rats, ovariectomy can impair, and estradiol administration can enhance, performance on a variety of cognitive tasks (Dohanich, 2002; Gibbs and Gabor, 2003; Korol, 2004; Luine et al., 2003). Many of these tasks (e.g., T-maze alternation, radial arm maze, working memory version of Morris water maze) are spatial tasks that rely heavily on hippocampal function.
Fewer studies have examined the effects of castration and gonadal hormone replacement on cognitive performance in male rodents; however, significant effects have been observed. For example, studies have shown that due to organizational effects of gonadal hormones during development, male rodents tend to outperform female rodents on spatial tasks (Dawson et al., 1975; Williams and Meck, 1991). Isgor and Sengelaub (2003) recently demonstrated a dose-dependent effect of neonatal testosterone administration on performance of adult rats in the Morris water maze. These findings are consistent with human studies which show that men tend to outperform women on tests of visual-spatial ability, mathematical reasoning ability, and targeting (Astur et al., 1998; Gallagher et al., 2000; Halpern, 1992).
In adult rats, castration has been shown to impair performance on radial arm maze (Daniel et al., 2003; Harrell et al., 1990), T-maze (Kritzer et al., 2001), and object recognition (Ceccarelli et al., 2001) tasks. Edinger et al. (2004) also recently reported effects of castration and hormone treatment on contextual, but not cued, fear conditioning. In several cases, treatment with testosterone was shown to restore or enhance performance in castrated males (Edinger et al., 2004; Kritzer et al., 2001). Some of these effects may be due to local aromatization of testosterone to estradiol (Bimonte-Nelson et al., 2003; Frye and Rhodes, 2002; Packard, 1998); however, recent work by Frye and co-workers demonstrates that 3-alpha-androstane-diol, a non-estrogenic metabolite of testosterone also can significantly affect performance on a variety of hippocampal tasks (Edinger et al., 2004; Frye et al., 2004), possibly via direct interaction with androgen receptors located in the hippocampus (Brown et al., 1995; Kerr et al., 1995; Simerly et al., 1990).
Previously, our own studies focused on effects of estradiol in female rats and have demonstrated significant estradiol-mediated enhancement in the rate at which ovariectomized rats learn a delayed matching-to-position T-maze task (Gibbs, 1999, 2002; Gibbs et al., 2004). The mechanisms that underlie this effect are not entirely clear. In females, both estradiol and testosterone have been shown to increase spine density and connectivity on CA1 pyramidal cells in the hippocampus (Leranth et al., 2004; Woolley, 2000). These are the primary output cells of the hippocampus, and loss of these cells is reported to produce an amnestic syndrome in humans (Zola-Morgan et al., 1986). At least one study suggests that effects of estradiol on spatial working memory performance in female rats may be related to estradiol-mediated increases in spine density on CA1 pyramidal cells (Sandstrom and Williams, 2001). In the study by Isgor and Sengelaub (2003) mentioned above, the effect of neonatal testosterone on Morris water maze performance correlated with increased length and branching of CA3 pyramidal cell dendrites. Our own studies have shown that cholinergic projections from the basal forebrain to the hippocampus also are involved, since loss of these projections eliminates the estradiol-mediated effect on DMP acquisition (Gibbs, 2002). Notably, these projections also appear to be necessary for the estradiol-mediated effects on spine density in area CA1 (Lam and Leranth, 2003), suggesting there may be a common mechanism underlying the effects of estradiol on different spatial tasks.
Recently, studies by Leranth et al. (2003) showed that administration of testosterone or DHT to gonadectomized male rats results in a significant increase in synaptic spine density on CA1 pyramidal cells, similar to effects of estradiol and testosterone in females. Notably, estradiol was not effective in males. Based on the hypothesis that the effects of acute hormone treatment on spine density in area CA1 of male rats contribute to subsequent effects on the performance of spatial tasks, we predicted that castration would impair, and that testosterone (but not estradiol) would enhance DMP acquisition in male rats. For comparison, the rats were also tested on a non-spatial configural association task, which studies suggest is less dependent on hippo-campal function (Rudy and Sutherland, 1995). Notably, estradiol, but not testosterone, enhanced DMP acquisition in male rats, much as we have observed in female rats. Conversely, testosterone, but not estradiol, appeared to enhance performance during increasing intertrial delays. In contrast, hormone treatments had no significant effect on acquisition of the configural association task. These findings are discussed in relation to potential mechanisms that underlie gonadal hormone effects on cognitive performance.
Methods
Eleven young adult (275–300 g) gonadally intact male Sprague–Dawley rats and 35 young adult (275–300 g) castrated rats were purchased and housed individually with food and water freely available. Animals were housed for 2–4 weeks prior to use. All procedures were carried out in accordance with PHS policies and with the approval of the University of Pittsburgh’s Institutional Animal Care and Use Committee.
Drug treatments
Castrated rats were anesthetized with isoflurane and received Silastic capsules (0.058 in. inner diameter, 0.077 in. outer diameter, Dow corning Corp., Midland, MI) containing either 17-β-estradiol (E; 5 mm capsule; n = 12) or testosterone propionate (T; 25 mm capsule; n = 12) implanted sc. The E capsules produce levels of estradiol in the physiological range (for a young adult female rat) for up to 2 months post-implantation. These levels have been shown to enhance DMP acquisition in young adult ovariectomized female rats (Gibbs, 1999, 2002; Gibbs et al., 2004). In contrast, the T capsules produce supra-physiological levels of testosterone and had to be replaced every 30 days. Since castrated rats have very low levels of T, and gonadally intact rats have physiological levels of T, we thought that treating rats with supraphysiological levels might provide additional information about potential dose-related effects of T. All remaining rats received blank capsules, and all capsules were replaced every 30 days.
Delayed matching-to-position training and testing
After 2 weeks of treatment, rats were food deprived to 85% body weight and trained on a delayed matching-to-position (DMP) T-maze task as previously described (Gibbs, 1999, 2002). The T-maze consisted of an approach alley (4 in. wide × 14 in. long) and two goal arms (4 in. wide × 12 in. long). The walls of the maze were 5 in. high and were constructed of black Plexiglas. The top of the maze was constructed of clear Plexiglas, which allowed the animals to view the surrounding room, and was attached to the walls of the maze by metal hinges. Manually operated sliding doors were positioned 8 in. down the approach alley and at the entrance to each goal arm.
Rats were adapted to the maze and trained to run to the ends of the goal arms by using a series of 6 forced ‘‘choices’’ per day for 4 days, each rewarded with 4 food pellets (Formula A/1 45 mg pellets, Research Diets, Inc., New Brunswick, NJ). Right and left arms were alternated in a random, balanced fashion to avoid the introduction of a side bias. Animals then began DMP training.
DMP training was performed in trial pairs. Initially, each rat received 8 trial pairs/day. The first trial of each pair consisted of a forced ‘‘choice’’ in which one goal arm was blocked, forcing the animal to enter the unblocked arm to receive food reward (2 pellets). The rat was then immediately (minimal delay; <5 s) returned to the approach alley for the second trial during which both goal arms were open. A choice was defined as an animal placing both front legs, and at least part of both rear legs, into a goal arm. Returning to the same arm visited on the forced trial resulted in food reward (4 pellets; the rat remained in the arm for 10–20 s while eating the food). Entering the incorrect arm resulted in no food reward and confinement to the arm for 60 s. Forced choices were selected in a random, balanced fashion to avoid the introduction of a side bias. Rats were run in squads of 4–6. After each trial pair, an animal was returned to its cage for 5–10 min while training proceeded with the other animals. Rats continued to receive 8 trial pairs/day until they reached a criterion of 15/16 correct choices over two consecutive days or until they had received 30 days of training.
One day after reaching criterion, animals received one trial pair during which the T-maze was rotated 180° (relative to extramaze cues) between the forced and open trial. This was done to assess whether rats were using a place strategy (relying on extramaze cues) or a response strategy (independent of extramaze cues) to perform the task. After rotating the maze, an animal that was using a response strategy (e.g., follow a left turn with another left turn, or a right turn with another right turn) would enter the previously entered arm, regardless of orientation to the extramaze cues. In contrast, an animal that was using a place strategy (e.g., enter the arm located in the position of the room where the previous choice arm was located) would enter the alternate arm, now located in the same position of the room that was occupied by the other arm during the previous trial. The following 3 days, rats received 8 trial pairs/day with increased intertrial delays of 30, 60, and 90 s, to assess working memory performance.
Configural association training
At the completion of DMP testing, rats were trained on an operant configural association (CA) negative patterning task (Butt et al., 2002). Training was performed in operant chambers (Med. Associates, Inc., Georgia, VT) connected to a computer running Med-PC software. Each operant chamber contained a dim red house light, a ventilation fan, a 6 W stimulus panel light, a speaker calibrated to present a 1500 Hz tone, a pellet dispenser, and a recessed food cup located immediately below the panel light. Entry into the food cup was monitored by a photosensor.
Rats were adapted to the chamber by receiving one 60 min session during which they received a total of 16 food pellets delivered at intervals ranging from 2 to 6 min. CA training began the following day. Each rat received one training session per day for a total of 24 days. Each session lasted for a maximum of 110 min. During the session, rats received 30 presentations of a tone conditioning stimulus (CS), 30 presentations of a light CS, and 30 presentations during which the tone and the light were presented simultaneously. If an animal entered the food cup within 10 s of presentation of the tone or the light, the CS was discontinued and the animal received a food reward (one 45-mg pellet). If an animal entered the food cup when the light and the tone were presented simultaneously, the stimuli were discontinued, the house light was turned off for 60 s, and no food was delivered. This is referred to as a time out. The CS presentations were randomly distributed throughout the session and occurred at one of 30 randomly selected intertrial intervals ranging from 12 to 70 s. During each session, the following measures were recorded: (a) number of responses to each CS, (b) time between presentation of each CS and entry into the food cup, (c) number of entrances into the food cup during the intertrial interval, and (d) percent of time during the intertrial interval spent in the food cup.
Hormone assays
At the completion of training, rats were euthanized with Nembutal (2 cc/kg). Trunk blood was collected and assayed by radioimmunoassay for serum levels of estradiol and testosterone by the Assay Core of the Center for Reproductive Physiology at the University of Pittsburgh. The testosterone assay had a minimum detection limit of approximately 20 pg/ml serum. The estradiol assay had a minimum detection limit of approximately 0.7 pg/ml serum.
Data analysis
Days to criterion on the DMP task were analyzed by Kruskal–Wallis non-parametric ANOVA and by the Dunn’s post-test. Performance during acquisition of the DMP task was grouped into eight 3-day blocks of training. Once an animal reached criterion, a value of 0.9375 (15/16) was recorded for performance on subsequent days. The blocked data were then analyzed by ANOVA with repeated measures on ‘block’. The effects of rotating the maze 180° were analyzed by contingency table and chi-square test. Performance during increased intertrial delays was analyzed by ANOVA with repeated measures on ‘delay’. Each measure recorded during CA training was analyzed by ANOVA with repeated measures on ‘day’. Statistics were performed using Systat 5.0, Prism 3.0, and JMP 5.1 for Macintosh. Statistical significance was defined as P < 0.05.
Results
Hormone levels
The mean circulating levels of testosterone and estradiol for each treatment group are summarized in Table 1. The mean circulating level of testosterone in the gonadally intact and T-treated rats was 3.5 ± 0.5 ng/ml and 14.4 ± 1.0 ng/ml, respectively. As expected, testosterone was undetectable in castrated rats that did not receive T-capsules. The levels of estradiol in both intact and castrated males were very low, ranging from 1.6 to 4.2 pg/ml. Rats that received E-capsules had a mean level of estradiol of 25.2 ± 3.6 pg/ml, which is within the physiological range for young adult, gonadally intact female rats.
Table 1.
Serum levels of gonadal hormones
| Tx Group | N | Testosterone (ng/ml) | Estradiol (pg/ml) |
|---|---|---|---|
| Intact | 11 | 3.5 ± 0.5 | 1.6 ± 0.5 |
| Castrate | 11 | <0.02 | 4.2 ± 0.9 |
| Castrate + T | 12 | 14.4 ± 1.0 | 4.2 ± 1.9 |
| Castrate + E | 12 | <0.02 | 25.2 ± 3.6 |
Values show mean ± SEM.
Effects on the DMP task
DMP acquisition
All rats reached criterion on the DMP task. Neither castration nor T-treatment had any significant effect on the rate at which rats acquired the task. E-treatment, however, appeared to enhance DMP acquisition in males. Analysis of the number of days to reach criterion (Fig. 1) revealed a significant effect of treatment (Kruskal–Wallis statistic = 10.8, P < 0.02) and a significant difference between E-treated and T-treated castrated rats ( P < 0.05 by Dunn’s post-test). The effects of estradiol, however, were most apparent in the learning curves (Fig. 2), which showed that all groups performed at comparable levels below chance at the start of training, but that E-treated animals improved at a significantly faster rate than all other groups. Statistical analysis revealed a significant effect of ‘treatment’ ( F[3,42] = 3.2, P = 0.03), a significant effect of ‘block’ ( F[7, 294] = 277, P < 0.0001), and a significant ‘treatment’ × ‘block’ interaction ( F[21, 294] = 2.3, P = 0.001). Post hoc analysis revealed that during block 3, E-treated animals differed significantly from all other groups ( P < 0.05).
Fig. 1.
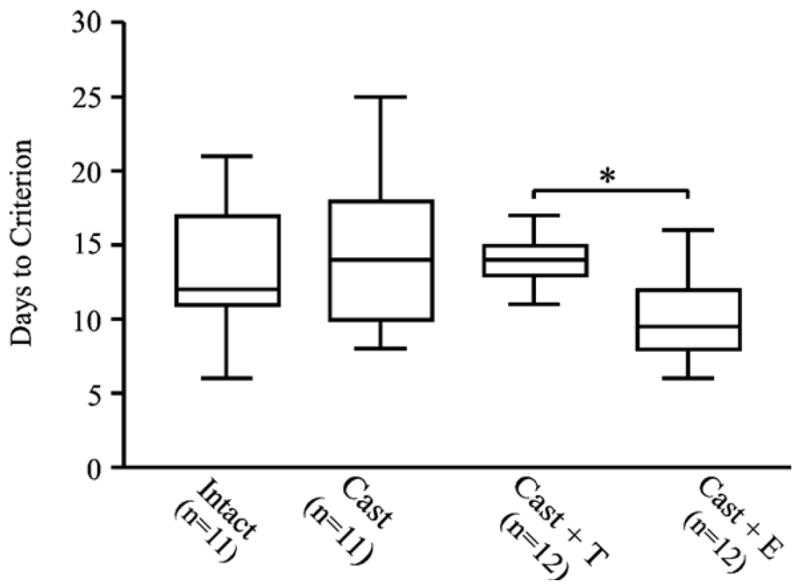
Box and whisker plots comparing the number of days to reach criterion for each of the four treatment groups. E-treated rats required fewer days to reach criterion than T-treated rats (*P < 0.05).
Fig. 2.
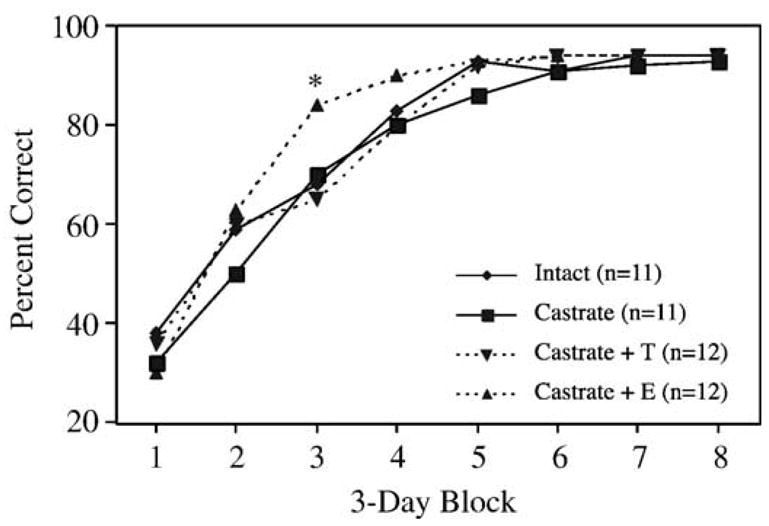
Learning curves showing acquisition of the DMP task by each treatment group as a function of time. Percent correct refers to the percentage of trials on which rats scored a correct response. Values represent the mean performance within a 3-day block of training for each treatment group. E-treated rats scored significantly better on block 3 of training than all other groups (*P < 0.05).
Effects of rotating the maze 180°
After reaching criterion, each rat received one trial pair during which the maze was rotated 180° relative to the extramaze cues between the forced and open trials. Performance was measured by the accuracy of arm choice with respect to position in the room as described in the Methods section. For example, a high level of correct entries would indicate that animals were using a strategy that does not rely on the position of the arm relative to extramaze cues (e.g., a response strategy), whereas a low level of correct entries would indicate that animals were using a strategy that does rely on the location of the arm relative to extramaze cues (e.g., a place strategy). Rotating the maze reduced the performance of all groups to chance levels (40–50%) (Fig. 3), and no significant effects of treatment were detected.
Fig. 3.
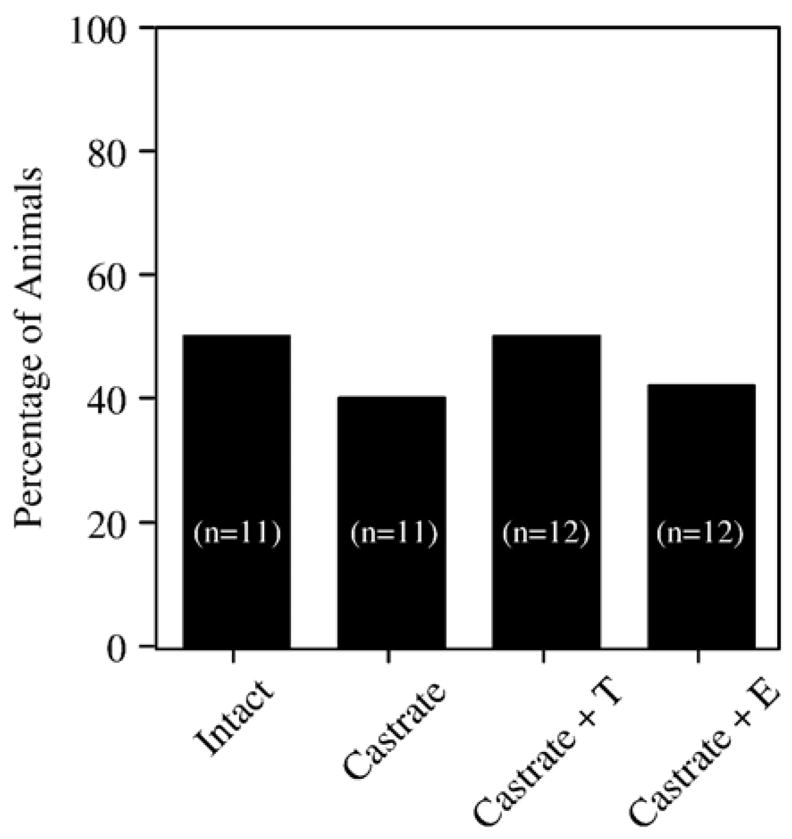
Effects of rotating the maze 180° between the forced and open choices on group performance after rats reached criterion. Bars indicate the percentage of rats in each group that entered the correct arm after rotating the maze 180° prior to the open choice.
Effects on working memory
One day after rotating the maze, rats received 3 days of testing with intertrial delays (delay between the forced and open choice) of 30, 60, and 90 s. The performance of all groups was decreased as a result of increasing the intertrial delay (Fig. 4). Notably, T-treated animals were less affected by increasing the delay than the other groups of castrates. Statistical analysis revealed a significant effect of ‘treatment’ ( F[3,42] = 3.9, P < 0.05) and a significant effect of ‘delay’ F[3,126] = 62.2, P < 0.0001), but no significant interaction between ‘treatment’ and ‘delay’ ( F[9,126] = 1.1, P = 0.37). Post hoc analysis revealed that T-treated animals performed better than castrates ( P < 0.05) and castrates + E ( P < 0.01), with the performance of gonadally intact animals falling between that of the T-treated and non-T-treated castrates.
Fig. 4.
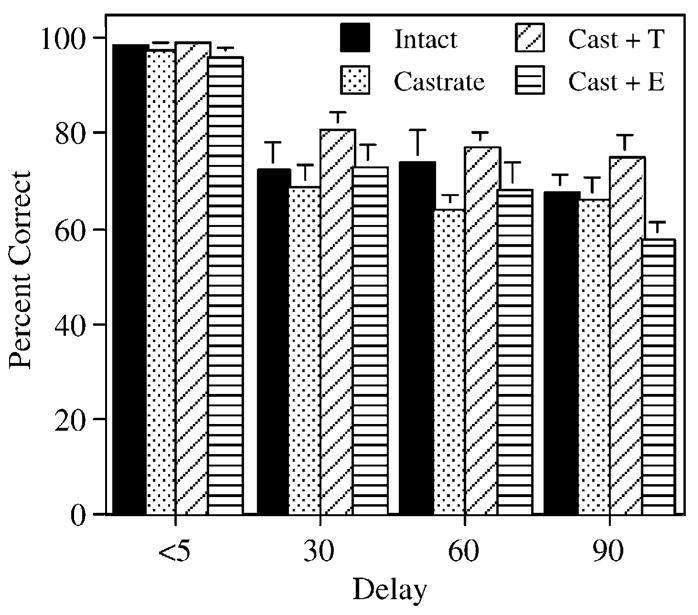
Effects on performance of increasing the delay between the forced and open choice to 30, 60, and 90 s. Percent correct refers to the percentage of trial pairs on which rats scored a correct response. Bars represent the mean percentage of correct responses ± SEM. Performance on the 2 days immediately preceding the increase in intertrial delay was used for the delay <5 s data. Note that T-treated and gonadally intact rats appear less affected by the increase in intertrial delay than the other treatment groups.
Effects on the CA task
Three rats (one E-treated animal, one T-treated animal, and one intact control) developed health problems and had to be removed from the study prior to completion of the CA training. In addition, CA data from three other rats (one T-treated animal, one intact animal, and one castrate control) had to be excluded due to technical problems with one of the operant chambers. Among the remaining 40 animals, all treatment groups demonstrated acquisition of both simple and configural associations in the CA task (Fig. 5). Acquisition of the simple associations (e.g., response to light, response to tone) occurred very rapidly and achieved a stable maximal response (30/30 correct responses) within 3–4 training sessions. Acquisition of the negative patterning component (e.g., lack of response to simultaneous presentation of light + tone) took longer and tended to stabilize at a group average of 18–22 incorrect responses out of 30 presentations after 24 training sessions. Pilot data (not shown) suggest that the performance of Sprague–Dawley rats does not improve significantly beyond this level even after 40 training sessions. Similar patterns were observed for response time. For example, the time to respond to a simple stimulus decreased during the first week of training and continued to decrease throughout training (Figs. 6A–D). In contrast, response time to the complex stimulus decreased during the first 4–5 days of training and then began increasing again. No significant effects of treatment on the number of responses to the complex stimulus (Fig. 5E) or on the average time to respond to the complex stimulus (Fig. 6E) were detected. For example, repeated measures analysis of the number of responses to light + tone on days 10 – 24 revealed no significant effect of ‘treatment’ ( F[3,36] = 0.1, P = 0.94), a significant effect of ‘day’ F[14,504] = 31.0, P < 0.0001), and no significant interaction between ‘treatment’ and ‘day’ ( F[42,504] = 1.3, P = 0.13). Repeated measures analysis of the time to respond to light + tone on days 5–16 likewise revealed no significant effect of ‘treatment’ ( F[3,36] = 1.9, P = 0.15), a significant effect of ‘day’ F[11,396] = 20.7, P < 0.0001), and no significant interaction between ‘treatment’ and ‘day’ ( F[33,396] = 0.77, P = 0.81).
Fig. 5.
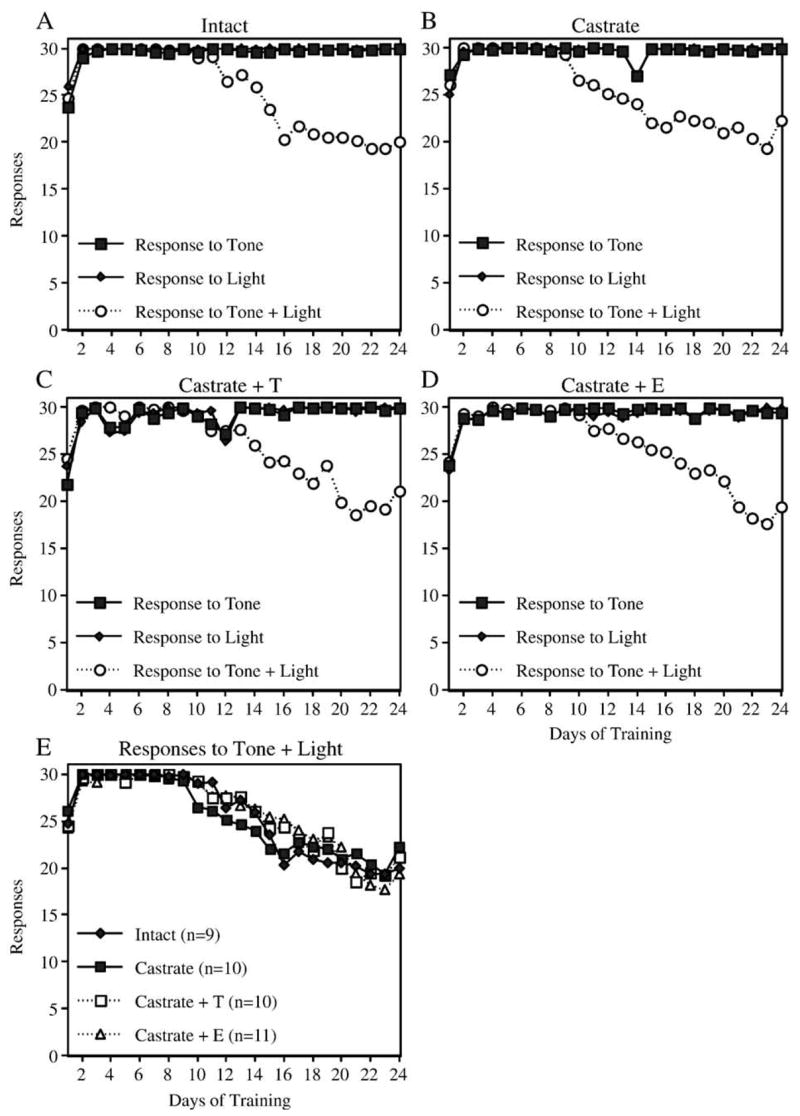
Learning curves showing responses during acquisition of the CA task. Panels A– D show the mean number of responses to the tone, the light, and the combination of tone + light for each treatment group. Note that rats in all groups rapidly learned to respond to the simple stimuli, and more gradually decreased their response to the combination stimulus. Panel E compares the decrease in response to the combination stimulus over time for all four treatment groups.
Fig. 6.
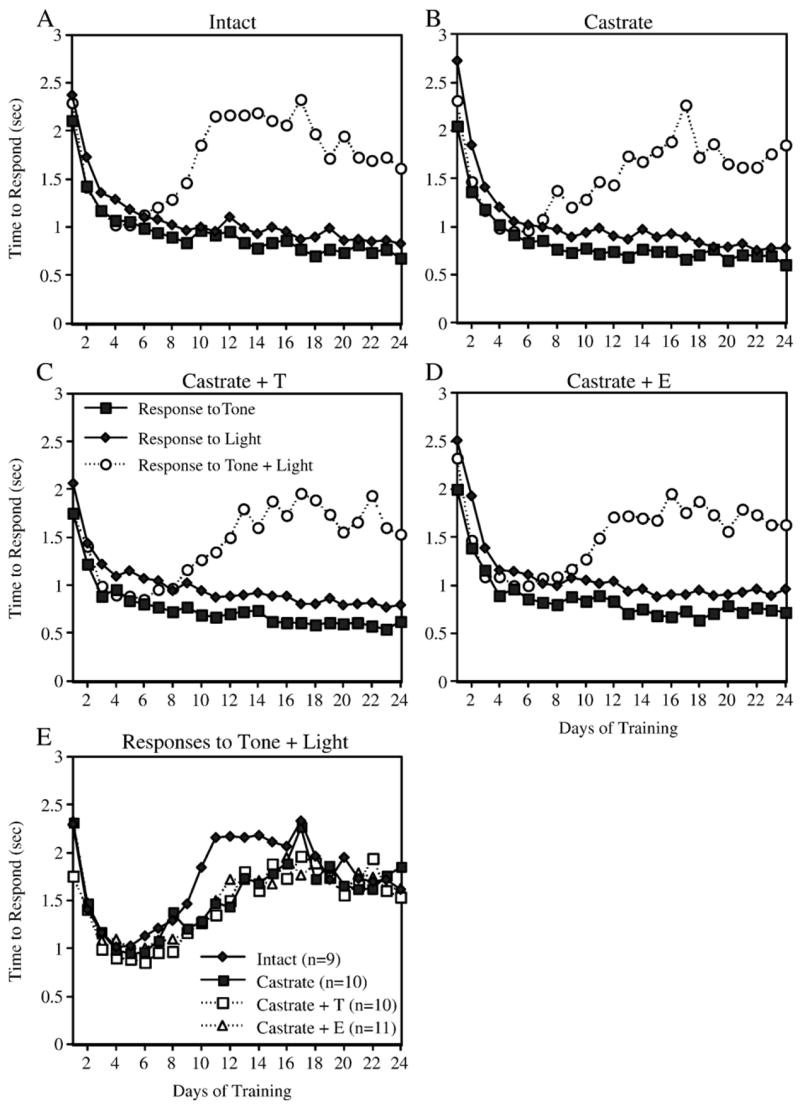
Learning curves showing response time during acquisition of the CA task. Panels A –D show the mean time between presentation of the stimulus and entry into the food cup following presentation of the tone, the light, and the combination of tone + light for each treatment group. Note that response time decreased dramatically at first and continued to decrease in response to the simple stimuli. In contrast, response time to the complex stimulus decreased during the first 4– 5 days of training, and then increased during the next 5– 10 days. Panel E compares response time to the combination stimulus for all four treatment groups.
Discussion
The data demonstrate that (a) hormone treatment can significantly affect cognitive performance in adult, castrated male rats, (b) the effects of hormone treatment on cognitive performance in male rats are task-specific, and (c) testosterone and estradiol have different effects on cognitive performance in male rats, suggesting that the effects of testosterone are not mediated via conversion to estradiol. These conclusions are supported by the observation that estradiol enhanced DMP acquisition in castrated male rats, but did not alter the effect of increasing the intertrial delay, whereas neither castration nor T-treatment had any significant effect on DMP acquisition but did influence the effect of increasing the intertrial delay.
Notably, these findings differ considerably from those reported by Kritzer et al. (2001), who reported that castration produced a significant impairment in acquisition of a delayed alternation T-maze task which was reversed by testosterone treatment, but not by estradiol. The reasons for the discrepancy are not clear. Motivational, attentional, and motor requirements of the two tasks are similar. Some major differences include the use of an alteration paradigm and water reward in the previous study versus use of a matching-to-position paradigm and food reward in the present study. One possibility is that the alternation and matching-to-position paradigms rely on different underlying circuitries to varying degrees. Kritzer and colleagues argue that effects on acquisition of the alteration task correlate with effects on dopaminergic innervation of the frontal cortex; whereas we have shown that estrogen effects on DMP acquisition in female rats are dependent upon cholinergic innervation of the hippocampus (Gibbs, 2002). Hence, the difference in results may reflect the degree to which specific cortical and subcortical circuits are involved in acquisition of the different paradigms.
Importantly, none of the treatments in the present study had any significant effect on acquisition of the CA task. The CA task incorporates many of the same motivational factors that drive performance on the DMP task, namely food deprivation and reward; however, in contrast to spatial tasks which, in rodents, rely heavily on hippocampal circuits, the CA task appears to rely more heavily on projections to frontal cortex (Butt and Hodge, 1997; Butt et al., 2002; Rudy and Sutherland, 1995). The fact that no significant treatment effects were observed on acquisition of the CA task indicates that all groups were equally willing to work for food reward and suggests that the effects of estradiol and testosterone on the DMP task are not due to differences in motivation. These data suggest that estradiol and testosterone each has distinct effects on cognitive performance in males and that the effects are limited to specific cognitive domains.
DMP acquisition versus working memory performance
The effects of estradiol on DMP acquisition in male rats is similar to our findings in female rats, where estradiol significantly enhances DMP acquisition without evidence for an effect on working memory (Gibbs, 1999, 2002; Gibbs et al., 2004). The observation that group performance is equivalent at the start of training, and that E-treated rats improve at a faster rate, demonstrates that E-treatment enhances the rate of learning as opposed to merely improving performance.
It should be noted that effects of estradiol on working memory in rats have been detected with other, more rigorous tasks (Bimonte and Denenberg, 1999; Daniel et al., 1999; Fader et al., 1999; Luine and Rodriguez, 1994). Previously, we hypothesized that the DMP task may not be sensitive enough to detect subtle changes in working memory performance; however, the observation that T-treated rats were less affected by increasing the intertrial delay than the non-T-treated castrates demonstrates that delay-dependent effects can, in fact, be detected and suggests that testosterone, but not estradiol, enhances working memory in males performing this task. Notably, Bimonte et al. (2003) also recently demonstrated that testosterone, but not DHT, enhances working memory performance in aged male rats. The observation that DHT was not effective raises the question of whether local conversion to estradiol is involved. While we cannot definitively rule out the possibility that local conversion of testosterone to estradiol within specific brain regions underlies the effect on DMP performance, the observation that estradiol treatment did not mimic the effect strongly suggests that conversion to estradiol is not sufficient. Likewise, the observation that estradiol, but not testosterone, enhanced DMP acquisition in these same rats supports the conclusion that effects on DMP acquisition and working memory performance are dissociable events and are affected differently by estradiol and testosterone. This is consistent with the findings by Frye and co-workers that show significant distinguishable effects of both estradiol and androgens, including 3-alpha-androstanediol (a metabolite of testosterone), on cognitive performance in rats (Edinger and Frye, 2004; Edinger et al., 2004; Frye and Rhodes, 2002; Frye et al., 2004).
Effects on strategy
Studies show that rodents can use different strategies to solve T-maze tasks (Dudchenko, 2001), and that the rate of acquisition may depend on the specific strategy employed. In general, place strategies (i.e., relying upon the use of extramaze cues) involving hippocampal circuits appear to be most efficient for solving visual spatial tasks (Packard and McGaugh, 1996). Korol and Kolo (2002) recently showed that elevated estrogen biases female rats towards use of a place versus a response strategy. In the present study, once rats had reached criterion, rotating the maze 180° between the forced and open choice significantly impaired performance to chance levels among all treatment groups. This suggests that by the time the animals reached criterion, rats in each treatment group relied on the position of the arms relative to extramaze cues to at least some degree in selecting which arm to enter on the open choice. No significant differences between treatment groups in the performance deficit produced by rotating the maze was observed, suggesting that the effect of estradiol on DMP acquisition was not due to a differential effect on the use of a place versus a response strategy, although a possible difference in the ability to adopt a particular strategy early on during training (i.e., problem solving) cannot be excluded.
Mechanism that may underlie differential effects on DMP acquisition and working memory performance
Several mechanisms by which hormone therapy may affect cognitive performance have been proposed. Two of these include hormone-mediated effects on hippocampal connectivity and function and hormone-mediated effects on basal forebrain cholinergic neurons.
Effects on hippocampal connectivity and function
Elegant studies by Woolley and co-workers (Woolley et al., 1997; Yankova et al., 2001) have shown that, in rats, the number of dendritic spines on CA1 hippocampal pyramidal cells varies across the estrous cycle and is acutely regulated by circulating levels of estradiol and progesterone. Effects on LTP and NMDA responses have also been described (Good et al., 1999; Gupta et al., 2001; Gureviciene et al., 2003; Woolley et al., 1997). Recent evidence suggests that these effects are related to an estrogen-mediated disinhibition of CA1 pyramidal cells via a reduction in GABA-mediated inhibition (Murphy et al., 1998; Rudick and Woolley, 2001; Rudick and Woolley, 2003). Using treatment paradigms analogous to those used by Woolley et al. (1997), Sandstrom and Williams (2001) showed that working memory in ovariectomized female rats was significantly affected by treatment with estradiol and progesterone, with the best performance corresponding with periods of high spine density, and worst performance corresponding with periods of low spine density. This suggests that the effects on hippocampal connectivity that have been described can mediate changes in memory performance. Recent studies by Leranth et al. (2003) have demonstrated that in male rats, castration decreases, and testosterone or dihydrotestosterone restores, dendritic spine density on CA1 pyramidal neurons, indicating that androgens affect spine density in males analogous to the effects of estradiol in females. Notably, estradiol did not restore spine density in male rats. This is consistent with the effect of testosterone, but not estradiol, on working memory in castrated male rats. Conversely, the observation that estradiol, but not castration or testosterone treatment, affected DMP acquisition suggests that this effect is not related to effects on hippocampal connectivity, at least in male rats.
Role of basal forebrain cholinergic neurons
We and others have shown that, in female rats, estradiol also has significant effects on basal forebrain cholinergic neurons (for a review, see Gibbs and Gabor, 2003). These neurons are the primary source of cholinergic innervation to the hippocampus and cortex (Woolf, 1991) and have been shown in many studies to play an important role in learning, memory, and attentional processes (Baxter and Chiba, 1999; Everitt and Robbins, 1997). Recently we showed that basal forebrain cholinergic projections are essential for estrogen-mediated enhancement of DMP acquisition in female rats (Gibbs, 2002). Notably, recent studies indicate that basal forebrain cholinergic projections also play an important role in the estrogen-mediated disinhibition of CA1 pyramidal cells (Rudick et al., 2003) as well as effects of estradiol on CA1 dendritic spine density (Lam and Leranth, 2003).
The extent to which gonadal hormones affect basal forebrain cholinergic neurons in male rats is less clear. One recent study reported that castration resulted in significantly fewer ChAT-immunoreactive cells detected in the medial septum and specific cortical areas relative to intact controls, and that this effect was reversed by testosterone treatment (Nakamura et al., 2002), suggesting that testosterone can affect ChAT expression in the male rat brain. Studies by Packard et al. (Packard and Teather, 1997; Packard et al., 1996) have demonstrated significant effects of systemic and intrahippocampal estradiol administration on memory consolidation in both male and female rats. These effects were blocked by systemic injection of the muscarinic receptor antagonist scopolamine, suggesting that the effects of estradiol depend upon an interaction with cholinergic systems. Therefore, there is evidence that estradiol, acting in the hippocampus via a cholinergic mechanism, can influence memory consolidation in both male and female rats. Since estradiol does not increase spine density on CA1 pyramidal cells in male rats (Leranth et al., 2003), we assume that the effect on memory consolidation involves some other effect of cholinergic afferents on hippocampal function. Likewise, we hypothesize that the effects on DMP acquisition involve cholinergic effects on hippocampal function distinct from those associated with effects on CA1 connectivity.
Summary
Collectively, these data demonstrate a dissociation between the effects of testosterone and estradiol on cognitive performance in male rats. Specifically, testosterone, but not estradiol, appeared to enhance working memory, whereas estradiol, but not testosterone, enhanced DMP acquisition. These effects were task specific and appeared to reflect distinct effects within specific cognitive domains. In contrast, treatments had no effect on acquisition of a configural association task, suggesting that effects on the DMP task were not due to effects on motivational factors. The selectivity of these effects may underlie in part the difficulties in detecting consistent effects of hormone therapy on cognitive performance in human studies, where more global assessments are commonly employed.
Acknowledgments
I wish to acknowledge Charles Kyle Ermer, Cathy Pazsint, and Douglas Nelson for their excellent technical assistance in the performance of these studies. Supported by NIH Grant #RO1 AG021471.
References
- Astur RS, Ortiz ML, Sutherland RJ. A characterization of performance by men and women in a virtual Morris water task: a large and reliable sex difference. Behav Brain Res. 1998;93 (1–2):185–190. doi: 10.1016/s0166-4328(98)00019-9. [DOI] [PubMed] [Google Scholar]
- Baxter MG, Chiba AA. Cognitive functions of the basal forebrain. Curr Opin Neurobiol. 1999;9 (2):178–183. doi: 10.1016/s0959-4388(99)80024-5. [DOI] [PubMed] [Google Scholar]
- Bimonte HA, Denenberg VH. Estradiol facilitates performance as working memory load increases. Psychoneuroendocrinology. 1999;24 (2):161–173. doi: 10.1016/s0306-4530(98)00068-7. [DOI] [PubMed] [Google Scholar]
- Bimonte HA, Nelson ME, Granholm AC. Age-related deficits as working memory load increases: relationships with growth factors. Neurobiol Aging. 2003;24 (1):37–48. doi: 10.1016/s0197-4580(02)00015-5. [DOI] [PubMed] [Google Scholar]
- Bimonte-Nelson HA, Singleton RS, Nelson ME, Eckman CB, Barber J, Scott TY, Granholm AC. Testosterone, but not nonaromatizable dihydrotestosterone, improves working memory and alters nerve growth factor levels in aged male rats. Exp Neurol. 2003;181 (2):301–312. doi: 10.1016/s0014-4886(03)00061-x. [DOI] [PubMed] [Google Scholar]
- Brown TJ, Sharma M, Heisler LE, Karsan N, Walters MJ, MacLusky NJ. In vitro labeling of gonadal steroid hormone receptors in brain tissue sections. Steroids. 1995;60:726–737. doi: 10.1016/0039-128x(95)00107-2. [DOI] [PubMed] [Google Scholar]
- Butt AE, Hodge GK. Simple and configural association learning in rats with bilateral quisqualic acid lesions of the nucleus basalis magnocellularis. Behav Brain Res. 1997;89 (1–2):71–85. doi: 10.1016/s0166-4328(97)00062-4. [DOI] [PubMed] [Google Scholar]
- Butt AE, Noble MM, Rogers JL, Rea TE. Impairments in negative patterning, but not simple discrimination learning, in rats with 192 IgG-saporin lesions of the nucleus basalis magnocellularis. Behav Neurosci. 2002;116 (2):241–255. doi: 10.1037//0735-7044.116.2.241. [DOI] [PubMed] [Google Scholar]
- Ceccarelli I, Scaramuzzino A, Aloisi AM. Effects of gonadal hormones and persistent pain on non-spatial working memory in male and female rats. Behav Brain Res. 2001;123 (1):65–76. doi: 10.1016/s0166-4328(01)00195-4. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Roberts SL, Dohanich GP. Effects of ovarian hormones and environment on radial maze and water maze performance of female rats. Physiol Behav. 1999;66 (1):11 –20. doi: 10.1016/s0031-9384(98)00272-8. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Winsauer PJ, Moerschbaecher JM. Castration in rats impairs performance during acquisition of a working memory task and exacerbates deficits in working memory produced by scopolamine and mecamylamine. Psychopharmacology (Berlin) 2003;170 (3):294–300. doi: 10.1007/s00213-003-1537-4. [DOI] [PubMed] [Google Scholar]
- Dawson JLM, Cheung YM, Lau RTS. Developmental effects of neonatal sex hormones on spatial and activity skills in the white rat. Biol Psychol. 1975;3:213–229. doi: 10.1016/0301-0511(75)90036-8. [DOI] [PubMed] [Google Scholar]
- Dohanich GP. Gonadal steroids, learning and memory. In: Pfaff DW, Arnold AP, Etgen AM, Farbach SE, Rubin RT, editors. Horm Brain Behav. Vol. 1. Academic Press; San Diego: 2002. pp. 265–327. [Google Scholar]
- Dudchenko PA. How do animals actually solve the T maze? Behav Neurosci. 2001;115 (4):850–860. [PubMed] [Google Scholar]
- Edinger KL, Frye CA. Testosterone’s analgesic, anxiolytic, and cognitive-enhancing effects may be due in part to actions of its 5alpha-reduced metabolites in the hippocampus. Behav Neurosci. 2004;118 (6):1352–1364. doi: 10.1037/0735-7044.118.6.1352. [DOI] [PubMed] [Google Scholar]
- Edinger KL, Lee B, Frye CA. Mnemonic effects of testosterone and its 5alpha-reduced metabolites in the conditioned fear and inhibitory avoidance tasks. Pharmacol Biochem Behav. 2004;78 (3):559–568. doi: 10.1016/j.pbb.2004.04.024. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Central cholinergic systems and cognition. Annu Rev Psychol. 1997;48:649–684. doi: 10.1146/annurev.psych.48.1.649. [DOI] [PubMed] [Google Scholar]
- Fader AJ, Johnson PEM, Dohanich GP. Estrogen improves working but not reference memory and prevents amnestic effects of scopolamine on a radial-arm maze. Pharmacol Biochem Behav. 1999;62 (4):711–717. doi: 10.1016/s0091-3057(98)00219-6. [DOI] [PubMed] [Google Scholar]
- Frye CA, Rhodes ME. Enhancing effects of estrogen on inhibitory avoidance performance may be in part independent of intracellular estrogen receptors in the hippocampus. Brain Res. 2002;956 (2):285–293. doi: 10.1016/s0006-8993(02)03559-x. [DOI] [PubMed] [Google Scholar]
- Frye CA, Edinger KL, Seliga AM, Wawrzycki JM. 5alpha-reduced androgens may have actions in the hippocampus to enhance cognitive performance of male rats. Psychoneuroendocrinology. 2004;29 (8):1019–1027. doi: 10.1016/j.psyneuen.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Gallagher AM, De Lisi R, Holst PC, McGillicuddy-De Lisi AV, Morely M, Cahalan C. Gender differences in advanced mathematical problem solving. J Exp Child Psychol. 2000;75 (3):165–190. doi: 10.1006/jecp.1999.2532. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Estrogen replacement enhances acquisition of a spatial memory task and reduces deficits associated with hippocampal muscarinic receptor inhibition. Horm Behav. 1999;36:222–233. doi: 10.1006/hbeh.1999.1541. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Basal forebrain cholinergic neurons are necessary for estrogen to enhance acquisition of a delayed matching-to-position T-maze task. Horm Behav. 2002;42:245–257. doi: 10.1006/hbeh.2002.1825. [DOI] [PubMed] [Google Scholar]
- Gibbs RB, Gabor R. Estrogen and cognition: applying preclinical findings to clinical perspectives. J Neurosci Res. 2003;74:637–643. doi: 10.1002/jnr.10811. [DOI] [PubMed] [Google Scholar]
- Gibbs RB, Gabor R, Cox T, Johnson DA. Effects of raloxifene and estradiol on hippocampal acetylcholine release and spatial learning in the rat. Psychoneuroendocrinology. 2004;29:741–748. doi: 10.1016/S0306-4530(03)00118-5. [DOI] [PubMed] [Google Scholar]
- Good M, Day M, Muir JL. Cyclical changes in endogenous levels of oestrogen modulate the induction of LTD and LTP in the hippocampal CA1 region. Eur J Neurosci. 1999;11 (12):4476–4480. doi: 10.1046/j.1460-9568.1999.00920.x. [DOI] [PubMed] [Google Scholar]
- Gupta RR, Sen S, Diepenhorst LL, Rudick CN, Maren S. Estrogen modulates sexually dimorphic contextual fear conditioning and hippocampal long-term potentiation (LTP) in rats(1) Brain Res. 2001;888 (2):356–365. doi: 10.1016/s0006-8993(00)03116-4. [DOI] [PubMed] [Google Scholar]
- Gureviciene I, Puolivali J, Pussinen R, Wang J, Tanila H, Ylinen A. Estrogen treatment alleviates NMDA-antagonist induced hippo-campal LTP blockade and cognitive deficits in ovariectomized mice. Neurobiol Learn Mem. 2003;79 (1):72–80. doi: 10.1016/s1074-7427(02)00012-6. [DOI] [PubMed] [Google Scholar]
- Halpern D. Sex Differences in Cognitive Abilities. 2. Lawrence Erlbaum Associates; Hillsdale, NJ: 1992. [Google Scholar]
- Harrell LE, Goyal M, Parsons DS, Peagler A. The effect of gonadal steroids on the behavioral and biochemical effects of hippo-campal sympathetic ingrowth. Physiol Behav. 1990;48 (4):507–513. doi: 10.1016/0031-9384(90)90291-b. [DOI] [PubMed] [Google Scholar]
- Isgor C, Sengelaub DR. Effects of neonatal gonadal steroids on adult CA3 pyramidal neuron dendritic morphology and spatial memory in rats. J Neurobiol. 2003;55 (2):179–190. doi: 10.1002/neu.10200. [DOI] [PubMed] [Google Scholar]
- Kerr JE, Allore RJ, Beck SG, Handa RJ. Distribution and hormonal regulation of androgen receptor (AR) and AR messenger ribonucleic acid in the rat hippocampus. Endocrinology. 1995;136 (8):3213–3221. doi: 10.1210/endo.136.8.7628354. [DOI] [PubMed] [Google Scholar]
- Korol DL. Role of estrogen in balancing contributions from multiple memory systems. Neurobiol Learn Mem. 2004;82 (3):309–323. doi: 10.1016/j.nlm.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Korol DL, Kolo LL. Estrogen-induced changes in place and response learning in young adult female rats. Behav Neurosci. 2002;116 (3):411–420. doi: 10.1037//0735-7044.116.3.411. [DOI] [PubMed] [Google Scholar]
- Kritzer MF, McLaughlin PJ, Smirlis T, Robinson JK. Gonadectomy impairs T-maze acquisition in adult male rats. Horm Behav. 2001;39 (2):167–174. doi: 10.1006/hbeh.2001.1645. [DOI] [PubMed] [Google Scholar]
- Lam TT, Leranth C. Role of the medial septum diagonal band of Broca cholinergic neurons in oestrogen-induced spine synapse formation on hippocampal CA1 pyramidal cells of female rats. Eur J Neurosci. 2003;17 (10):1997–2005. doi: 10.1046/j.1460-9568.2003.02637.x. [DOI] [PubMed] [Google Scholar]
- Leranth C, Petnehazy O, MacLusky NJ. Gonadal hormones affect spine synaptic density in the CA1 hippocampal subfield of male rats. J Neurosci. 2003;23 (5):1588–1592. doi: 10.1523/JNEUROSCI.23-05-01588.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leranth C, Hajszan T, MacLusky NJ. Androgens increase spine synapse density in the CA1 hippocampal subfield of ovariectomized female rats. J Neurosci. 2004;24 (2):495–499. doi: 10.1523/JNEUROSCI.4516-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine V, Rodriguez M. Effects of estradiol on radial arm maze performance of young and aged rats. Behav Neural Biol. 1994;62 (3):230–236. doi: 10.1016/s0163-1047(05)80021-4. [DOI] [PubMed] [Google Scholar]
- Luine VN, Jacome LF, Maclusky NJ. Rapid enhancement of visual and place memory by estrogens in rats. Endocrinology. 2003;144 (7):2836–2844. doi: 10.1210/en.2003-0004. [DOI] [PubMed] [Google Scholar]
- Murphy DD, Cole NB, Greenberger V, Segal M. Estradiol increases dendritic spine density by reducing GABA neurotransmission in hippocampal neurons. J Neurosci. 1998;18 (7):2550–2559. doi: 10.1523/JNEUROSCI.18-07-02550.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura N, Fujita H, Kawata M. Effects of gonadectomy on immunoreactivity for choline acetyltransferase in the cortex, hippocampus, and basal forebrain of adult male rats. Neuroscience. 2002;109 (3):473–485. doi: 10.1016/s0306-4522(01)00513-9. [DOI] [PubMed] [Google Scholar]
- Packard MG. Posttraining estrogen and memory modulation. Horm Behav. 1998;34 (2):126–139. doi: 10.1006/hbeh.1998.1464. [DOI] [PubMed] [Google Scholar]
- Packard MG, McGaugh JL. Inactivation of hippocampus or caudate nucleus with lidocaine differentially affects expression of place and response learning. Neurobiol Learn Mem. 1996;65 (1):65–72. doi: 10.1006/nlme.1996.0007. [DOI] [PubMed] [Google Scholar]
- Packard MG, Teather LA. Posttraining estradiol injections enhance memory in ovariectomized rats: cholinergic blockade and synergism. Neurobiol Learn Mem. 1997;68:172–188. doi: 10.1006/nlme.1997.3785. [DOI] [PubMed] [Google Scholar]
- Packard MG, Kohlmaier JR, Alexander GM. Posttraining intrahippocampal estradiol injections enhance spatial memory in male rats: interaction with cholinergic systems. Behav Neurosci. 1996;110 (3):626–632. doi: 10.1037//0735-7044.110.3.626. [DOI] [PubMed] [Google Scholar]
- Rudick CN, Woolley CS. Estrogen regulates functional inhibition of hippocampal CA1 pyramidal cells in the adult female rat. J Neurosci. 2001;21 (17):6532–6543. doi: 10.1523/JNEUROSCI.21-17-06532.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudick CN, Woolley CS. Selective estrogen receptor modulators regulate phasic activation of hippocampal CA1 pyramidal cells by estrogen. Endocrinology. 2003;144 (1):179–187. doi: 10.1210/en.2002-220581. [DOI] [PubMed] [Google Scholar]
- Rudick CN, Gibbs RB, Woolley CS. A role for the basal forebrain cholinergic system in estrogen-induced disinhibition of hippocampal pyramidal cells. J Neurosci. 2003;23 (11):4479–4490. doi: 10.1523/JNEUROSCI.23-11-04479.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy JW, Sutherland RJ. Configural association theory and the hippocampal formation: an appraisal and reconfiguration. Hippocampus. 1995;5 (5):375–389. doi: 10.1002/hipo.450050502. [DOI] [PubMed] [Google Scholar]
- Sandstrom NJ, Williams CL. Memory retention is modulated by acute estradiol and progesterone replacement. Behav Neurosci. 2001;115 (2):384–393. [PubMed] [Google Scholar]
- Simerly RB, Chang C, Muramatsu M, Swanson LW. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study. J Comp Neurol. 1990;294:76–95. doi: 10.1002/cne.902940107. [DOI] [PubMed] [Google Scholar]
- Williams CL, Meck WH. The organizational effects of gonadal steroids on sexually dimorphic spatial ability. Psychoneuroendocrinology. 1991;16:155–176. doi: 10.1016/0306-4530(91)90076-6. [DOI] [PubMed] [Google Scholar]
- Woolf NJ. Cholinergic systems in mammalian brain and spinal cord. Prog Neurobiol. 1991;37:475–524. doi: 10.1016/0301-0082(91)90006-m. [DOI] [PubMed] [Google Scholar]
- Woolley CS. Effects of oestradiol on hippocampal circuitry. Novartis Found Symp. 2000;230:173–180. 181–7. doi: 10.1002/0470870818.ch13. [DOI] [PubMed] [Google Scholar]
- Woolley CS, Weiland NG, McEwen BS, Schwartzkroin PA. Estradiol increases the sensitivity of hippocampal CA1 pyramidal cells to NMDA receptor-mediated synaptic input: correlation with dendritic spine density. J Neurosci. 1997;17 (5):1848–1859. doi: 10.1523/JNEUROSCI.17-05-01848.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yankova M, Hart SA, Woolley CS. Estrogen increases synaptic connectivity between single presynaptic inputs and multiple postsynaptic CA1 pyramidal cells: a serial electron-microscopic study. Proc Natl Acad Sci U S A. 2001;98 (6):3525–3530. doi: 10.1073/pnas.051624598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zola-Morgan S, Squire LR, Amaral DG. Human amnesia and the medial temporal region: enduring memory impairment following a bilateral lesion limited to field CA1 of the hippocampus. J Neurosci. 1986;6 (10):2950–2967. doi: 10.1523/JNEUROSCI.06-10-02950.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]


